Experimental Study on the Adhesion of Abalone to Surfaces with Different Morphologies
Abstract
:1. Introduction
2. Materials and Methods
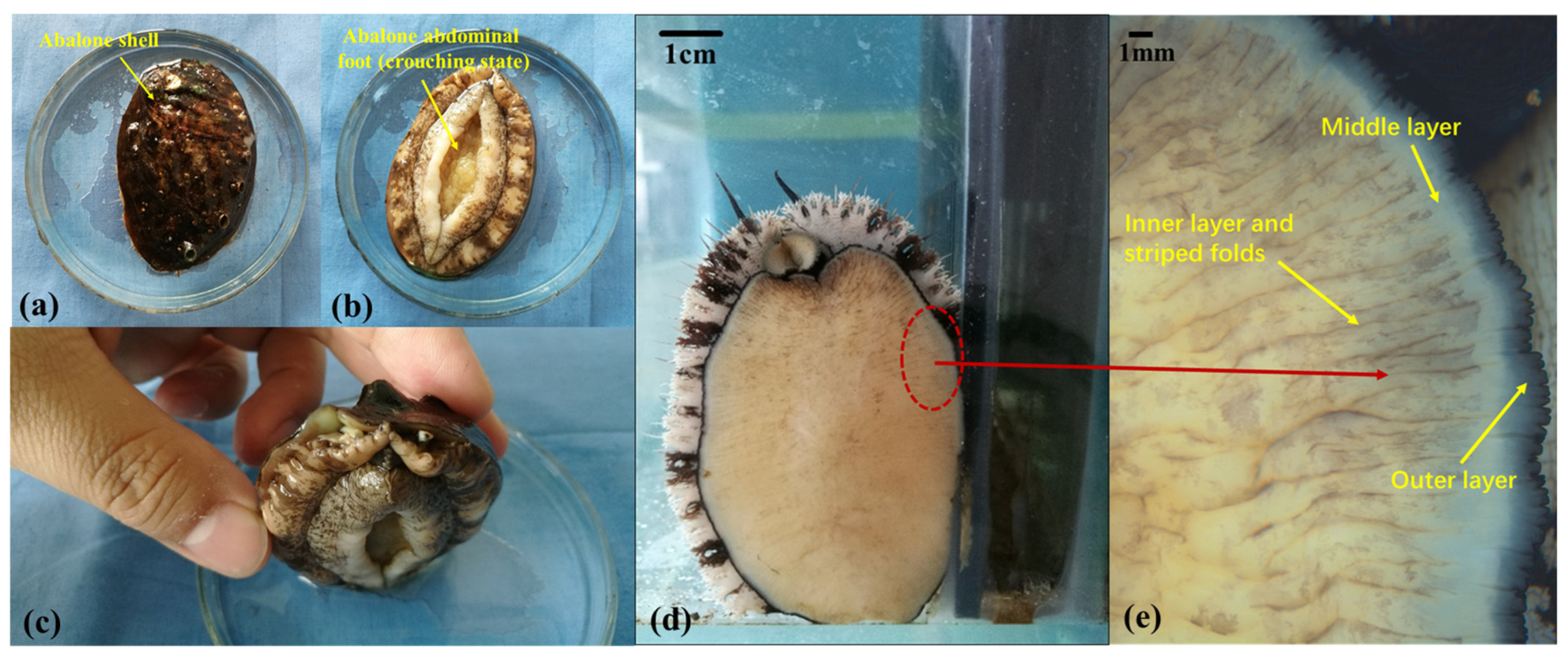
2.1. Observation of the Abalone’s Abdominal Foot
