Biomimetic Antifungal Materials: Countering the Challenge of Multidrug-Resistant Fungi
Abstract
1. Introduction
2. Understanding Multidrug-Resistant Fungi and the Challenges for Developing New Antifungals
3. Biomimetic Materials: An Overview
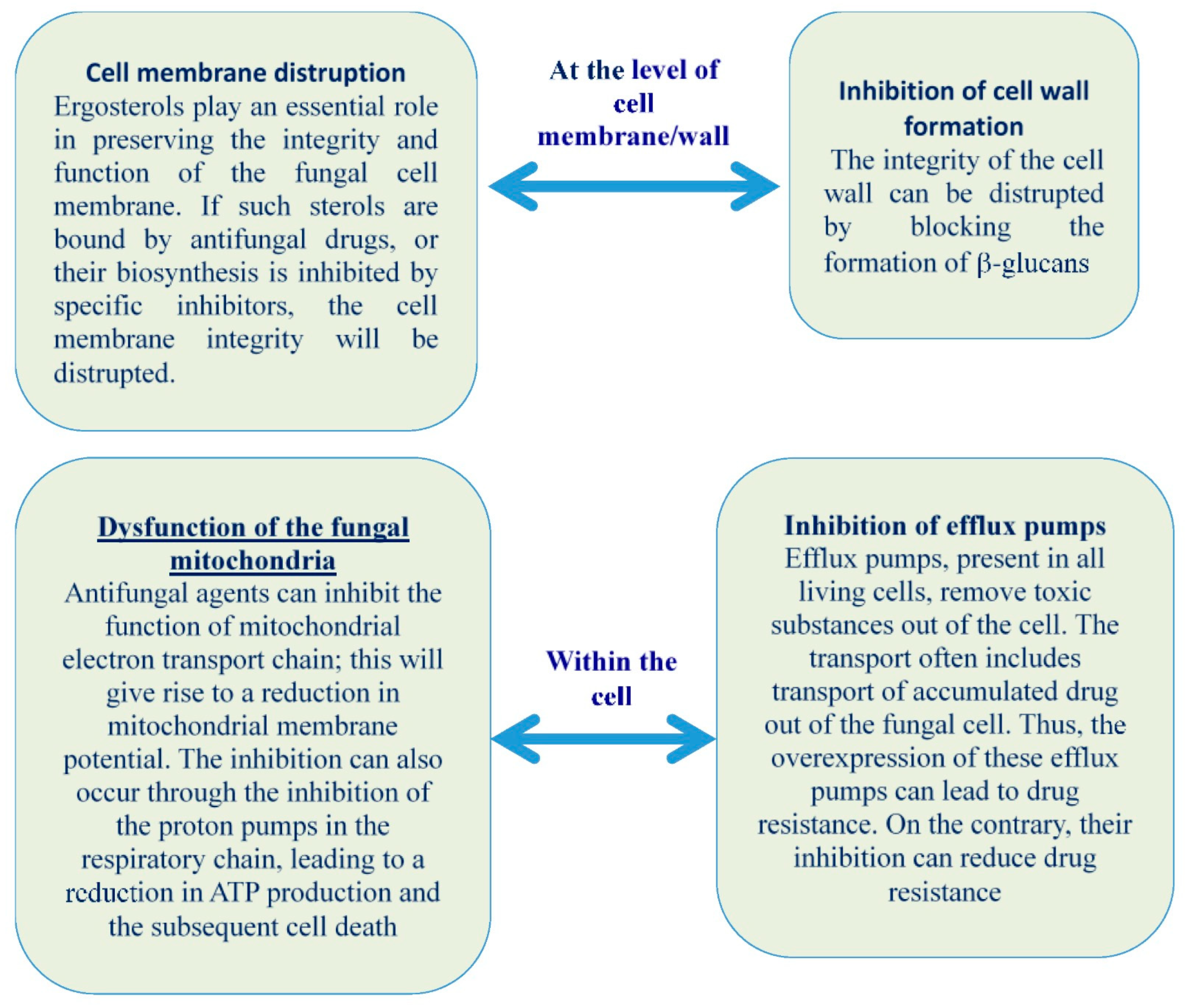
4. Types of Biomimetic Antifungal Agents and Strategies
4.1. Antifungal Peptides
4.1.1. Cecropin Peptides
4.1.2. Defensins and Defensin-like Peptides
4.1.3. Cathelicidins
4.1.4. Dermaseptins
4.2. Alginate-Based Antifungals
4.3. Chitosan and Chitosan Derivatives
4.4. Nanoparticles
4.5. Plant-Derived Polyphenols
4.6. Probiotic Bacteria
4.7. Graphene-Based Materials
4.8. Other Biomimetic Antifungal Agents
4.8.1. Essential Oils
4.8.2. Enzymatic Treatments
4.8.3. Lysozyme
4.8.4. Phospholipid-Based Liposomes
4.8.5. Propolis
4.8.6. Silk Fibroin-Based Materials
5. Challenges and Future Directions
5.1. Antifungal Peptides Limitations
5.2. Alginate-Based Hydrogels and Other Biomimetic Hydrogels Limitations
5.3. Chitosan and Chitosan Derivatives Limitations
5.4. Nanoparticles Limitations
5.5. Plant-Derived Polyphenols Limitations
5.6. Graphene-Based Materials Limitations
5.7. Probiotics Limitations
6. Research Gaps in the Biomimetic Antifungal Materials
6.1. Mechanisms of Action
6.2. Optimization of Biocompatibility and Toxicity
6.3. Long-Term Efficacy and Resistance Development
6.4. Scalability and Cost-Effectiveness
6.5. In Vivo Studies and Clinical Trials
6.6. Multifunctional and Hybrid Materials
6.7. Environmental Impact and Degradation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khalifa, H.O.; Watanabe, A.; Kamei, K. Azole and echinocandin resistance mechanisms and genotyping of Candida tropicalis in Japan: Cross-boundary dissemination and animal–human transmission of C. tropicalis infection. Clin. Microbiol. Infect. 2022, 28, 302.e5–302.e8. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Hubka, V.; Watanabe, A.; Nagi, M.; Miyazaki, Y.; Yaguchi, T.; Kamei, K. Prevalence of antifungal resistance, genetic basis of acquired azole and echinocandin resistance, and genotyping of Candida krusei recovered from an international collection. Antimicrob. Agents Chemother. 2022, 66, e01856-21. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Watanabe, A.; Kamei, K. Genetic mutations in FKS1 gene associated with acquired echinocandin resistance in Candida parapsilosis complex. Mycopathologia 2024, 189, 40. [Google Scholar] [CrossRef]
- Mudenda, S. Global Burden of fungal infections and antifungal resistance from 1961 to 2024: Findings and future implications. Pharmacol. Pharm. 2024, 15, 81–112. [Google Scholar] [CrossRef]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Watanabe, A.; Kamei, K. Antifungal resistance and genotyping of clinical Candida parapsilosis complex in Japan. J. Fungi 2023, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Arai, T.; Majima, H.; Watanabe, A.; Kamei, K. Genetic basis of azole and echinocandin resistance in clinical Candida glabrata in Japan. Antimicrob. Agents Chemother. 2020, 64, e00783-20. [Google Scholar] [CrossRef]
- Denning, D. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, E428–E438. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Volume 1, pp. 1–48. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 1 July 2024).
- Negm, E.M.; Mohamed, M.S.; Rabie, R.A.; Fouad, W.S.; Beniamen, A.; Mosallem, A.; Tawfik, A.E.; Salama, H.M. Fungal infection profile in critically ill COVID-19 patients: A prospective study at a large teaching hospital in a middle-income country. BMC Infect. Dis. 2023, 23, 246. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal Infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and drug resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Majima, H.; Watanabe, A.; Kamei, K. In vitro characterization of twenty-one antifungal combinations against echinocandin-resistant and-susceptible Candida glabrata. J. Fungi 2021, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular evolution of antifungal drug resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Kamimoto, M.; Shimamoto, T.; Shimamoto, T. Antimicrobial effects of blueberry, raspberry, and strawberry aqueous extracts and their effects on virulence gene expression in Vibrio cholerae. Phytother. Res. 2015, 29, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Daptomycin: Mechanisms of Action and Resistance, and Biosynthetic Engineering. Curr. Opin. Chem. Biol. 2009, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gauwerky, K.; Borelli, C.; Korting, H.C. Targeting virulence: A new paradigm for antifungals. Drug Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Brown, G.D.; Netea, M.G.; Zelante, T.; Gresnigt, M.S.; van de Veerdonk, F.L.; Levitz, S.M. Immunotherapeutic approaches to treatment of fungal diseases. Lancet Infect. Dis. 2017, 17, E393–E402. [Google Scholar] [CrossRef]
- Sandhu, Z.A.; Raza, M.A.; Alqurashi, A.; Sajid, S.; Ashraf, S.; Imtiaz, K.; Aman, F.; Alessa, A.H.; Shamsi, M.B.; Latif, M. Advances in the optimization of Fe nanoparticles: Unlocking antifungal properties for biomedical applications. Pharmaceutics 2024, 16, 645. [Google Scholar] [CrossRef]
- Jangjou, A.; Zareshahrabadi, Z.; Abbasi, M.; Talaiekhozani, A.; Kamyab, H.; Chelliapan, S.; Vaez, A.; Golchin, A.; Tayebi, L.; Vafa, E.; et al. Time to conquer fungal infectious diseases: Employing nanoparticles as powerful and versatile antifungal nanosystems against a wide variety of fungal species. Sustainability 2022, 14, 12942. [Google Scholar] [CrossRef]
- Bigham, A.; Zarepour, A.; Safarkhani, M.; Huh, Y.; Khosravi, A.; Rabiee, N.; Iravani, S.; Zarrabi, A. Inspired by nature: Bioinspired and biomimetic photocatalysts for biomedical applications. Nano Mater. Sci. 2024. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Du, F.; Sun, L.; Shi, J.; Long, M.; Chen, Z. Activity and mechanism of action of antifungal peptides from microorganisms: A review. Molecules 2021, 26, 3438. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125 (Suppl. S1), S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Arendrup, M.C. Acquired antifungal drug resistance in Aspergillus fumigatus: Epidemiology and detection. Med. Mycol. 2011, 49 (Suppl. S1), S90–S95. [Google Scholar] [CrossRef] [PubMed]
- Hagen, F.; Hare Jensen, R.; Meis, J.F.; Arendrup, M.C. Molecular epidemiology and in vitro antifungal susceptibility testing of 108 clinical Cryptococcus neoformans Sensu Lato and Cryptococcus gattii Sensu Lato isolates from Denmark. Mycoses 2016, 59, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A. Results from the ARTEMIS DISK global antifungal surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI atandardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef]
- Onishi, J.; Meinz, M.; Thompson, J.; Curotto, J.; Dreikorn, S.; Rosenbach, M.; Douglas, C.; Abruzzo, G.; Flattery, A.; Kong, L.; et al. Discovery of novel antifungal (1,3)-β-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 2000, 44, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in incidence and antifungal drug resistance in candidemia: Results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 2012, 55, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Lortholary, O.; Desnos-Ollivier, M.; Sitbon, K.; Fontanet, A.; Bretagne, S.; Dromer, F. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: A prospective multicenter study involving 2441 patients. Antimicrob. Agents Chemother. 2011, 55, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Arai, T.; Majima, H.; Watanabe, A.; Kamei, K. Evaluation of Surveyor Nuclease for rapid identification of FKS genes mutations in Candida glabrata. J. Infect. Chemother. 2021, 27, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Delma, F.Z.; Al-Hatmi, A.M.; Brüggemann, R.J.; Melchers, W.J.; de Hoog, S.; Verweij, P.E.; Buil, J.B. Molecular mechanisms of 5-fluorocytosine resistance in yeasts and filamentous fungi. J. Fungi 2021, 7, 909. [Google Scholar] [CrossRef] [PubMed]
