Investigating the Mechanical Performance of Bionic Wings Based on the Flapping Kinematics of Beetle Hindwings
Abstract
1. Introduction
2. Materials and Methods
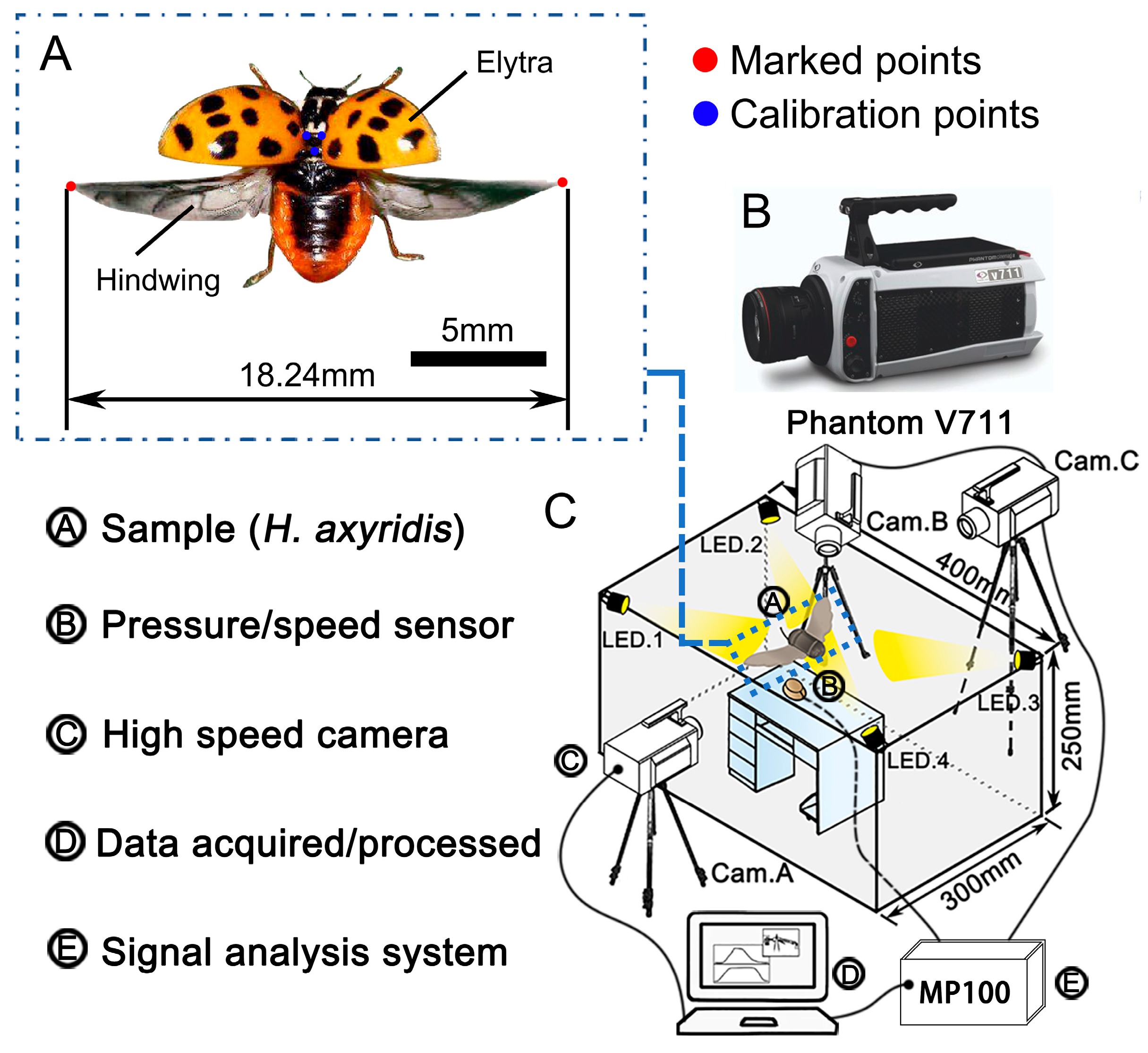
2.1. Specimens
2.2. Feature Parameters
2.3. Dynamic Capture
2.4. Wind Tunnel
3. Results
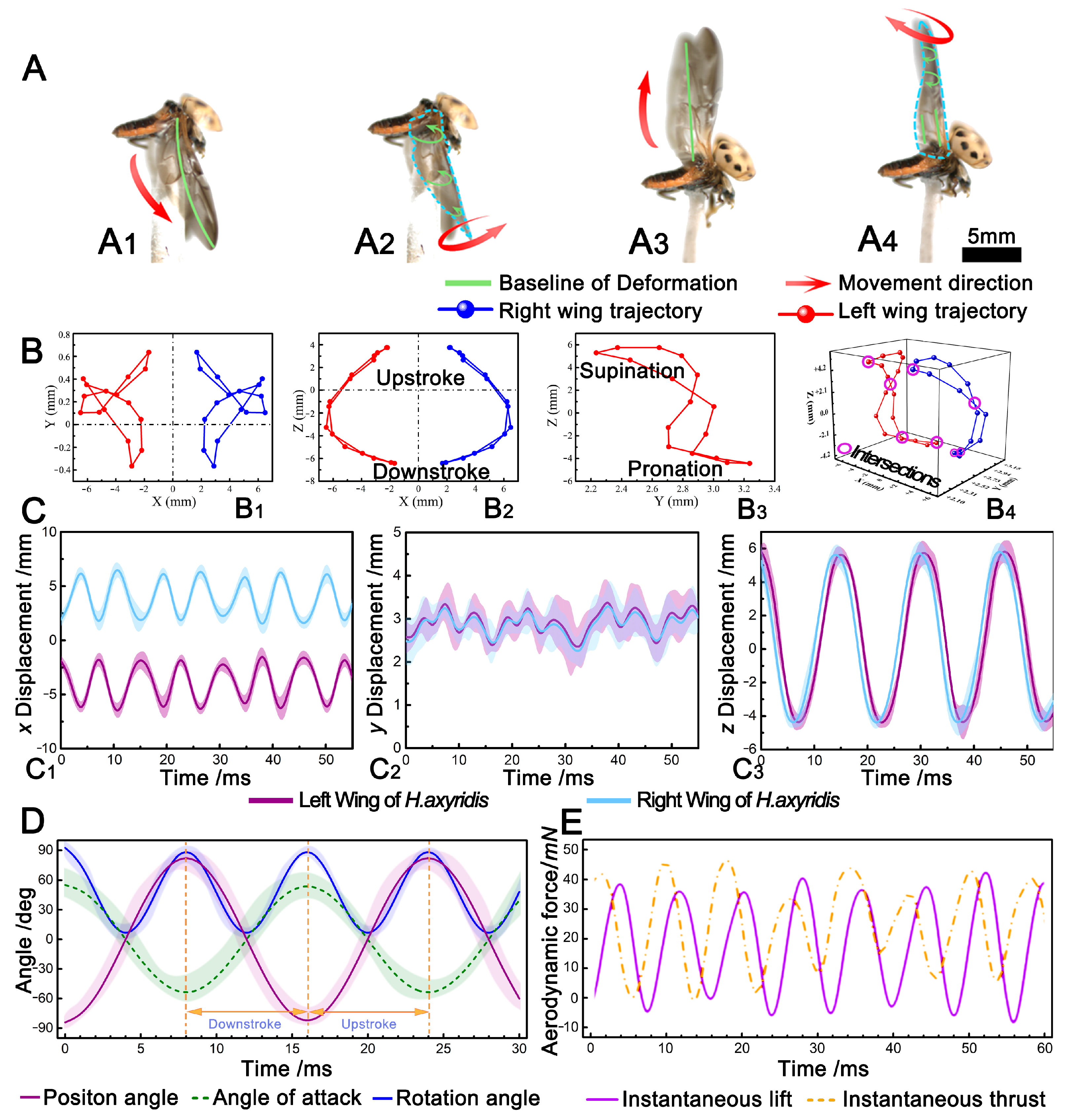
3.1. Hindwing Wingbeat Motion
3.2. Wing Tip Trajectories
3.3. Aerodynamics
3.4. Establishment of the Flapping Kinematic Model
4. Discussion
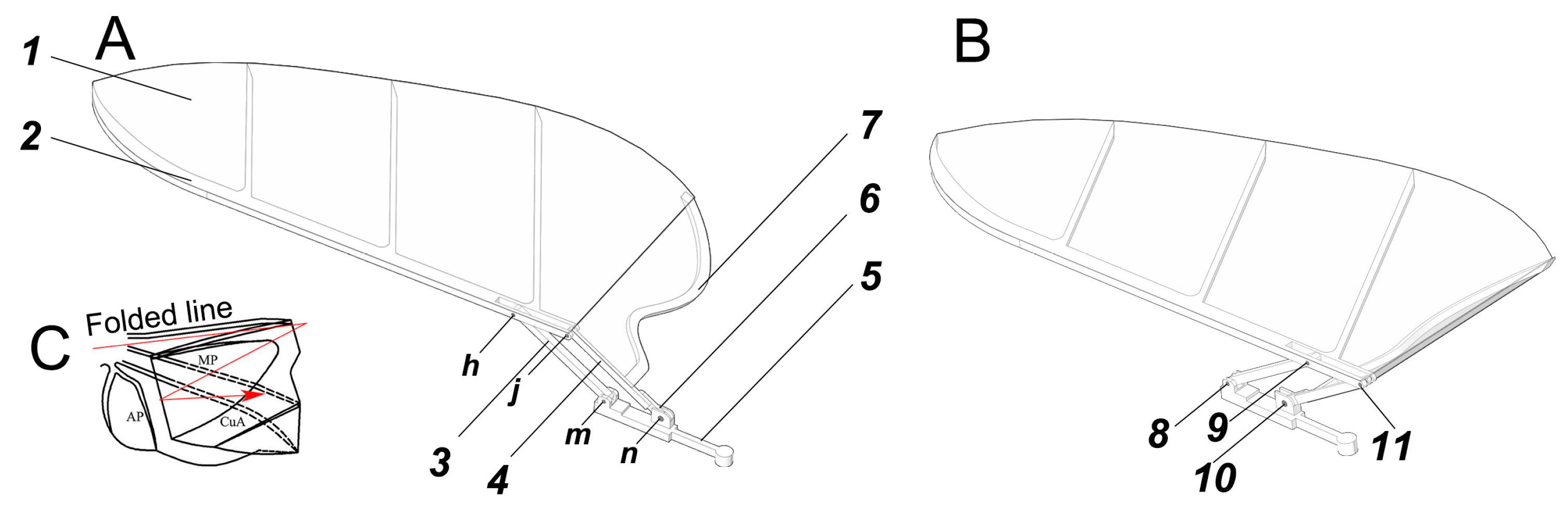
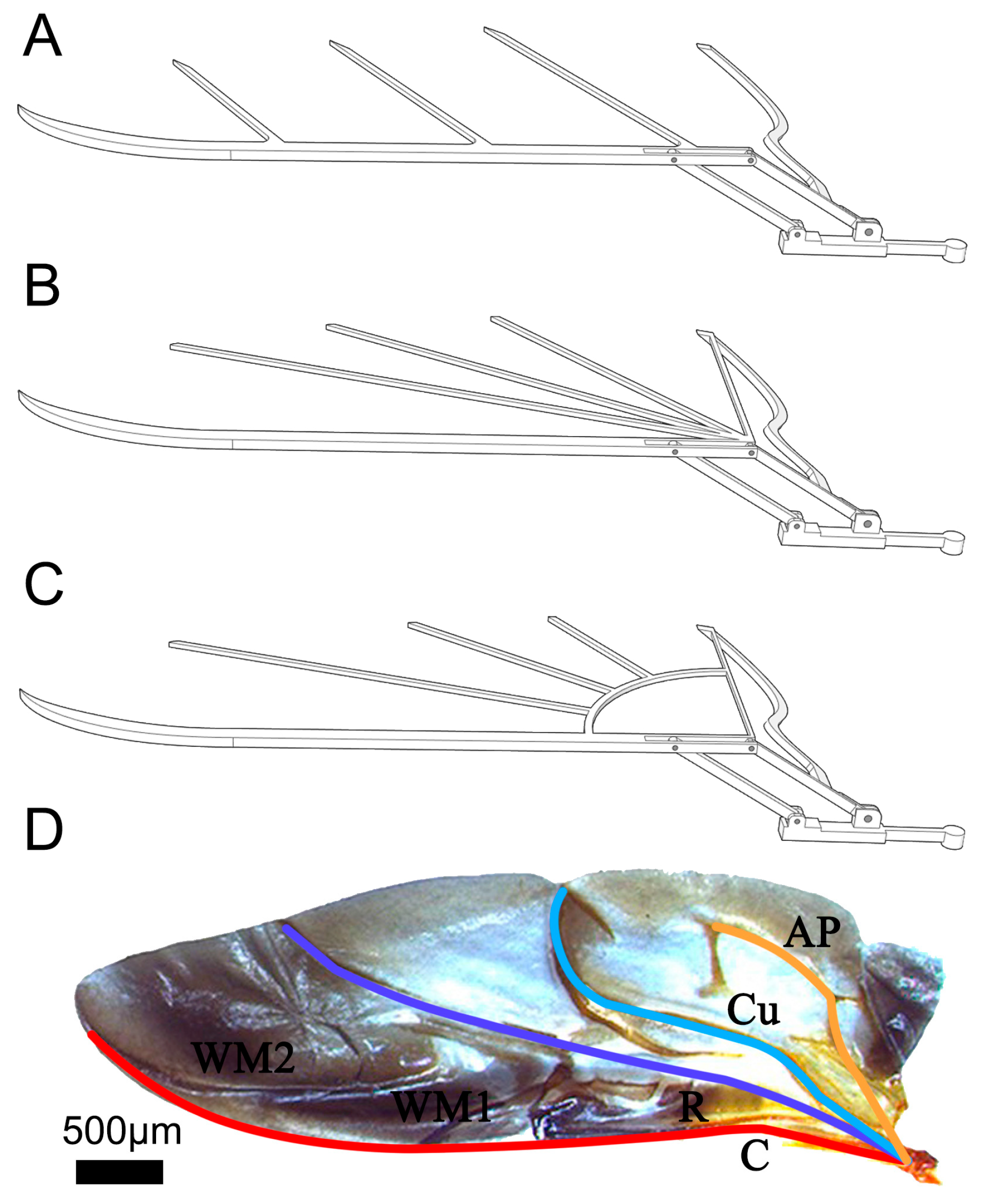
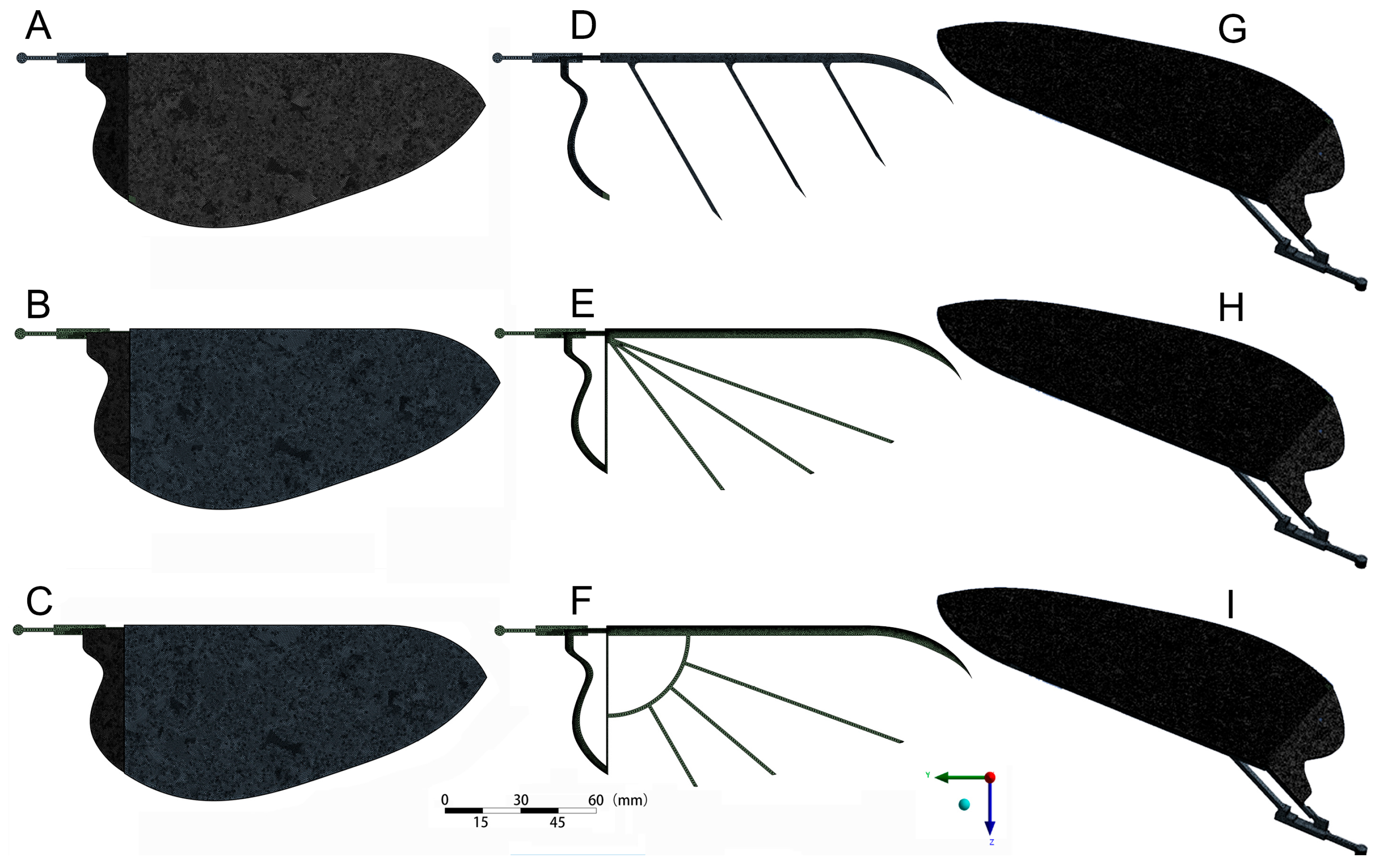
4.1. Design of Bionic Wing
4.2. Mechanical Performance Analysis of the Bionic Wing Model
4.2.1. Model and Mesh
4.2.2. Mechanical Performance Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.Y.; Liu, C.; Bhushan, B. A review of beetle hindwings: Structure, mechanical properties, mechanism and bioinspiration. J. Mech. Behav. Biomed. 2019, 94, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.D.; Percin, M.; Karasek, M.; van Oudheusden, B. Flow visualization around a flapping-wing micro air vehicle in free flight using large-scale PIV. Aerospace 2018, 5, 99. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, L.; Liu, H.; Yin, C.Y. Measuring wing kinematics, flight trajectory and body attitude during forward flight and turning maneuvers in dragonflies. J. Exp. Biol. 2003, 206, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, J.M.; Ellington, C.P. Dragonfly