2.1.1. Abalone Sample Preparation
2.1.2. Observations of the Abalone’s Abdominal Foot Surface Morphology
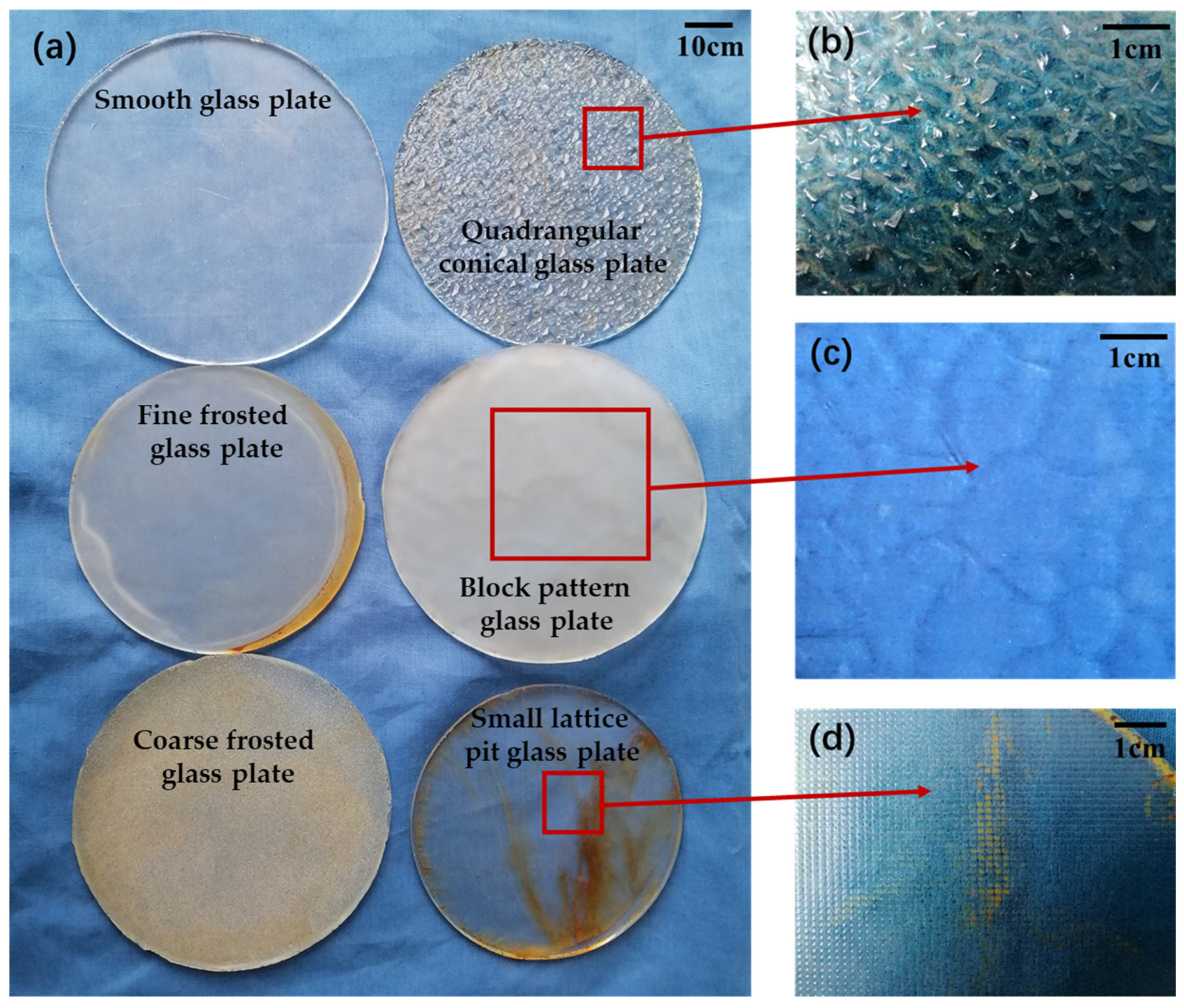
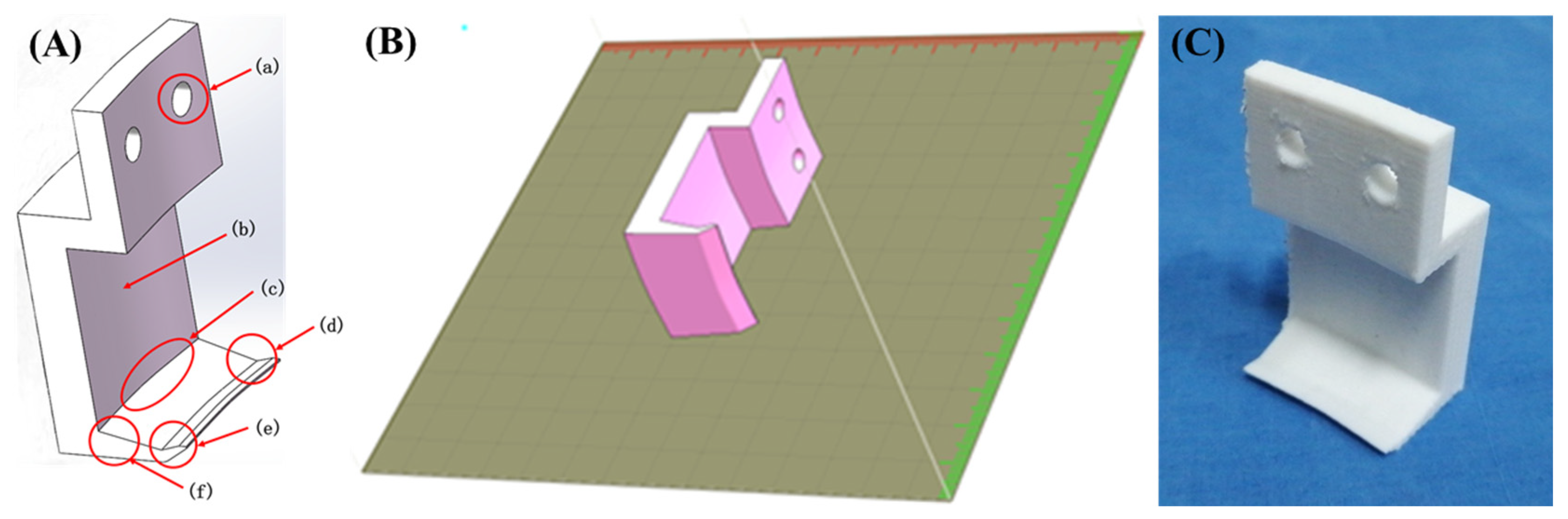
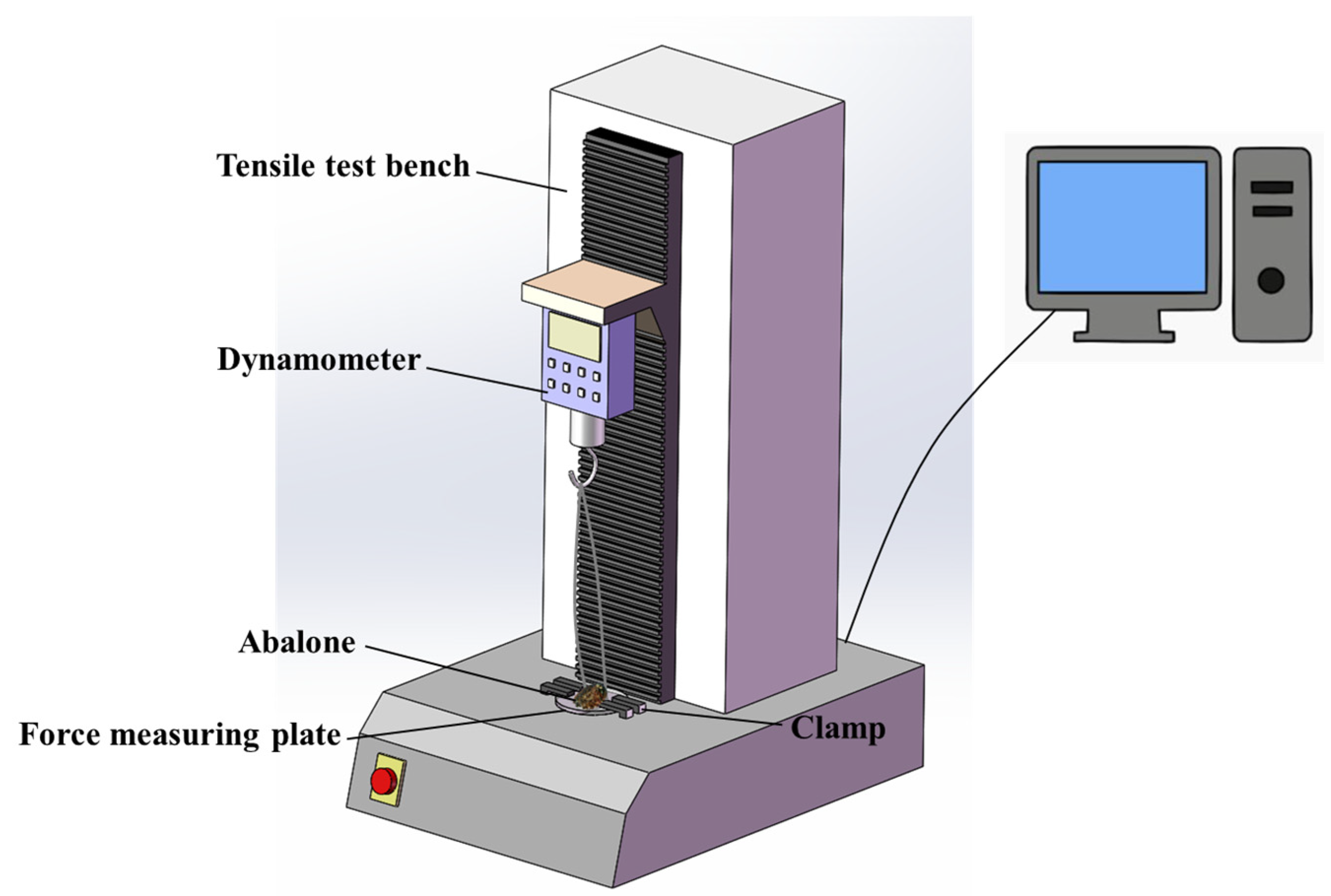