- Vermes, A.; Guchelaar, H.J.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Ghannoum, M.A. Flucytosine treatment and resistance mechanisms. In Antimicrobial Drug Resistance; Mayers, D.L., Sobel, J.D., Ouellette, M., Kaye, K.S., Marchaim, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 407–413. [Google Scholar]
- Pfaller, M.; Neofytos, D.; Diekema, D.; Azie, N.; Meier-Krisesche, H.-U.; Quan, S.-P.; Horn, D. Epidemiology and outcomes of candidemia in 3648 patients: Data for the prospective antifungal therapy (PATH Alliancew) registry, 2004–2008. Diagn. Microbiol. Infect. Dis. 2012, 74, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Rex, J.H.; Walsh, T.J.; Nettleman, M.; Anaissie, E.J.; Bennett, J.E.; Bow, E.J.; Carillo-Munoz, A.J.; Chavanet, P.; Cloud, G.A.; Denning, D.W.; et al. Need for alternative trial designs and evaluation strategies for therapeutic studies of invasive mycoses. Clin. Infect. Dis. 2001, 33, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rahamim, V.; Azagury, A. Bioengineered biomimetic and bioinspired noninvasive drug delivery systems. Adv. Funct. Mater. 2021, 31, 2102033. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics: Biologically Inspired Technologies; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; ISBN 9780849331633. [Google Scholar]
- Das, D.; Noh, I. Overviews of biomimetic medical materials. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Cham, Switzerland, 2018; pp. 3–24. [Google Scholar]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: Focusing on cartilage tissue engineering. J. Biol. Eng. 2019, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Chee, E.; Brown, A.C. Biomimetic antimicrobial material strategies for combating antibiotic resistant bacteria. Biomater. Sci. 2020, 8, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Okanda, T.; Abd El-Hafeez, A.A.; Abd El Latif, A.; Habib, A.G.; Yano, H.; Kato, Y.; Matsumoto, T. Comparative evaluation of five assays for detection of carbapenemases with a proposed scheme for their precise application. J. Mol. Diagn. 2020, 22, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Soliman, A.M.; Saito, T.; Kayama, S.; Yu, L.; Hisatsune, J.; Sugai, M.; Nariya, H.; Ahmed, A.M.; Shimamoto, T.; et al. First report of foodborne Klebsiella pneumoniae coharboring blaVIM-1, blaNDM-1, and mcr-9. Antimicrob. Agents Chemother. 2020, 64, e00882-20. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Wang, J.; Vermerris, W. Antimicrobial nanomaterials derived from natural products—A review. Materials 2016, 9, 255. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S.; Van Eps, J.L.; Cabrera, F.J.; Barbosa, Z.; Del Rosal, G.M.; Weiner, B.K.; Ellsworth, W.A., IV; Tasciotti, E. Platelet-rich plasma: A biomimetic approach to enhancement of surgical wound healing. J. Surg. Res. 2017, 207, 33–44. [Google Scholar] [CrossRef]
- Espina, L.; Pagán, R.; López, D.; García-Gonzalo, D. Individual constituents from essential oils inhibit biofilm mass production by multi-drug resistant Staphylococcus aureus. Molecules 2015, 20, 11357–11372. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Aslanli, A.; Stepanov, N.; Senko, O.; Maslova, O. Various biomimetics, including peptides as antifungals. Biomimetics 2023, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Bentz, M.L.; Nunnally, N.; Lockhart, S.R.; Sexton, D.J.; Berkow, E.L. Antifungal activity of nikkomycin Z against Candida auris. J. Antimicrob. Chemother. 2021, 76, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Kočendová, J.; Vaňková, E.; Volejníková, A.; Nešuta, O.; Buděšínský, M.; Socha, O.; Hájek, M.; Hadravová, R.; Čerovský, V. Antifungal activity of analogues of antimicrobial peptides isolated from bee venoms against vulvovaginal Candida spp. FEMS Yeast Res. 2019, 19, foz013. [Google Scholar] [CrossRef] [PubMed]
- Heymich, M.-L.; Nißl, L.; Hahn, D.; Noll, M.; Pischetsrieder, M. Antioxidative, antifungal and additive activity of the antimicrobial peptides Leg1 and Leg2 from chickpea. Foods 2021, 10, 585. [Google Scholar] [CrossRef]
- Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect cecropins, antimicrobial peptides with potential therapeutic applications. Int. J. Mol. Sci. 2019, 20, 5862. [Google Scholar] [CrossRef]
- Ki, M.R.; Kim, S.H.; Park, T.I.; Pack, S.P. Self-entrapment of antimicrobial peptides in silica particles for stable and effective antimicrobial peptide delivery system. Int. J. Mol. Sci. 2023, 24, 16423. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liu, Y.; Shui, L.; Zhao, Z.; Mao, X.; Liu, Z. Mechanisms of action of the antimicrobial peptide cecropin in the killing of Candida albicans. Life 2022, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, J.; Bulet, P.; Charlet, M.; Lowenberger, C.; Blass, C.; Müller, H.M.; Richman, A. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 2000, 9, 75–84. [Google Scholar] [CrossRef]
- Lowenberger, C.; Charlet, M.; Vizioli, J.; Kamal, S.; Richman, A.; Christensen, B.M.; Bulet, P. Antimicrobial activity spectrum, cDNA cloning, and mRNA expression of a newly isolated member of the cecropin family from the mosquito vector Aedes aegypti. J. Biol. Chem. 1999, 274, 20092–20097. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, H.; Ma, D.; Wu, J.; Wang, Y.; Song, Y.; Lai, R. Toward an understanding of the molecular mechanism for successful blood feeding by coupling proteomics analysis with pharmacological testing of horsefly salivary glands. Mol. Cell. Proteom. 2008, 7, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.; Hultmark, D. Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol. 1999, 29, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Munks, R.J.; Hamilton, J.V.; Vovelle, F.; Brun, R.; Lehane, M.J.; Bulet, P. Epithelial innate immunity: A novel antimicrobial peptide with antiparasitic activity in the blood-sucking insect Stomoxys calcitrans. J. Biol. Chem. 2002, 277, 49921–49926. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, A.J.; Bland, J.M.; Jacks, T.J.; Grimm, C.; Walsh, T.J. Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin. Med. Mycol. 1998, 36, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Geng, T.; Hou, C.; Huang, Y.; Qin, G.; Guo, X. Bombyx mori cecropin A has a high antifungal activity to entomopathogenic fungus Beauveria bassiana. Gene 2016, 583, 29–35. [Google Scholar] [CrossRef]
- Lee, E.; Kim, J.K.; Jeon, D.; Jeong, K.W.; Shin, A.; Kim, Y. Functional roles of aromatic residues and helices of papiliocin in its antimicrobial and anti-inflammatory activities. Sci. Rep. 2015, 5, 12048. [Google Scholar] [CrossRef]
- Yoe, S.M.; Kang, C.S.; Han, S.S.; Bang, I.S. Characterization and cDNA cloning of hinnavin II, a cecropin family antibacterial peptide from the cabbage butterfly, Artogeia rapae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 199–205. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, A.J.; Bland, J.M.; Vigo, C.B.; Jacks, T.J.; Peter, J.; Walsh, T.J. D-Cecropin B: Proteolytic resistance, lethality for pathogenic fungi and binding properties. Med. Mycol. 2000, 38, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, W.; Zhang, L.; Zhang, Y.; Cao, B. Cecropin A–melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem. Biophys. Res. Commun. 2014, 451, 650–655. [Google Scholar] [CrossRef]
- Lee, J.K.; Seo, C.H.; Luchian, T.; Park, Y. Antimicrobial peptide CMA3 derived from the CA-MA hybrid peptide: Antibacterial and anti-inflammatory activities with low cytotoxicity and mechanism of action in Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; Gonçalves, S.; Santos, N.C. Defensins: Antifungal lessons from eukaryotes. Front. Microbiol. 2014, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Shi, M.; Ye, X.Q.; Chen, M.Y.; Chen, X.X. Identification, characterization and expression of a defensin-like antifungal peptide from the Whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Insect Mol. Biol. 2013, 22, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; del Carmen Rodriguez, M.; Lanz-Mendoza, H.; Zhu, S. AdDLP, a Bacterial defensin-like peptide, exhibits anti-plasmodium activity. Biochem. Biophys. Res. Commun. 2017, 494, 642–647. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, S. Alteration of the mode of antibacterial action of a defensin by the amino-terminal loop substitution. Biochem. Biophys. Res. Commun. 2012, 426, 630–635. [Google Scholar] [CrossRef]