flight. III. Lift and power requirements. J. Exp. Biol. 1997, 200, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, D.; Onishi, M.; Sugikawa, K. Vein-membrane interaction in cambering of flapping insect wings. Biomimetics 2023, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Shirakawa, N.; Ishihara, D. Novel computational design of polymer micromachined insect-mimetic wings for flapping-wing nano air vehicles. Biomimetics 2024, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, B.; Eisner, T. Hindwings are unnecessary for flight but essential for execution of normal evasive flight in Lepidoptera. Proc. Natl. Acad. Sci. USA 2008, 105, 16636–16640. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, H.Y.; Ma, T.; Ning, J.G.; Gorb, S. Wing coupling mechanism in the butterfly Pieris rapae (Lepidoptera, Pieridae) and its role in taking off. J. Insect Physiol. 2021, 131, 104212. [Google Scholar] [CrossRef] [PubMed]
- Muijres, F.T.; Iwasaki, N.A.; Elzinga, M.J.; Melis, J.M.; Dickinson, M.H. Flies compensate for unilateral wing damage through modular adjustments of wing and body kinematics. Interface Focus 2017, 7, 20160103. [Google Scholar] [CrossRef]
- Lyu, Y.Z.; Zhu, H.J.; Sun, M. Wing kinematic and aerodynamic compensations for unilateral wing damage in a small phorid fly. Phys. Rev. E 2020, 101, 012412. [Google Scholar] [CrossRef]
- Ha, N.S.; Jin, T.L.; Goo, N.S.; Park, H.C. Anisotropy and non-homogeneity of an Allomyrina Dichotoma beetle hind wing membrane. Bioinspir. Biomim. 2011, 6, 046003. [Google Scholar] [CrossRef] [PubMed]