2.2. Adhesion Test
2.2.1. Preparation of the Force Measuring Plate
2.2.2. Design and Processing of the Hook
2.2.3. Abalone Abdominal Foot Adhesion Test
3. Results and Discussion
3.1. Calculation and Analysis of Test Results
3.2. Adhesion Mechanism Analysis
4. Conclusions
- (1)
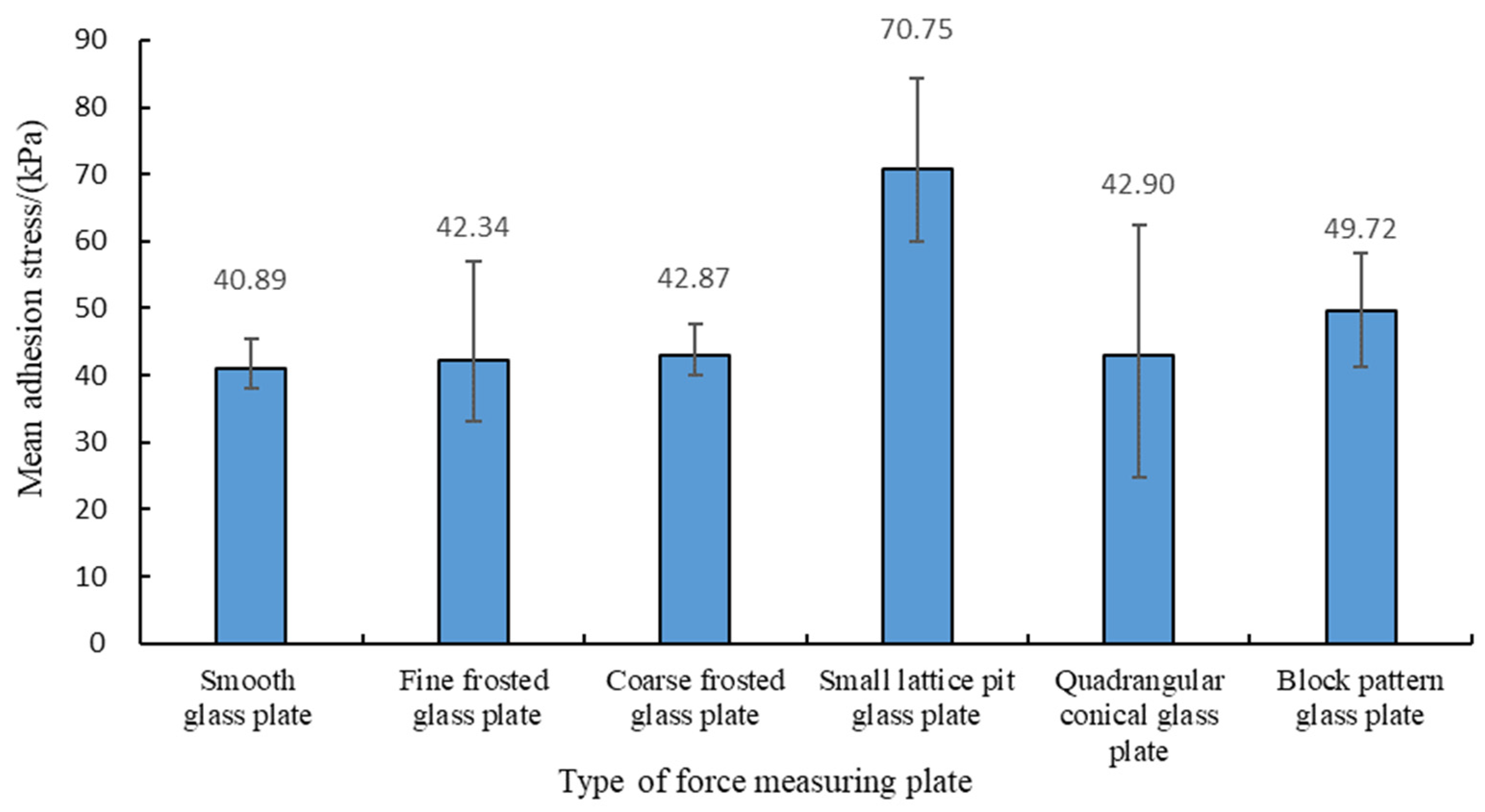
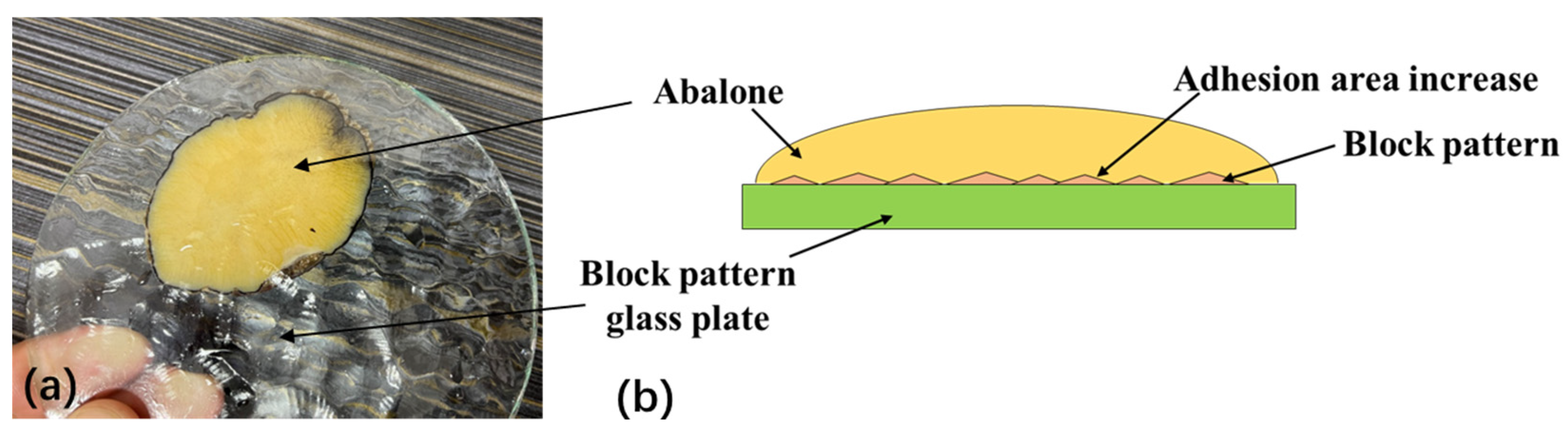
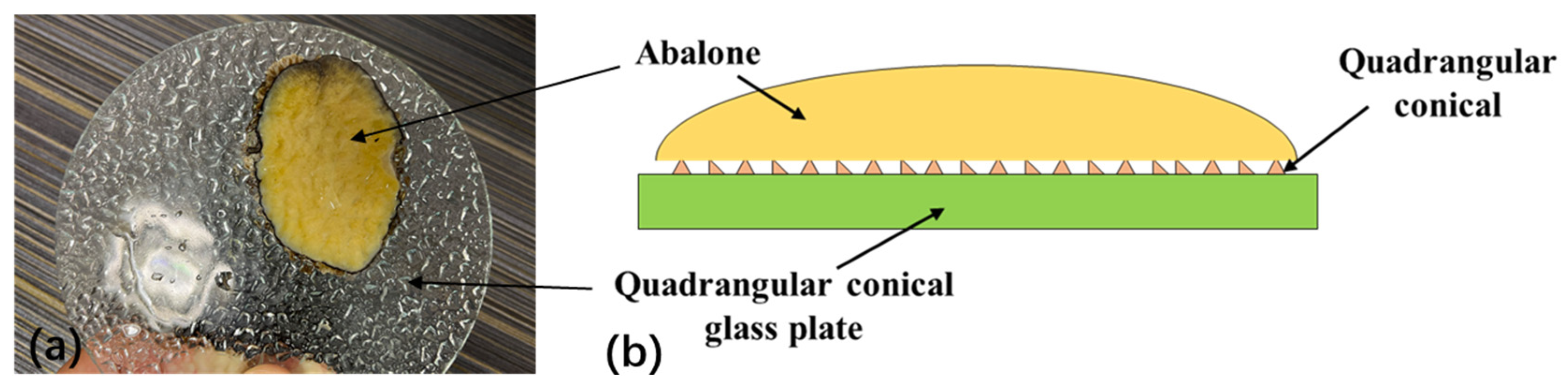
- There was no significant difference in the adhesion of the abalone to the fine frosted glass plate, coarse frosted glass plate, quadrangular conical glass plate, or smooth glass plate. However, adhesion to the small lattice pit glass plate and block pattern glass plate was significantly different.