- de Oliveira Carvalho, A.; Moreira Gomes, V. Plant defensins and defensin-like peptides-biological activities and biotechnological applications. Curr. Pharm. Des. 2011, 17, 4270–4293. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.F.; Vasconcelos, É.A.; Pelegrini, P.B.; Grossi de Sa, M.F. Antifungal defensins and their role in plant defense. Front. Microbiol. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Di Luca, M.; Esin, S.; Florio, W.; Brancatisano, F.L.; Bottai, D.; Campa, M.; Batoni, G. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides 2008, 29, 1–6. [Google Scholar] [CrossRef]
- McPhee, J.B.; Scott, M.G.; Hancock, R.E. Design of host defence peptides for antimicrobial and immunity enhancing activities. Comb. Chem. High Throughput Screen. 2005, 8, 257–272. [Google Scholar] [CrossRef]
- Zhao, C.; Nguyen, T.; Boo, L.M.; Hong, T.; Espiritu, C.; Orlov, D.; Wang, W.; Waring, A.; Lehrer, R.I. RL-37, an Alpha-helical antimicrobial peptide of the rhesus monkey. Antimicrob. Agents Chemother. 2001, 45, 2695–2702. [Google Scholar] [CrossRef]
- Termen, S.; Tollin, M.; Olsson, B.; Svenberg, T.; Agerberth, B.; Gudmundsson, G.H. Phylogeny, processing and expression of the rat cathelicidin rCRAMP: A model for innate antimicrobial peptides. Cell. Mol. Life Sci. 2003, 60, 536–549. [Google Scholar] [CrossRef]
- Tomasinsig, L.; Zanetti, M. The Cathelicidins-structure, function and evolution. Curr. Protein Pept. Sci. 2005, 6, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Holani, R.; Rathnayaka, C.; Blyth, G.A.; Babbar, A.; Lahiri, P.; Young, D.; Dufour, A.; Hollenberg, M.D.; McKay, D.M.; Cobo, E.R. Cathelicidins induce toll-interacting protein synthesis to prevent apoptosis in colonic epithelium. J. Innate Immun. 2023, 15, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, J.; Liu, X.; Yang, H.; Liu, R.; Wu, J.; Wang, A.; Lin, D.; Lai, R. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotic. PLoS ONE 2008, 3, e3217. [Google Scholar] [CrossRef] [PubMed]
- Broekman, D.C.; Guðmundsson, G.H.; Maier, V.H. Differential regulation of cathelicidin in salmon and cod. Fish Shellfish Immunol. 2013, 35, 532–538. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.; Molhoek, E.M.; Bikker, F.J.; Yu, P.-L.; Veldhuizen, E.J.A.; Haagsman, H.P. Avian cathelicidins: Paradigms for the development of anti-infectives. Vet. Microbiol. 2011, 153, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Memariani, M.; Memariani, H. Antifungal properties of cathelicidin LL-37: Current knowledge and future research directions. World J. Microbiol. Biotechnol. 2024, 40, 34. [Google Scholar] [CrossRef]
- Van Eijk, M.; Boerefijn, S.; Cen, L.; Rosa, M.; Morren, M.J.; Van Der Ent, C.K.; Kraak, B.; Dijksterhuis, J.; Valdes, I.D.; Haagsman, H.P.; et al. Cathelicidin-inspired antimicrobial peptides as novel antifungal compounds. Med. Mycol. 2020, 58, 1073–1084. [Google Scholar] [CrossRef]
- Bartels, E.J.H.; Dekker, D.; Amiche, M. Dermaseptins, multifunctional antimicrobial peptides: A review of their pharmacology, effectivity, mechanism of action, and possible future directions. Front. Pharmacol. 2019, 10, 465831. [Google Scholar] [CrossRef]
- Nicolas, P.; El Amri, C. The dermaseptin superfamily: A gene-based combinatorial library of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1537–1550. [Google Scholar] [CrossRef]