- Kassner, Z.; Dafni, E.; Ribak, G. Kinematic compensation for wing loss in flying damselflies. J. Insect Physiol. 2016, 85, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.Y.; Lai, Y.H.; Yang, J.T. Effects of phase lag on the hovering flight of damselfly and dragonfly. Phys. Rev. E 2019, 100, 063102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Zhao, C.X.; Zhang, Y.L.; Zhang, Y. Effect of flapping trajectories on the dragonfly aerodynamics. Chinese Sci. Bull. 2006, 51, 777–784. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, H.L.; Rajabi, H.; Zhao, H.Y.; Ning, J.G.; Gorb, S. Structure, properties and functions of the forewing-hindwing coupling of honeybees. J. Insect Physiol. 2019, 118, 103936. [Google Scholar] [CrossRef]
- Li, X.; Guo, C. Wing-kinematics measurement and flight modelling of the bamboo weevil C. buqueti. IET Nanobiotechnol. 2020, 14, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Meresman, Y.; Husak, J.F.; Ben-Shlomo, R.; Ribak, G. Morphological diversification has led to inter-specific variation in elastic wing deformation during flight in scarab beetles. Roy. Soc. Open Sci. 2020, 7, 200277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Sun, M. Wing kinematics measurement and aerodynamics of hovering droneflies. J. Exp. Biol. 2008, 211, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.L.; Liu, Y.P.; Sun, M. Wing motion measurement and aerodynamics of hovering true hoverflies. J. Exp. Biol. 2011, 214, 2832–2844. [Google Scholar] [CrossRef]
