Efficient Bioactive Surface Coatings with Calcium Minerals: Step-Wise Biomimetic Transformation of Vaterite to Carbonated Apatite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glass Cleaning
2.3. Preparation of Calcium Carbonate Vaterite (CCV)
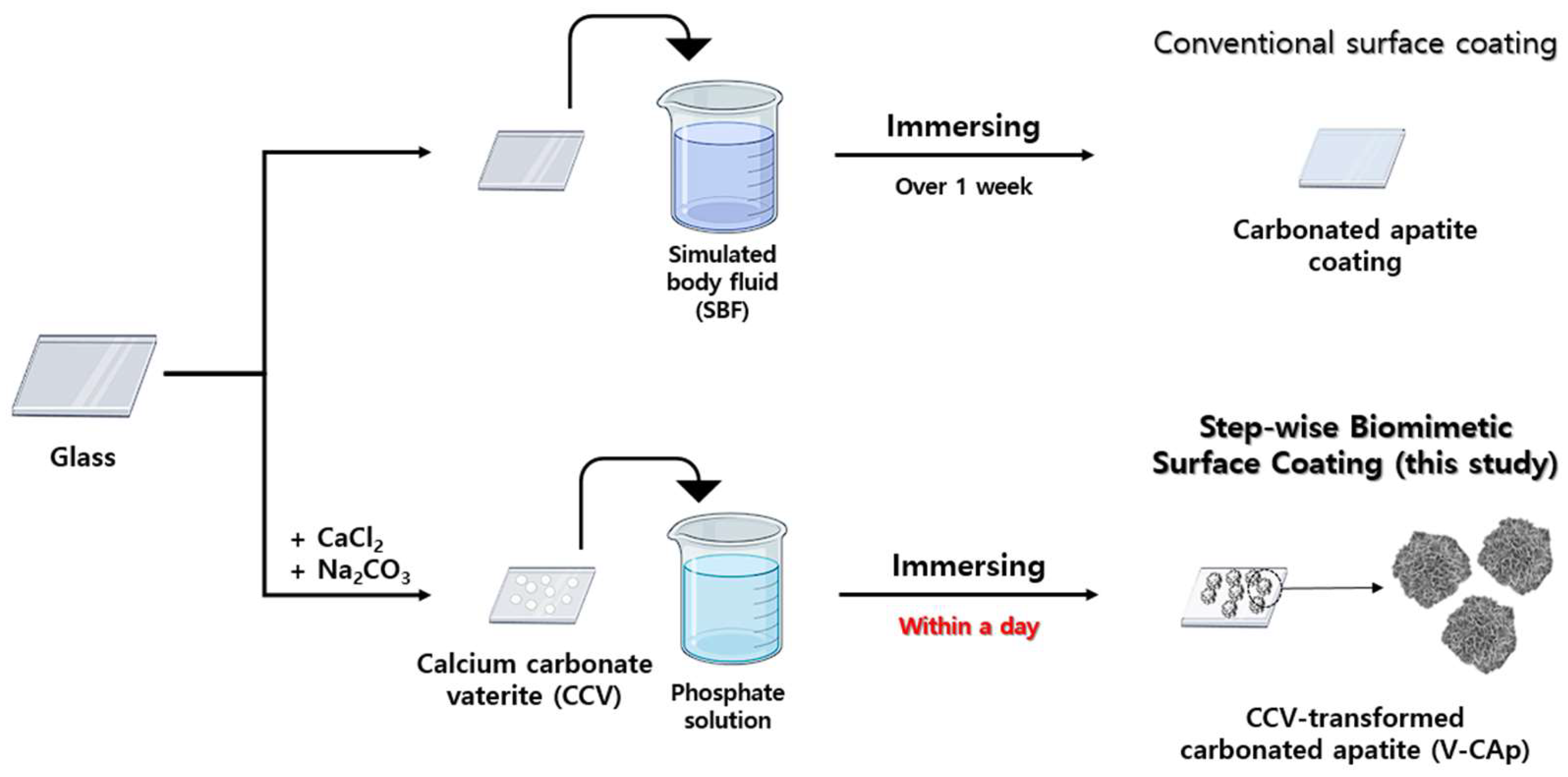
2.4. Transformation of Carbonated Apatite (V-CAp) from Calcium Carbonate Vaterite (CCV)
2.5. Characterization of Carbonated Apatite
2.6. In Vitro Cell Culture Experiments
2.7. Cell Proliferation Assay
2.8. Cell Differentiation Assay
2.9. Statistical Analysis
3. Results and Discussion
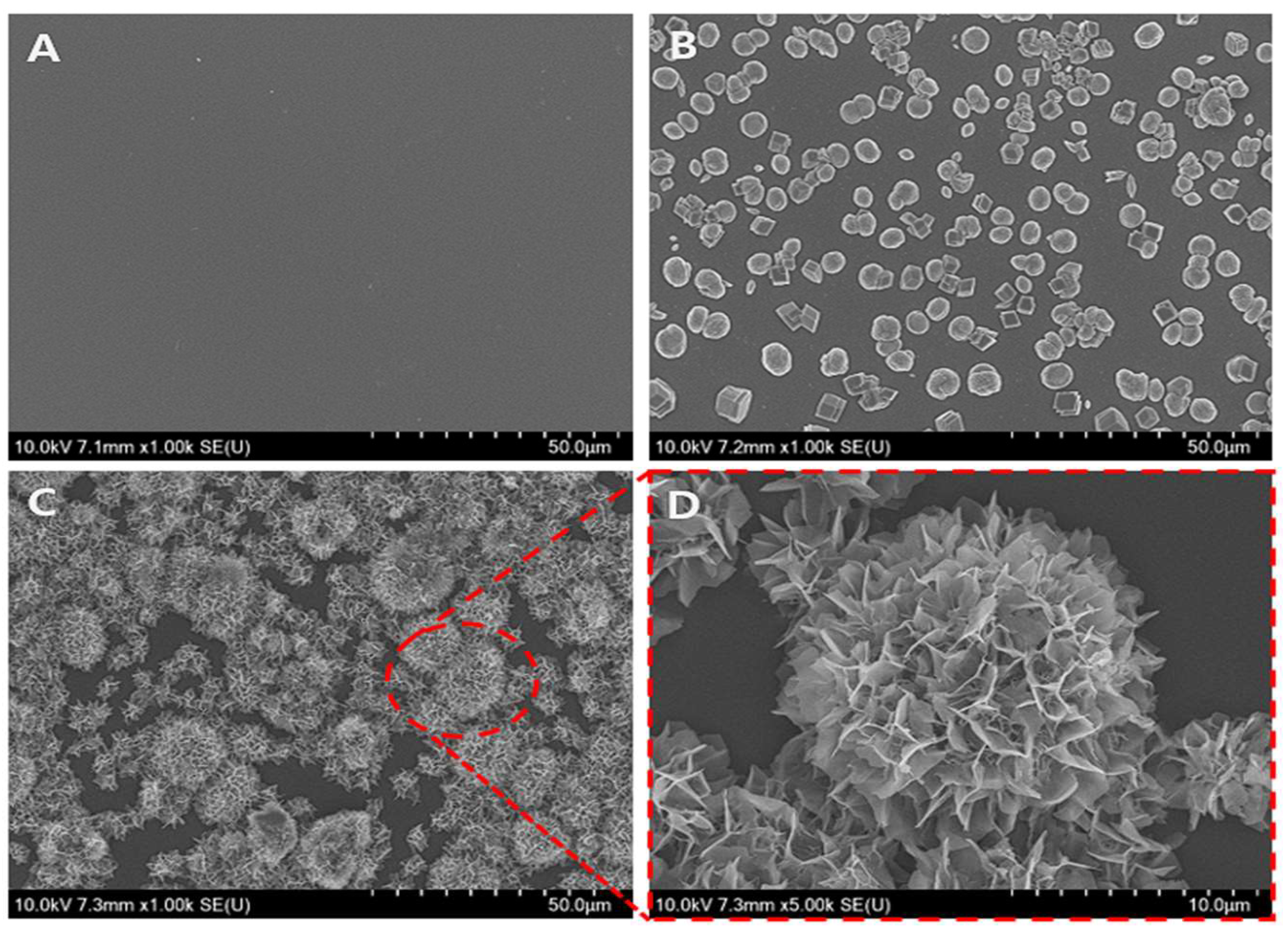
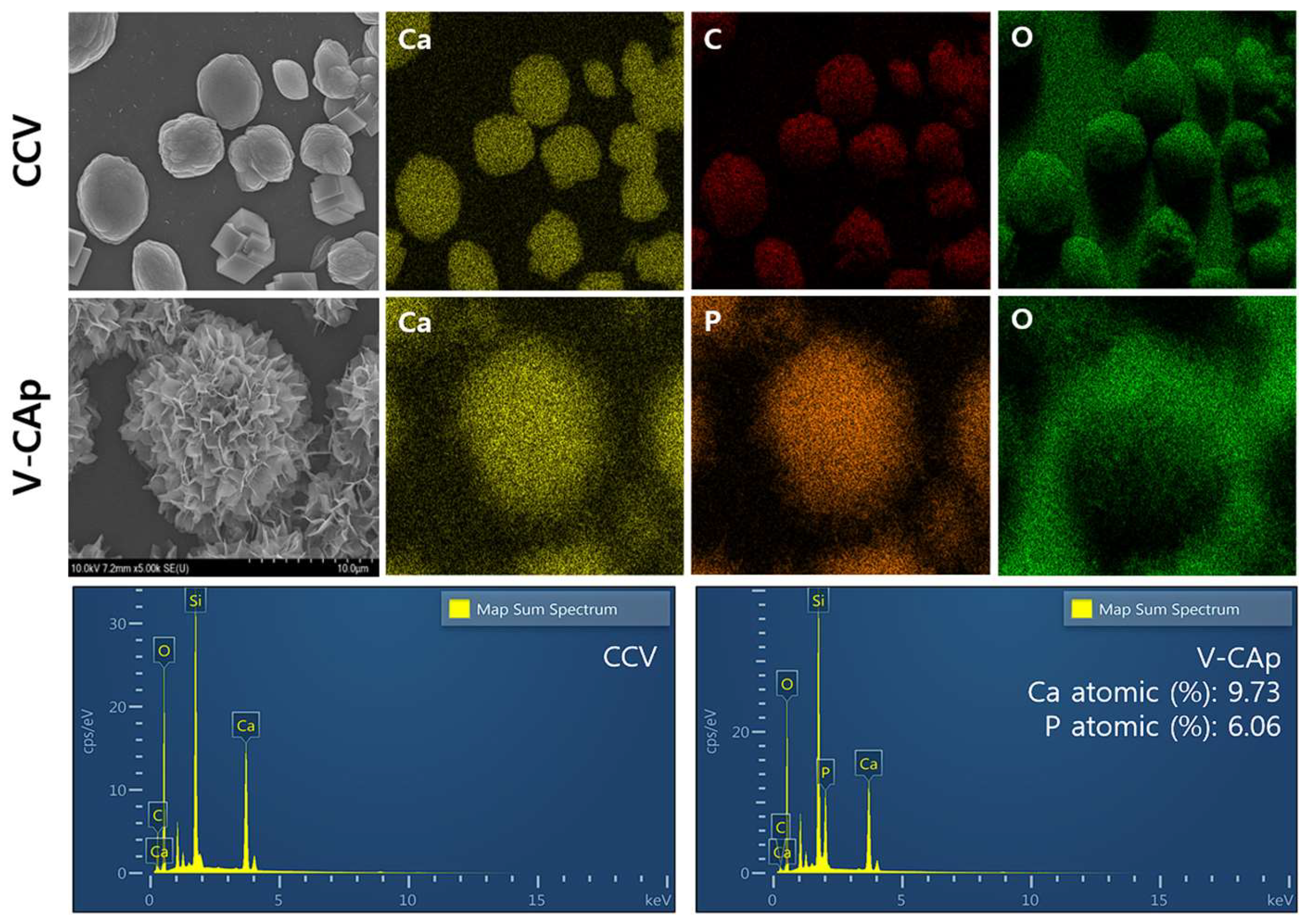
3.1. Morphological Analysis of Vaterite-Transformed Carbonated Apatite (V-CAp)
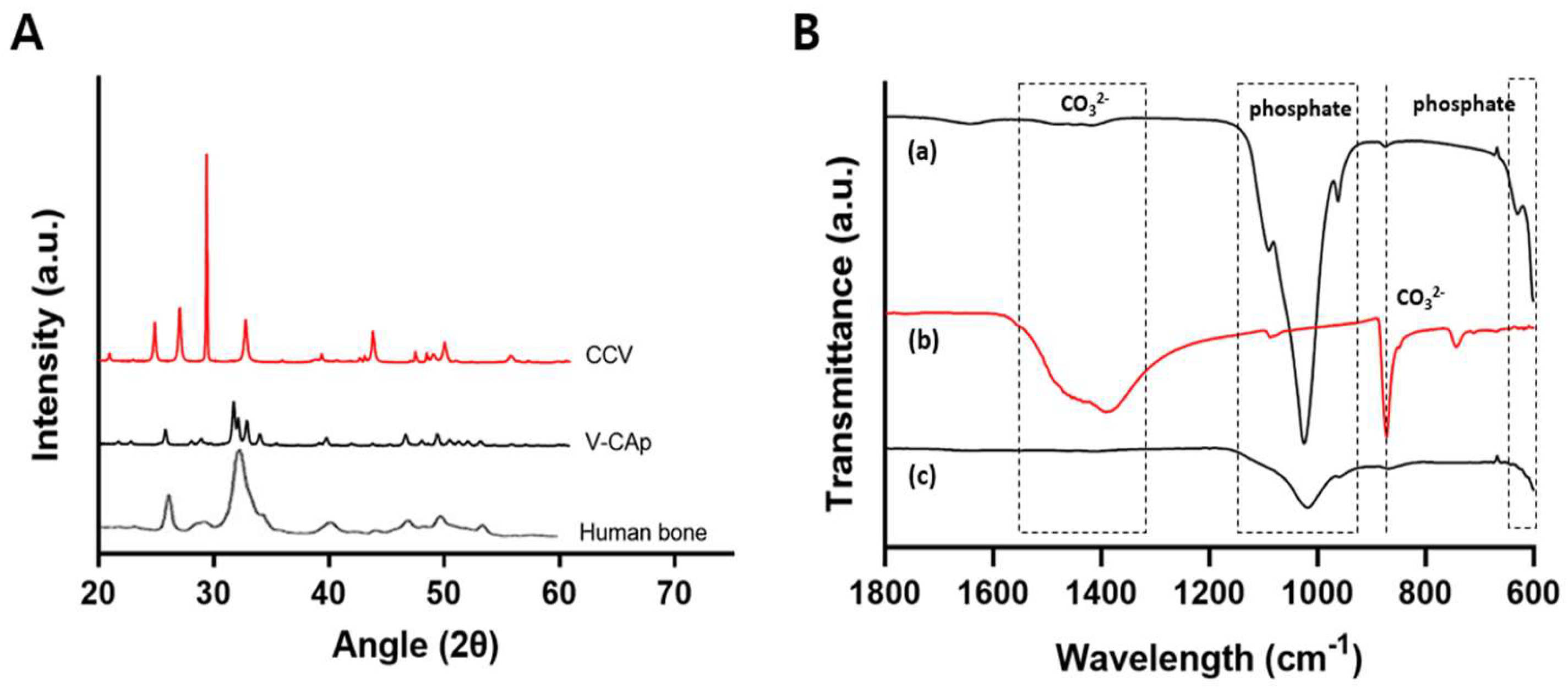
3.2. Characterization of Generated V-CAp
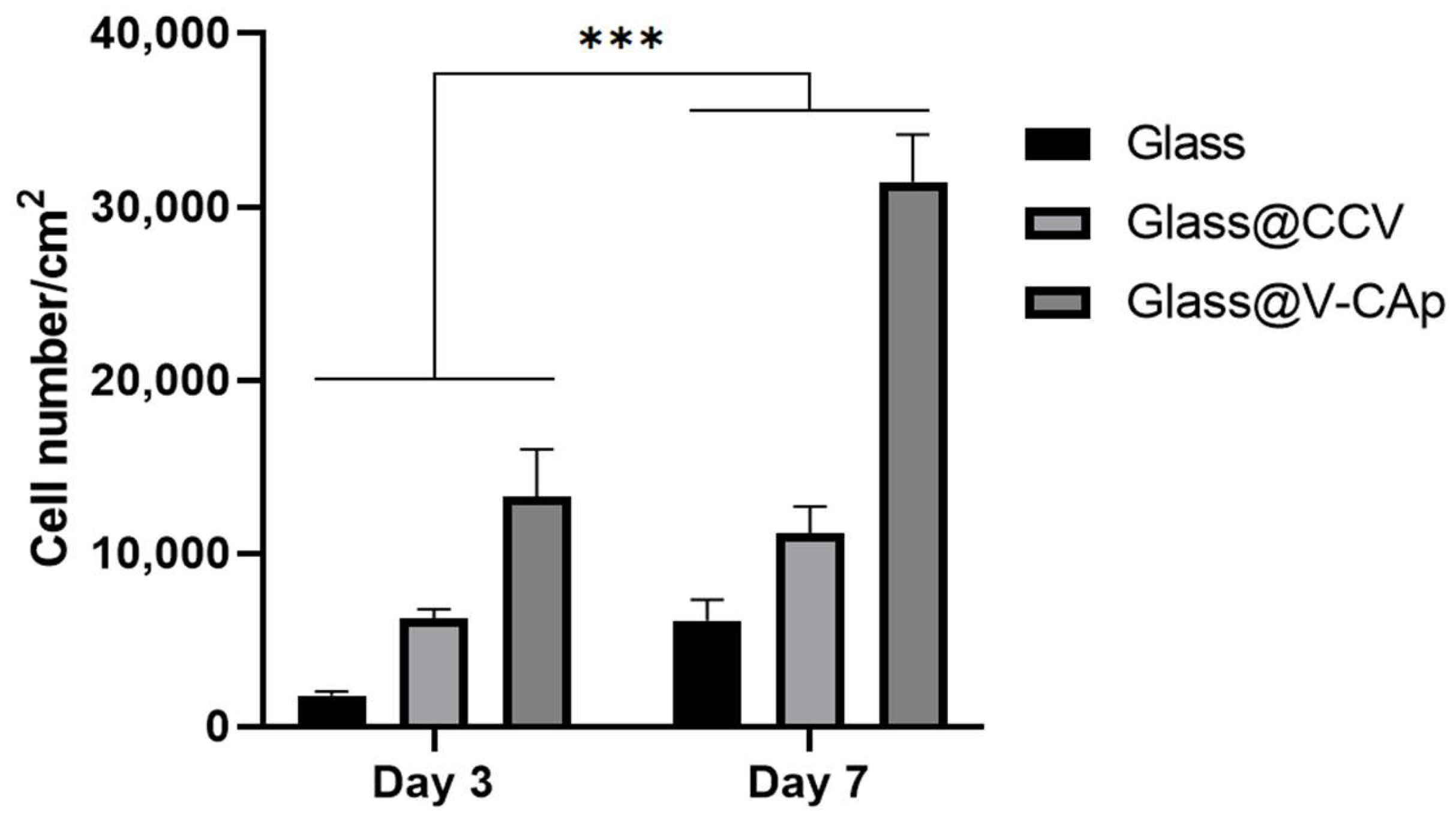
3.3. In Vitro Proliferation and ALP Activity on the V-CAp Coated Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Taton, T.A. Boning up on biology. Nature 2001, 412, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2014, 12, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-Y.; Lo, S.-C.; Shih, C.-S.; Yang, Y.-C.; Feng, H.-P.; Lin, Y.-C. Titanium surface modified by hydroxyapatite coating for dental implants. Surf. Coat. Technol. 2013, 231, 337–345. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, R.; Tang, W.; Yang, F.; Tian, H.; Peng, S.; Pan, H.; Shuai, C. Structural and Functional Adaptive Artificial Bone: Materials, Fabrications, and Properties. Adv. Funct. Mater. 2023, 33, 2214726. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2020, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Q.; Xu, T.; Zhang, H.; Zhang, M.; Lu, L.; Hao, Y.; Fuh, J.; Zhao, X. Photocrosslinkable nanocomposite ink for printing strong, biodegradable and bioactive bone graft. Biomaterials 2020, 263, 120378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, J.; Zou, L.; Xu, G.; Geng, Y. Facile one-step bioinspired mineralization by chitosan functionalized with graphene oxide to activate bone endogenous regeneration. Chem. Eng. J. 2019, 378, 122174. [Google Scholar] [CrossRef]
- Guo, F.; Wang, E.; Yang, Y.; Mao, Y.; Liu, C.; Bu, W.; Li, P.; Zhao, L.; Jin, Q.; Liu, B.; et al. A natural biomineral for enhancing the biomineralization and cell response of 3D printed polylactic acid bone scaffolds. Int. J. Biol. Macromol. 2023, 242, 124728. [Google Scholar] [CrossRef] [PubMed]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone mineral: New insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef]
- Ariani, M.D.; Salim, S. In vitro and in vivo evaluation of carbonate apatite-collagen scaffolds with some cytokines for bone tissue engineering. J. Indian Prosthodont. Soc. 2015, 15, 349–355. [Google Scholar] [CrossRef]
- Mondal, S.; Pal, U.; Dey, A. Natural origin hydroxyapatite scaffold as potential bone tissue engineering substitute. Ceram. Int. 2016, 42, 18338–18346. [Google Scholar] [CrossRef]
- Jayasree, R.; Madhumathi, K.; Rana, D.; Ramalingam, M.; Nankar, R.P.; Doble, M.; Kumar, T.S.S. Development of Egg Shell Derived Carbonated Apatite Nanocarrier System for Drug Delivery. J. Nanosci. Nanotechnol. 2018, 18, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Kajander, K.; Sirkiä, S.V.; Vallittu, P.K.; Heino, T.J.; Määttä, J.A. Bioactive glasses promote rapid pre-osteoblastic cell migration in contrast to hydroxyapatite, while carbonated apatite shows migration inhibiting properties. Sci. Rep. 2023, 13, 20587. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, C.; Hara, H.; Takano, I.; Hayakawa, T.; Sato, M. Application of carbonated apatite coating on a Ti substrate by aqueous spray method. Mater. Sci. Eng. C 2013, 33, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.B.; Ma, J.; Boey, F. Nanosized hydroxyapatite powders derived from coprecipitation process. J. Mater. Sci. 2002, 37, 1131–1134. [Google Scholar] [CrossRef]
- Liu, D.-M.; Troczynski, T.; Tseng, W.J. Water-based sol–gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.K.; Haq, E.U.; Licciulli, A. Rapid synthesis and characterization of silicon substituted nano hydroxyapatite using microwave irradiation. Curr. Appl. Phys. 2014, 14, 87–92. [Google Scholar] [CrossRef]
- Liang, W.; Zhan, L.; Piao, L.; Rüssel, C. Lead and copper removal from aqueous solutions by porous glass derived calcium hydroxyapatite. Mater. Sci. Eng. B 2011, 176, 1010–1014. [Google Scholar] [CrossRef]
- Midorikawa, K.; Hiromoto, S.; Yamamoto, T. Carbonate content control in carbonate apatite coatings of biodegradable magnesium. Ceram. Int. 2024, 50, 6784–6792. [Google Scholar] [CrossRef]
- Earl, J.S.; Wood, D.J.; Milne, S.J. Hydrothermal synthesis of hydroxyapatite. J. Phys. Conf. Ser. 2006, 26, 268–271. [Google Scholar] [CrossRef]
- Minh, D.P.; Tran, N.D.; Nzihou, A.; Sharrock, P. Novel one-step synthesis and characterization of bone-like carbonated apatite from calcium carbonate, calcium hydroxide and orthophosphoric acid as economical starting materials. Mater. Res. Bull. 2014, 51, 236–243. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Donskaya, N.O.; Valeev, D.V.; Fomin, A.S.; Murzakhanov, F.F.; Leonov, A.V.; Konovalov, A.A.; Antonova, O.S.; Shoppert, A.A.; Kudryavtsev, E.A.; et al. Mesoporous molybdate-substituted hydroxyapatite nanopowders obtained via a hydrothermal route. Ceram. Int. 2024, 50, 17404–17418. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.; Kol’tsov, A.; Kuz’mina, M.; Zorina, M.; Poritskaya, L. Ion substitutions and non-stoichiometry of carbonated apatite-(CaOH) synthesised by precipitation and hydrothermal methods. J. Mol. Struct. 2011, 992, 9–18. [Google Scholar] [CrossRef]