- Morton, C.O.; Dos Santos, S.C.; Coote, P.J. An Amphibian-derived, cationic, alpha-helical antimicrobial peptide kills yeast by caspase-independent but aif-dependent programmed cell death. Mol. Microbiol. 2007, 65, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Belmadani, A.; Semlali, A.; Rouabhia, M. Dermaseptin-S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic protease genes. J. Appl. Microbiol. 2018, 125, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Han, S.S. Dual-crosslinked poly (vinyl alcohol)/sodium alginate/silver nanocomposite beads–a promising antimicrobial material. Food Chem. 2017, 234, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Spadari, C.D.C.; Lopes, L.B.; Ishida, K. Potential use of alginate-based carriers as antifungal delivery system. Front. Microbiol. 2017, 8, 241872. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.; Tahir, M.H.; Farooqi, F.; Manzoor, S.; Khan, S.U. Strategies adopted for the preparation of sodium alginate-based nanocomposites and their role as catalytic, antibacterial, and antifungal agents. Rev. Chem. Eng. 2023, 39, 1359–1391. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinović-Runić, J.; Vukomanović, M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles incorporated hydrogels for delivery of antimicrobial agents: Developments and trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Taran, M.; Imani, M.M. Preparation, structural characterization, thermal properties and antifungal activity of alginate-CuO bionanocomposite. Mater. Sci. Eng. C 2019, 101, 323–329. [Google Scholar] [CrossRef]
- Xiang, S.; Ma, X.; Shi, H.; Ma, T.; Tian, C.; Chen, Y.; Chen, H.; Chen, X.; Luo, K.; Cai, L. Green synthesis of an alginate-coated silver nanoparticle shows high antifungal activity by enhancing its cell membrane penetrating ability. ACS Appl. Bio Mater. 2019, 2, 4087–4096. [Google Scholar] [CrossRef]
- Gong, Y.; Han, G.; Zhang, Y.; Pan, Y.; Li, X.; Xia, Y.; Wu, Y. Antifungal activity and cytotoxicity of zinc, calcium, or copper alginate fibers. Biol. Trace Elem. Res. 2012, 148, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Streit, F.; Koch, F.; Laranjeira, M.; Ninow, J.L. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Braz. J. Microbiol. 2009, 40, 20–25. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hamdine, M.; Heuzey, M.C.; Bégin, A. Effect of organic and inorganic acids on concentrated chitosan solutions and gels. Int. J. Biol. Macromol. 2005, 37, 134–142. [Google Scholar] [CrossRef]
- Hernández-Lauzardo, A.N.; Bautista-Baños, S.; Velazquez-Del Valle, M.G.; Méndez-Montealvo, M.G.; Sánchez-Rivera, M.M.; Bello-Perez, L.A. Antifungal effects of chitosan with different molecular weights on in vitro development of Rhizopus stolonifer (Ehrenb.: Fr.) Vuill. Carbohydr. Polym. 2008, 73, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, R.; Liu, S.; Qin, Y.; Meng, X.; Li, P. Microwave-assisted degradation of chitosan for a possible use in inhibiting crop pathogenic fungi. Int. J. Biol. Macromol. 2012, 51, 767–773. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hjeljord, L.G.; Aam, B.B.; Sørlie, M.; Tronsmo, A. Antifungal effect of chito-oligosaccharides with different degrees of polymerization. Eur. J. Plant Pathol. 2015, 141, 147–158. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, H.T.V.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Shih, P.Y.; Liao, Y.T.; Tseng, Y.K.; Deng, F.S.; Lin, C.H. A potential antifungal effect of chitosan against Candida albicans is mediated via the inhibition of SAGA complex component expression and the subsequent alteration of cell surface integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Galvan Marquez, I.; Akuaku, J.; Cruz, I.; Cheetham, J.; Golshani, A.; Smith, M.L. Disruption of protein synthesis as antifungal mode of action by chitosan. Int. J. Food Microbiol. 2013, 164, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chen, C.Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Cuero, R.G.; Duffus, E.; Osuji, G.; Pettit, R. Aflatoxin control in preharvest maize: Effects of chitosan and two microbial agents. J. Agric. Sci. 1991, 117, 165–169. [Google Scholar] [CrossRef]
- Laflamme, P.; Benhamou, N.; Bussières, G.; Dessureault, M. Differential effect of chitosan on root rot fungal pathogens in forest nurseries. Can. J. Bot. 2000, 77, 1460–1468. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Alburquenque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Zhang, X.; Peng, N.; Mei, Y.; Liang, Y. Low molecular weight chitosan is an effective antifungal agent against Botryosphaeria sp. and preservative agent for pear (Pyrus) fruits. Int. J. Biol. Macromol. 2017, 95, 1135–1143. [Google Scholar] [CrossRef]