- Kesel, A.B.; Philippi, U.; Nachtigall, W. Biomechanical aspects of the insect wing: An analysis using the finite element method. Comput. Biol. Med. 1998, 28, 423–437. [Google Scholar] [CrossRef]
- Xiang, J.W.; Du, J.X.; Li, D.C.; Zhen, C. Functional morphology and structural characteristics of wings of the ladybird beetle, Coccinella septempunctata (L.). Microsc. Res. Tech. 2016, 79, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.H.; Wang, X.S.; Li, X.D.; Chen, Y.L. Effects of dragonfly wing structure on the dynamic performances. J. Bionic Eng. 2013, 10, 28–38. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Liang, Y.; Ren, L.; Jie, M.; Yang, Z. Antifatigue properties of dragonfly Pantala flavescens wings. Microsc. Res. Tech. 2014, 77, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Darvizeh, A.; Shafiei, A.; Taylor, D.; Dirks, J.H. Numerical investigation of insect wing fracture behaviour. J. Biomech. 2015, 48, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Bhayu, P.R.; Nguyen, Q.V.; Park, H.C.; Goo, N.S.; Byun, D. Artificial cambered-wing for a beetle-mimicking flapper. J. Bionic Eng. 2010, 7, S130–S136. [Google Scholar] [CrossRef]