- (2)
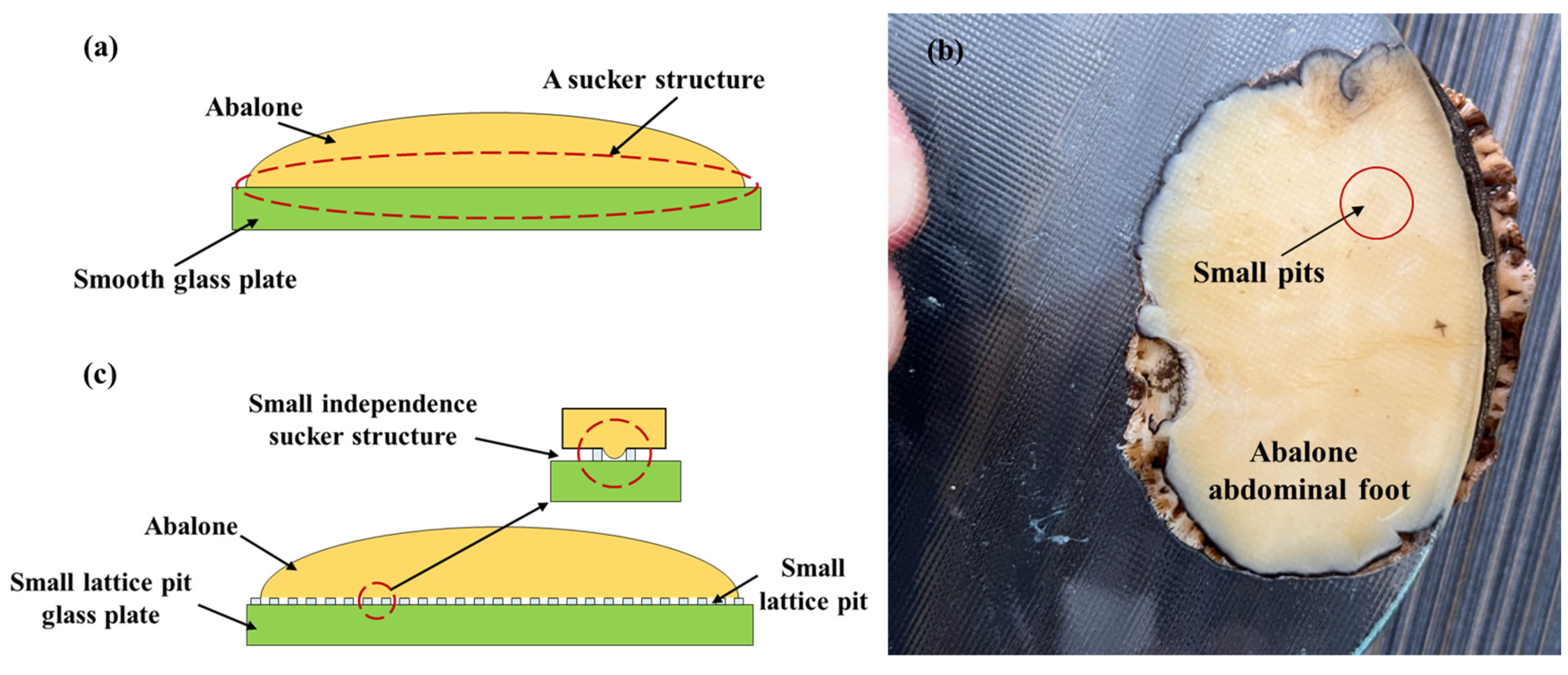
- The quadrangular conical shape in the quadrangular conical glass plate changed rapidly, making it difficult for the abalone’s abdominal foot to fully adhere to the morphological surface of this plate. Conversely, the surface morphology of the block pattern glass plate changed slowly, enabling the abalone’s abdominal foot to fully adhere to this plate’s surface. When the abalone adhered to the small lattice pit glass plate, each small lattice pit was enclosed, excluded some of the gas in the pit, forming an independent sucker system due to the stretching characteristics of the abdominal foot and resulting in a significant increase in the adhesion of the abdominal foot.
- (3)
- Changes in the stretching of the abdominal foot created difficulties in achieving small morphological size changes based on the roughness, leading to no significant differences in the adhesion of abalone to force measuring plates with different types of roughness.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Ma, C.; Ji, J.; Li, J. Underwater adhesion mechanisms and biomimetic study of Marine Life. Tribology 2020, 40, 816–830. [Google Scholar] [CrossRef]
- Maie, T.; Blob, R.W. Adhesive force and endurance of the pelvic sucker across different modes of waterfall-climbing in gobiid fishes: Contrasting climbing mechanisms share aspects of ontogenetic change. Zoology 2021, 149, 125969. [Google Scholar] [CrossRef]
- Palecek, A.M.; Schoenfuss, H.L.; Blob, R.W. Sucker shapes, skeletons, and bioinspiration: How hard and soft tissue morphology generates adhesive performance in waterfall climbing goby fishes. Integr. Comp. Biol. 2022, 62, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Luo, H.Y.; Linghu, C.H.; Song, J. Elastic energy storage enabled magnetically actuated, octopus-inspired smart adhesive. Adv. Funct. Mater. 2021, 31, 2009217.1–2009217.9. [Google Scholar] [CrossRef]
- Baik, S.; Hwang, G.W.; Jang, S.; Kim, K.H.; Yang, T.H.; Pang, C.H. Bioinspired microsphere-embedded adhesive architectures for an electrothermally actuating transport device of dry/wet pliable surfaces. ACS Appl. Mater. Interfaces 2021, 13, 6930–6940. [Google Scholar] [CrossRef]
- Huie, J.M.; Summers, A.P. The effects of soft and rough substrates on suction-based adhesion. J. Exp. Biol. 2022, 225, jeb243773. [Google Scholar] [CrossRef]
- Kazuma, T.; Yasutaka, M.; Masatsugu, S.; Yuji, H. A new concept for an adhesive material inspired by clingfish sucker nanofilaments. Langmuir ACS J. Surf. Colloids 2022, 38, 1215–1222. [Google Scholar] [CrossRef]
- Popov, V.L.; Filippov, A.E.; Gorb, S.N. Biological microstructures with high adhesion and friction. Numerical approach. Phys. Uspekhi 2016, 59, 829–845. [Google Scholar] [CrossRef]
- Bai, L.; Song, Z.; Wang, P. Simulation analysis of internal flow field of vacuum sucker under different leakage conditions. Mech. Eng. 2018, 7, 33–35+38. [Google Scholar] [CrossRef]
- Qin, J.; Deng, C.; Wang, D.; Li, W. Influence of different layouts based on finite element on vacuum adhesion device. J. Guilin Univ. Technol. 2017, 37, 713–717. [Google Scholar]
- Zhen, J.; Yang, Z.; Wang, X.; Song, H. Design of an intelligent vacuum sucker device. J. Vac. Sci. Technol. 2017, 37, 1038–1043. [Google Scholar]