- Monga, S.; Hoang, T.X.; Park, J.K.; Kim, J.Y. Antifungal activity of chitosan against Trichophyton rubrum. J. Chitin Chitosan 2020, 25, 157–161. [Google Scholar]
- Ke, Y.; Ding, B.; Zhang, M.; Dong, T.; Fu, Y.; Lv, Q.; Ding, W.; Wang, X. Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus flavus and Aspergillus fumigatus. Carbohydr. Polym. 2022, 275, 118673. [Google Scholar] [CrossRef]
- Peng, Y.; Han, B.; Liu, W.; Xu, X. Preparation and antimicrobial activity of hydroxypropyl chitosan. Carbohydr. Res. 2005, 340, 1846–1851. [Google Scholar] [CrossRef]
- de Oliveira Pedro, R.; Takaki, M.; Gorayeb, T.C.C.; Del Bianchi, V.L.; Thomeo, J.C.; Tiera, M.J.; de Oliveira Tiera, V.A. Synthesis: Characterization and antifungal activity of quaternary derivatives of chitosan on Aspergillus flavus. Microbiol. Res. 2013, 168, 50–55. [Google Scholar] [CrossRef]
- Tajdini, F.; Amini, M.A.; Nafissi-Varcheh, N.; Faramarzi, M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int. J. Biol. Macromol. 2010, 47, 180–183. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Balicka-Ramisz, A.; Wojtasz-Pajak, A.; Pilarckyk, B.; Ramisz, A.; Laurans, L. Antibacterial and antifugal activity of chitosan. In Proceedings of the 12th ISAH Congress on Animal Hygiene, Warszawa, Poland, 4–8 September 2005; pp. 406–409. [Google Scholar]
- Zakrzewska, A.; Boorsma, A.; Delneri, D.; Brul, S.; Oliver, S.G.; Klis, F.M. Cellular processes and pathways that protect Saccharomyces cerevisiae cells against the plasma membrane-perturbing compound chitosan. Eukaryot. Cell 2007, 6, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.S.; Pal, A. Synthesis of chitosan-based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef]
- Ziani, K.; Fernández-Pan, I.; Royo, M.; Maté, J.I. Antifungal activity of films and solutions based on chitosan against typical seed fungi. Food Hydrocoll. 2009, 23, 2309–2314. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- Hiba, H.; Thoppil, J.E. Medicinal herbs as a panacea for biogenic silver nanoparticles. Bull. Natl. Res. Cent. 2022, 46, 9. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cervantes, J.A.; Gallegos-Alvarado, D.Y.; Osorio-Concepción, M.; Morones-Ramírez, J.R. Nanomaterial-based antifungal therapies to combat fungal diseases aspergillosis, coccidioidomycosis, mucormycosis, and candidiasis. Pathogens 2021, 10, 1303. [Google Scholar] [CrossRef]
- Parveen, J.; Sultana, T.; Kazmi, A.; Malik, K.; Ullah, A.; Ali, A.; Qayyum, B.; Raja, N.I.; Mashwani, Z.U.R.; Rehman, S.U. Phytosynthesized nanoparticles as novel antifungal agent for sustainable agriculture: A mechanistic approach, current advances, and future directions. J. Nanotechnol. 2023, 2023, 8011189. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Żarowska, B.; Koźlecki, T.; Piegza, M.; Jaros-Koźlecka, K.; Robak, M. New look on antifungal activity of silver nanoparticles (AgNPs). Pol. J. Microbiol. 2019, 68, 515–525. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Terea, H.; Selloum, D.; Rebiai, A.; Bouafia, A.; Ben Mya, O. Preparation and characterization of cellulose/ZnO nanoparticles extracted from peanut shells: Effects on antibacterial and antifungal activities. Biomass Convers. Biorefin. 2023, 1–12. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical applications and bioavailability of curcumin—An updated overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.M.; Getso, M.I.; Farahyar, S.; Khodavaisy, S.; Roudbary, M.; Mahabadi, V.P.; Mahmoudi, S. Antifungal activity of green-synthesized curcumin-coated silver nanoparticles alone and in combination with fluconazole and itraconazole against Candida and Aspergillus species. Curr. Med. Mycol. 2023, 9, 38. [Google Scholar]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin nanoparticles as promising therapeutic agents for drug targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, X.; Nie, J.; Zhao, H.; Li, W. Nano-antimicrobial peptides based on constitutional isomerism-dictated self-assembly. Biomacromolecules 2022, 23, 1302–1313. [Google Scholar] [CrossRef]