- Liu, Z.W.; Yan, X.J.; Qi, M.J.; Zhu, Y.S.; Huang, D.W.; Zhang, X.Y.; Lin, L.W. Artificial insect wings with biomimetic wing morphology and mechanical properties. Bioinspir. Biomim. 2017, 12, 056007. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, N.E.; Palazotto, A. The evaluation of a bio-logically inspired engineered MAV wing compared to the Manduca sexta wing under simulated flapping conditions. Int. J. Micro. Air. Veh. 2011, 3, 149–168. [Google Scholar] [CrossRef]
- Fardisi, M.; Mason, L.J. Influence of temperature, gender, age, and mating status on cigarette beetle (Lasioderma serricorne (F.)) (Coleoptera: Anobiidae) flight initiation. J. Stored Prod. Res. 2013, 52, 93–99. [Google Scholar] [CrossRef]
- Haas, F.; Beutel, R.G. Wing folding and the functional morphology of the wing base in Coleoptera. Zoology 2001, 104, 123–141. [Google Scholar] [CrossRef]
- Hasan, J.; Roy, A.; Chatterjee, K.; Yarlagadda, P.K.D.V. Mimicking insect wings: The roadmap to bioinspiration. ACS Biomater. Sci. Eng. 2019, 5, 3139–3160. [Google Scholar] [CrossRef]
- Sane, S.P.; Dickinson, M.H. The control of flight force by a flapping wing: Lift and drag production. J. Exp. Biol. 2001, 24, 2607–2626. [Google Scholar] [CrossRef] [PubMed]
- Muijres, F.T.; Elzinga, M.J.; Melis, J.M.; Dickinson, M.H. Flies evade looming targets by executing rapid visually directed banked turns. Science 2014, 344, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, X.; Luo, P.; Huang, P. Optimization design and dynamic analysis on the drive mechanisms of flapping-wing air vehicles based on flapping trajectories. J. Phys. Conf. Ser. 2017, 916, 012008. [Google Scholar] [CrossRef]