- Tonegawa, T.; Ikoma, T.; Suetsugu, Y.; Igawa, N.; Matsushita, Y.; Yoshioka, T.; Hanagata, N.; Tanaka, J. Thermal expansion of type A carbonate apatite. Mater. Sci. Eng. B 2010, 173, 171–175. [Google Scholar] [CrossRef]
- Li, M.; Wu, G.; Wang, M.; Hunziker, E.B.; Liu, Y. Crystalline Biomimetic Calcium Phosphate Coating on Mini-Pin Implants to Accelerate Osseointegration and Extend Drug Release Duration for an Orthodontic Application. Nanomaterials 2022, 12, 2439. [Google Scholar] [CrossRef]
- Kokubo, T. Surface chemistry of bioactive glass-ceramics. J. Non-Cryst. Solids 1990, 120, 138–151. [Google Scholar] [CrossRef]
- Duan, H.; Cao, C.; Wang, X.; Tao, J.; Li, C.; Xin, H.; Yang, J.; Song, Y.; Ai, F. Magnesium-alloy rods reinforced bioglass bone cement composite scaffolds with cortical bone-matching mechanical properties and excellent osteoconductivity for load-bearing bone in vivo regeneration. Sci. Rep. 2020, 10, 18193. [Google Scholar] [CrossRef] [PubMed]
- Mythili, P.; Madalina, P.; Roxana, P.; Alain, L. Fabrication Methodologies of Biomimetic and Bioactive Scaffolds for Tissue Engineering Applications. In Materials, Technologies and Clinical Applications; Francesco, B., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 3–30. [Google Scholar]
- Li, M.; Wang, M.; Wei, L.; Werner, A.; Liu, Y. Biomimetic calcium phosphate coating on medical grade stainless steel improves surface properties and serves as a drug carrier for orthodontic applications. Dent. Mater. 2023, 39, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, J.; Qi, S.; He, L.; Zhao, J.; Zhang, X. Carbonate apatite coating on titanium induced rapidly by precalcification. Biomaterials 2001, 23, 173–179. [Google Scholar] [CrossRef]