- Zhang, J.; Xue, W.; Liang, Y.; Yang, M. Vacuum adhesion technology and its application in construction machinery assembly. Constr. Mach. Maint. 2015, S1, 302–305. [Google Scholar]
- Nie, J.; Wang, T.; Xu, Y.; Chen, Z.; Ma, Q. Vacuum adhesion performance test of flexible sucker. Hydraul. Pneum. 2020, 131–137. [Google Scholar] [CrossRef]
- Chen, L.; Yun, Z.; Jiang, Y. Design and performance analysis of bionic sucker based on remora adhesion principle. J. Beijing Univ. Chem. Technol. Nat. Sci. Ed. 2018, 45, 100–105. [Google Scholar]
- Li, J.; Liang, D.; Lu, B.; Li, G. Research on non-contact handling sucker system. J. Instrum. 2018, 39, 108–116. [Google Scholar] [CrossRef]
- Francesca, T.; Esther, A.; Barbara, M.; Stanislav, N.G. Hairy suckers: The surface microstructure and its possible functional significance in the Octopus vulgaris sucker Francesca. Beilstein J. Nanotechnol. 2014, 5, 561–565. [Google Scholar] [CrossRef]
- Francesca, T.; Nicola, M.P.; Michael, J.K.; Barbara, M. Unveiling the morphology of the acetabulum in octopus suckers and its role in attachment. Interface Focus 2014, 5, 20140050. [Google Scholar] [CrossRef]
- Francesca, T.; Lucia, B.; Michael, K.; Alessandro, G.; Angelo, B.; Barbara, M. The Morphology and Adhesion Mechanism of Octopus vulgaris Suckers. PLoS ONE 2013, 8, e65074. [Google Scholar] [CrossRef]
- Ditsche, P.; Wainwright, D.K.; Summers, A.P. Attachment to challenging substrates—Fouling, roughness and limits of adhesion in the northern clingfish (Gobiesox maeandricus). J. Exp. Biol. 2014, 217, 2548–2554. [Google Scholar] [CrossRef]
- Ditsche, P.; Summers, A.P. Learning from Northern clingfish (Gobiesox maeandricus): Bioinspired suction cups attach to rough surfaces. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190204. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chang, H.K.; Liu, G.L.; Chen, P.Y. Climbing upstream: Multi-scale structural characterization and underwater adhesion of the Pulin river loach (Sinogastromyzon puliensis). J. Mech. Behav. Biomed. Mater. 2017, 73, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Zuo, P.; Xu, X.; Liu, J. Adhesion Behaviors of Abalone Under the Action of Water Flow. Front. Mech. Eng. 2021, 7, 659468. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zuo, P.; Li, J.; Liu, J. A mechanics study on the self-righting of abalone from the substrate. Appl. Bionics Biomech. 2020, 2020, 8825451. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, C.; Liu, J.; Dong, X.; Liu, J. The co-effect of microstructures and mucus on the adhesion of abalone from a mechanical perspective. Biosurf. Biotribol. 2021, 7, 180–186. [Google Scholar] [CrossRef]
- Liu, X. Biological characteristics and artificial breeding technology of abalone. Anhui Agric. Sci. 2009, 37, 5872–5874. [Google Scholar]
- Lin, A.; Brunner, R.; Chen, P.; Talke, F.; Meyers, M. Underwater adhesion of abalone: The role of van der Waals and capillary forces. Acta Mater. 2009, 57, 4178–4185. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, S.; Liu, J. Insights into adhesion of abalone: A mechanical approach. J. Mech. Behav. Biomed. Mater. 2018, 77, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Xi, P.; Ye, S.; Cong, Q. Abalone adhesion: The role of various adhesion forces and their proportion to total adhesion force. PLoS ONE 2023, 18, e0286567. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, M. Abalone aquaculture technology. Agric. Eng. Technol. 2016, 36, 72. [Google Scholar]