- Sudo, S.; Tsuyuki, K.; Kanno, K. Wing characteristics and flapping behavior of flying insects. Exp. Mech. 2005, 45, 550–555. [Google Scholar] [CrossRef]
- Noda, R.; Nakata, T.; Liu, H. Effect of hindwings on the aerodynamics and passive dynamic stability of a hovering hawkmoth. Biomimetics 2023, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Sackey, J.; Berthier, S.; Maaza, M.; Beuvier, T.; Gibaud, A. Comparative study on nanostructured order-disorder in the wing eyespots of the giant owl butterfly, Caligo memnon. IET Nanobiotechnol. 2018, 12, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ji, A.H.; Li, Q.; Wang, W.; Qin, G.D.; Han, Q.F. The Unique Strategies of Flight Initiation Adopted by Butterflies on Vertical Surfaces. J. Bionic Eng. 2021, 18, 840–856. [Google Scholar] [CrossRef]
- Sun, J.; Du, R.; Liu, X.; Bechkoum, K.; Tong, J.; Chen, D.H. A simulation of the flight characteristics of the deployable hindwings of beetle. J. Bionic Eng. 2017, 14, 296–306. [Google Scholar] [CrossRef]
- Nakata, T.; Liu, H. Aerodynamic performance of a hovering hawk moth with flexible wings: A computational approach. P. Roy. Soc. B-Biol. Sci. 2012, 279, 722–731. [Google Scholar]
- Liu, C.; Sun, J.Y.; Li, F.D.; Bhushan, B.; Wu, W.; Tong, J. Effect of vein microstructure and nanomechanical behaviors on wind-resistant performance of Asian ladybeetle hindwing. Tribol. Int. 2020, 142, 105719. [Google Scholar]