- Leena, M.; Rana, D.; Webster, T.J.; Ramalingam, M. Accelerated synthesis of biomimetic nano hydroxyapatite using simulated body fluid. Mater. Chem. Phys. 2016, 180, 166–172. [Google Scholar] [CrossRef]
- Chaka, A.M. Ab Initio Thermodynamics of Hydrated Calcium Carbonates and Calcium Analogues of Magnesium Carbonates: Implications for Carbonate Crystallization Pathways. ACS Earth Space Chem. 2018, 2, 210–224. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.S.; Volodkin, D. CaCO3 crystals as versatile carriers for controlled delivery of antimicrobials. J. Control. Release 2020, 328, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, D.H.; Pack, S.P. Size Control of Biomimetic Curved-Edge Vaterite with Chiral Toroid Morphology via Sonochemical Synthesis. Biomimetics 2024, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium Carbonate Formation and Dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef] [PubMed]
- Donatan, S.; Yashchenok, A.; Khan, N.; Parakhonskiy, B.; Cocquyt, M.; Pinchasik, B.-E.; Khalenkow, D.; Möhwald, H.; Konrad, M.; Skirtach, A. Loading Capacity versus Enzyme Activity in Anisotropic and Spherical Calcium Carbonate Microparticles. ACS Appl. Mater. Interfaces 2016, 8, 14284–14292. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.-S.; Zong, J.-Y.; Zhao, D.; Li, F.; Zhuo, R.-X.; Cheng, S.-X. Calcium Carbonate/Carboxymethyl Chitosan Hybrid Microspheres and Nanospheres for Drug Delivery. J. Phys. Chem. C 2010, 114, 18940–18945. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Liu, X.; He, W.; Huang, Q.; Li, S.; Feng, Q. Calcium carbonate nanoparticles promote osteogenesis compared to adipogenesis in human bone-marrow mesenchymal stem cells. Prog. Nat. Sci. 2018, 28, 598–608. [Google Scholar] [CrossRef]