- Sun, L. Study on high adhesion performance of abalone and design of bionic sucker. Master’s Thesis, Jilin University, Changchun, China, 2017. [Google Scholar]
| Maximum Adhesion Force/N | Type of Force Measuring Plate | |||||
|---|---|---|---|---|---|---|
| Test Times | Smooth Glass Plate | Fine Frosted Glass Plate | Coarse Frosted Glass Plate | Small Lattice Pit Glass Plate | Quadrangular Conical Glass Plate | Block Pattern Glass Plate |
| 1 | 80.5 | 110.9 | 105.2 | 176.1 | 61.17 | 126.6 |
| Abalone mass/g | 49.1 | 67.5 | 54.9 | 56 | 57.4 | 60.2 |
| 2 | 89.13 | 95.92 | 89.46 | 149.5 | 175.6 | 125.1 |
| Abalone mass/g | 48.3 | 67 | 51.5 | 57.2 | 65.3 | 60.3 |
| 3 | 116.9 | 142.9 | 103.2 | 204 | 108.2 | 113.3 |
| Abalone mass/g | 59.7 | 58.1 | 56.9 | 56 | 56 | 63.6 |
| 4 | 96.9 | 114.6 | 112.7 | 144.9 | 94.7 | 129.4 |
| Abalone mass/g | 57.4 | 60.3 | 54.7 | 56 | 59.4 | 51.5 |
| 5 | 101.6 | 102.5 | 92.64 | 191.6 | 114.5 | 116.1 |
| Abalone mass/g | 60.1 | 60.3 | 53.8 | 58.5 | 58 | 51.5 |
| Adhesion Stress/kPa | Type of Force Measuring Plate | |||||
|---|---|---|---|---|---|---|
| Test Times | Smooth Glass Plate | Fine Frosted Glass Plate | Coarse Frosted Glass Plate | Small Lattice Pit Glass Plate | Quadrangular Conical Glass Plate | Block Pattern Glass Plate |
| 1 | 38.00 | 38.08 | 44.41 | 72.88 | 24.70 | 48.74 |
| 2 | 42.77 | 33.18 | 40.26 | 60.57 | 62.32 | 48.08 |
| 3 | 45.38 | 57.00 | 42.03 | 84.42 | 44.78 | 41.28 |
| 4 | 39.12 | 44.04 | 47.75 | 59.97 | 36.95 | 58.23 |
| 5 | 39.18 | 39.39 | 39.91 | 75.90 | 45.75 | 52.24 |
| Average value | 40.89 | 42.34 | 42.87 | 70.75 | 42.90 | 49.72 |
| Type of Force Measuring Plate | p Value | Explanation |
|---|---|---|
| Fine frosted glass plate | 0.917 | Comparison with smooth glass plate |
| Coarse frosted glass plate | 0.251 | Comparison with smooth glass plate |
| Small lattice pit glass plate | 0.009 | Comparison with smooth glass plate |
| Quadrangular conical glass plate | 0.754 | Comparison with smooth glass plate |
| Block pattern glass plate | 0.028 | Comparison with smooth glass plate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, P.; Qiao, Y.; Cong, Q.; Cui, Q. Experimental Study on the Adhesion of Abalone to Surfaces with Different Morphologies. Biomimetics 2024, 9, 206. https://doi.org/10.3390/biomimetics9040206
Xi P, Qiao Y, Cong Q, Cui Q. Experimental Study on the Adhesion of Abalone to Surfaces with Different Morphologies. Biomimetics. 2024; 9(4):206. https://doi.org/10.3390/biomimetics9040206
Chicago/Turabian StyleXi, Peng, Yanqi Qiao, Qian Cong, and Qingliang Cui. 2024. "Experimental Study on the Adhesion of Abalone to Surfaces with Different Morphologies" Biomimetics 9, no. 4: 206. https://doi.org/10.3390/biomimetics9040206
APA StyleXi, P., Qiao, Y., Cong, Q., & Cui, Q. (2024). Experimental Study on the Adhesion of Abalone to Surfaces with Different Morphologies. Biomimetics, 9(4), 206. https://doi.org/10.3390/biomimetics9040206