- Shen, H.; Ji, A.H.; Li, Q.; Li, X.; Ma, Y.P. Tensile mechanical properties and finite element simulation of the wings of the butterfly Tirumala limniace. J. Comp. Physiol. A. 2023, 209, 239–251. [Google Scholar] [CrossRef] [PubMed]

| Feature Parameters | Title 2 | Title 3 | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Mass (mg) | 2.95 | 3.05 | 3.01 | 2.88 | 3.16 | 3.01 ± 0.09 |
| Body length (mm) | 6.05 | 6.15 | 7.13 | 6.82 | 7.05 | 6.64 ± 0.45 |
| Body width (mm) | 4.78 | 4.89 | 4.81 | 4.92 | 4.95 | 4.87 ± 0.06 |
| Hindwing length (mm) | 9.35 | 9.74 | 9.88 | 9.82 | 9.76 | 9.71 ± 0.19 |
| Hindwing width (mm) | 2.89 | 2.75 | 2.79 | 2.73 | 2.88 | 2.81 ± 0.07 |
| Hindwing area (mm2) | 23.56 | 22.85 | 23.46 | 21.73 | 22.57 | 22.83 ± 0.66 |
| Folding ratio | 2.41 | 2.25 | 2.38 | 2.17 | 2.23 | 2.29 ± 0.09 |
| Test Section Parameters | Value |
|---|---|
| Working section shape | Rectangle |
| Working section area (mm2) | 650 × 450 |
| Length of working section (mm) | 1000 |
| Turbulence intensity (%) | <0.3 |
| Form of wind speed regulator | Hot-wire sensor |
| Range of wind speed (m/s) | 0–10 |
| Airflow nonuniformity of working section (%) | <3 |
| Wingbeat Phase | Stroke Plane Angle (°) | Flapping Amplitude (°) | Flapping Frequency (Hz) | |||
|---|---|---|---|---|---|---|
| Downstroke | Supination | Upstroke | Pronation | |||
| 43.8% | 18.6% | 31.3% | 6.3% | 2.1 | 168.4 ± 3.1 | 62.5 ± 1.6 |
| K | f (Hz) | c (mm) | vT (m/s) | R (mm) | θ (°) | |
|---|---|---|---|---|---|---|
| H. axyridis | 0.15 | 62.5 | 2.77 | 3.52 | 9.71 | 168.4 |