- Vikulina, A.; Webster, J.; Voronin, D.; Ivanov, E.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Mesoporous additive-free vaterite CaCO3 crystals of untypical sizes: From submicron to Giant. Mater. Des. 2020, 197, 109220. [Google Scholar] [CrossRef]
- Minh, D.P.; Nzihou, A.; Sharrock, P. Carbonated hydroxyapatite starting from calcite and different orthophosphates under moderate hydrothermal conditions: Synthesis and surface reactivity in simulated body fluid. Mater. Res. Bull. 2014, 60, 292–299. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Effect of carbonate content and buffer type on calcium phosphate formation in SBF solutions. J. Mater. Sci. Mater. Med. 2006, 17, 697–707. [Google Scholar] [CrossRef]
- Wong, S.L.; Deymier, A.C. Phosphate and buffer capacity effects on biomimetic carbonate apatite. Ceram. Int. 2023, 49, 12415–12422. [Google Scholar] [CrossRef]
- Minh, D.P.; Lyczko, N.; Sebei, H.; Nzihou, A.; Sharrock, P. Synthesis of calcium hydroxyapatite from calcium carbonate and different orthophosphate sources: A comparative study. Mater. Sci. Eng. B 2012, 177, 1080–1089. [Google Scholar] [CrossRef]
- Sandin, K.; Kloo, L.; Nevsten, P.; Wallenberg, R.L.; Olsson, L.-F. Formation of carbonated apatite particles from a supersaturated inorganic blood serum model. J. Mater. Sci. Mater. Med. 2009, 20, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, C.J.S.; Chernyshov, D.; Birkedal, H. Apatite Formation from Amorphous Calcium Phosphate and Mixed Amorphous Calcium Phosphate/Amorphous Calcium Carbonate. Chem. A Eur. J. 2016, 22, 12347–12357. [Google Scholar] [CrossRef]
- Myszka, B.; Schüßler, M.; Hurle, K.; Demmert, B.; Detsch, R.; Boccaccini, A.R.; Wolf, S.E. Phase-specific bioactivity and altered Ostwald ripening pathways of calcium carbonate polymorphs in simulated body fluid. RSC Adv. 2019, 9, 18232–18244. [Google Scholar] [CrossRef]
- Jaramillo-Martínez, S.; Vargas-Requena, C.; Rodríguez-Gónzalez, C.; Hernández-Santoyo, A.; Olivas-Armendáriz, I. Effect of extrapallial protein of Mytilus californianus on the process of in vitro biomineralization of chitosan scaffolds. Heliyon 2019, 5, e02252. [Google Scholar] [CrossRef] [PubMed]
- Pastero, L.; Bruno, M.; Aquilano, D. About the Genetic Mechanisms of Apatites: A Survey on the Methodological Approaches. Minerals 2017, 7, 139. [Google Scholar] [CrossRef]
- Benataya, K.; Lakrat, M.; Elansari, L.; Mejdoubi, E. Synthesis of B-type carbonated hydroxyapatite by a new dissolution-precipitation method. Mater. Today Proc. 2020, 31, S83–S88. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, W.; Su, X.; Li, G.; Zhou, Y.; Kundu, S.C.; Yao, J.; Cai, Y. Degradation pattern of porous CaCO3 and hydroxyapatite microspheres in vitro and in vivo for potential application in bone tissue engineering. Colloids Surf. B Biointerfaces 2016, 143, 56–63. [Google Scholar] [CrossRef]
- Al-Hamdan, S.H.; Al-Hamdan, K.; Junker, R.; A Jansen, J. Effect of implant surface properties on peri-implant bone healing: Implant stability and microcomputed tomographic analysis. Int. J. Oral Maxillofac. Implant. 2012, 27, 77–83. [Google Scholar]
- Kubies, D.; Himmlová, L.; Riedel, T.; Chánová, E.; Balík, K.; Douděrová, M.; Bártová, J.; Pešáková, V. The interaction of osteoblasts with bone-implant materials: 1. The effect of physicochemical surface properties of implant materials. Physiol. Res. 2011, 60, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Crosby, K.; Sawicki, M.; Shaw, L.L.; Wang, Y. Effects of Surface Roughness of Hydroxyapatite on Cell Attachment and Proliferation. J. Biotechnol. Biomater. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Xun, X.; Li, Y.; Ni, M.; Xu, Y.; Li, J.; Zhang, D.; Chen, G.; Ao, H.; Luo, H.; Wan, Y.; et al. Calcium crosslinked macroporous bacterial cellulose scaffolds with enhanced in situ mineralization and osteoinductivity for cranial bone regeneration. Compos. Part B Eng. 2024, 275, 111277. [Google Scholar] [CrossRef]
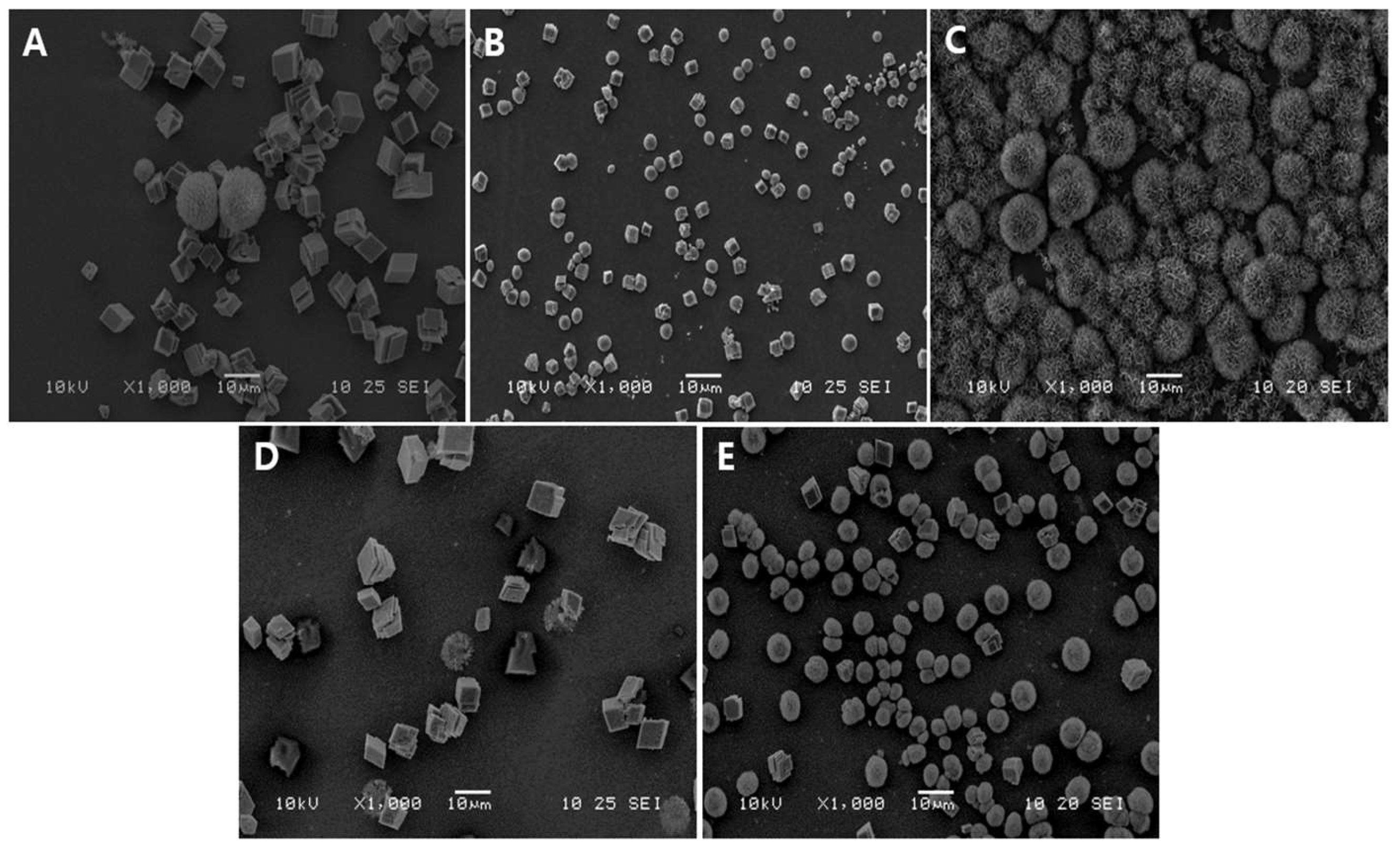
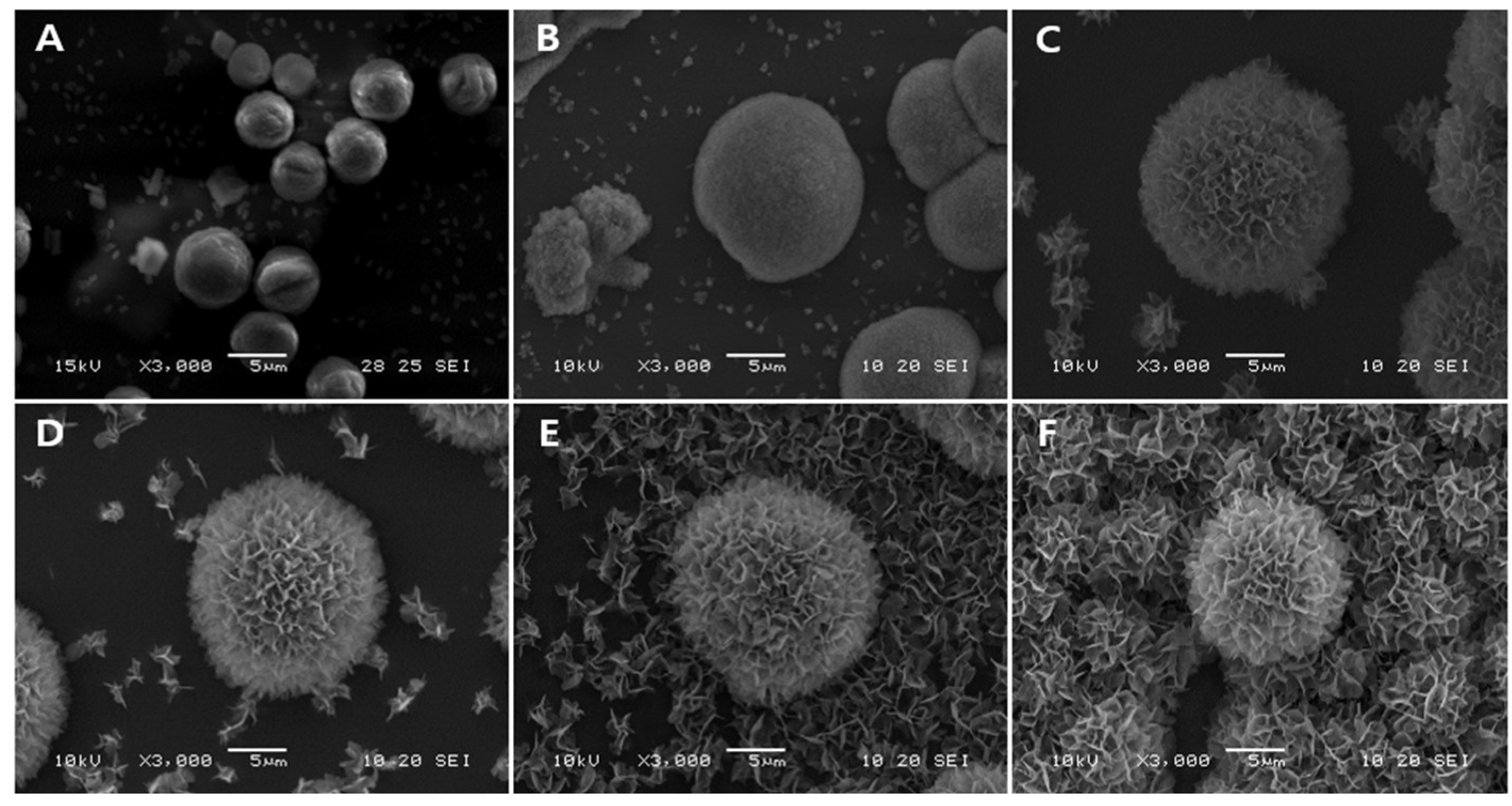

| Phosphate Solution | Phosphate Ingredient |
|---|---|
| P buffer (pH 7.6) | Monosodium phosphate, disodium phosphate |
| PS-1 (Adjusted pH 7.6) | Monosodium phosphate |
| PS-2 (Adjusted pH 7.6) | Disodium phosphate |
| Commercial PBS (pH 7.6) | Monosodium phosphate, disodium phosphate |
| SBF (pH 6.7) | Disodium phosphate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Min, K.H.; Pack, S.P. Efficient Bioactive Surface Coatings with Calcium Minerals: Step-Wise Biomimetic Transformation of Vaterite to Carbonated Apatite. Biomimetics 2024, 9, 402. https://doi.org/10.3390/biomimetics9070402
Kim DH, Min KH, Pack SP. Efficient Bioactive Surface Coatings with Calcium Minerals: Step-Wise Biomimetic Transformation of Vaterite to Carbonated Apatite. Biomimetics. 2024; 9(7):402. https://doi.org/10.3390/biomimetics9070402
Chicago/Turabian StyleKim, Dong Hyun, Ki Ha Min, and Seung Pil Pack. 2024. "Efficient Bioactive Surface Coatings with Calcium Minerals: Step-Wise Biomimetic Transformation of Vaterite to Carbonated Apatite" Biomimetics 9, no. 7: 402. https://doi.org/10.3390/biomimetics9070402
APA StyleKim, D. H., Min, K. H., & Pack, S. P. (2024). Efficient Bioactive Surface Coatings with Calcium Minerals: Step-Wise Biomimetic Transformation of Vaterite to Carbonated Apatite. Biomimetics, 9(7), 402. https://doi.org/10.3390/biomimetics9070402