| a0 | a1 | a2 | a3 | a4 | b1 | b2 | b3 | b4 | |
|---|---|---|---|---|---|---|---|---|---|
| α(t) | −0.83 | −34.83 | 7.17 | 1.96 | 2.49 | 7.25 | 4.03 | 3.11 | 2.59 |
| θ(t) | 7.49 | 32.03 | −3.31 | 3.12 | 3.93 | −3.43 | 0.56 | 1.24 | 2.34 |
| φ(t) | 40.18 | 4.67 | 38.12 | 2.60 | 3.78 | 3.57 | −8.86 | 5.67 | 2.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Shen, T.; Shen, H.; Ling, M.; Chen, G.; Lu, B.; Chen, F.; Wang, Z. Investigating the Mechanical Performance of Bionic Wings Based on the Flapping Kinematics of Beetle Hindwings. Biomimetics 2024, 9, 343. https://doi.org/10.3390/biomimetics9060343
Liu C, Shen T, Shen H, Ling M, Chen G, Lu B, Chen F, Wang Z. Investigating the Mechanical Performance of Bionic Wings Based on the Flapping Kinematics of Beetle Hindwings. Biomimetics. 2024; 9(6):343. https://doi.org/10.3390/biomimetics9060343
Chicago/Turabian StyleLiu, Chao, Tianyu Shen, Huan Shen, Mingxiang Ling, Guodong Chen, Bo Lu, Feng Chen, and Zhenhua Wang. 2024. "Investigating the Mechanical Performance of Bionic Wings Based on the Flapping Kinematics of Beetle Hindwings" Biomimetics 9, no. 6: 343. https://doi.org/10.3390/biomimetics9060343
APA StyleLiu, C., Shen, T., Shen, H., Ling, M., Chen, G., Lu, B., Chen, F., & Wang, Z. (2024). Investigating the Mechanical Performance of Bionic Wings Based on the Flapping Kinematics of Beetle Hindwings. Biomimetics, 9(6), 343. https://doi.org/10.3390/biomimetics9060343