- Tatli Seven, P.; Seven, I.; Gul Baykalir, B.; Iflazoglu Mutlu, S.; Salem, A.Z. Nanotechnology and nano-propolis in animal production and health: An overview. Ital. J. Anim. Sci. 2018, 17, 921–930. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal activity of phenolic and polyphenolic compounds from different matrices of Vitis vinifera L. against human pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Janeczko, M.; Gmur, D.; Kochanowicz, E.; Górka, K.; Skrzypek, T. Inhibitory effect of a combination of baicalein and quercetin flavonoids against Candida albicans strains isolated from the female reproductive system. Fungal Biol. 2022, 126, 407–420. [Google Scholar] [CrossRef]
- Sadeghi-Ghadi, Z.; Vaezi, A.; Ahangarkani, F.; Ilkit, M.; Ebrahimnejad, P.; Badali, H. Potent in vitro activity of curcumin and quercetin co-encapsulated in nanovesicles without hyaluronan against Aspergillus and Candida isolates. J. Mycol. Med. 2020, 30, 101014. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.D.C.O.; Gullo, F.P.; Freires, I.A.; de Souza Pitangui, N.; Segalla, M.P.; Fusco-Almeida, A.M.; Rosalen, P.L.; Regasini, L.O.; Mendes-Giannini, M.J.S. Synthesis, antifungal activity of caffeic acid derivative esters, and their synergism with fluconazole and nystatin against Candida spp. Diagn. Microbiol. Infect. Dis. 2016, 86, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Alfarrayeh, I.; Pollák, E.; Czéh, Á.; Vida, A.; Das, S.; Papp, G. Antifungal and anti-biofilm effects of caffeic acid phenethyl ester on different Candida species. Antibiotics 2021, 10, 1359. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Meng, X.; Lin, X.; Duan, N.; Wang, Z.; Wu, S. Antifungal activity and inhibitory mechanisms of ferulic acid against the growth of Fusarium graminearum. Food Biosci. 2023, 52, 102414. [Google Scholar] [CrossRef]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Kulik, T.; Buśko, M.; Pszczółkowska, A.; Perkowski, J.; Okorski, A. Plant lignans inhibit growth and trichothecene biosynthesis in Fusarium graminearum. Lett. Appl. Microbiol. 2014, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.V.; Tran, N.T.; Nguyen, P.L.; Nguyen, N.N.; Nguyen, N.T.; Nguyen, T.T.; Tran, T.T.; Nguyen, V.P.; Thai, H.T.; Hoang, D. Sustainable lignin-based nano hybrid biomaterials with high-performance antifungal activity. ACS Omega 2023, 8, 37540–37548. [Google Scholar] [CrossRef]
- Divyashree, S.; Shruthi, B.; Vanitha, P.R.; Sreenivasa, M.Y. Probiotics and their postbiotics for the control of opportunistic fungal pathogens: A review. Biotechnol. Rep. 2023, 38, e00800. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Gottlieb, A. Probiotics for oral and vulvovaginal candidiasis: A review. Dermatol. Ther. 2019, 32, e12970. [Google Scholar] [CrossRef]
- Allonsius, C.N.; van den Broek, M.F.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]
- Poon, Y.; Hui, M. Inhibitory effect of lactobacilli supernatants on biofilm and filamentation of Candida albicans, Candida tropicalis, and Candida parapsilosis. Front. Microbiol. 2023, 14, 1105949. [Google Scholar] [CrossRef] [PubMed]
- Arasu, V.M.; Jung, M.W.; Ilavenil, S.; Jane, M.; Kim, D.H.; Lee, K.D.; Park, H.S.; Hur, T.Y.; Choi, G.J.; Lim, Y.C.; et al. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013, 115, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Mackie, J.; Donachie, G.E.; Chapuis, A.; Mezerová, K.; Lenardon, M.D.; Brown, A.J.; Duncan, S.H.; Walker, A.W. Human gut bifidobacteria inhibit the growth of the opportunistic fungal pathogen Candida albicans. FEMS Microbiol. Ecol. 2022, 98, fiac095. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.H.; Mayer, M.P.; Miyazima, T.Y.; Matsubara, V.H.; Silva, E.G.; Paula, C.R.; Campos, T.T.; Nakamae, A.E. A multispecies probiotic reduces oral Candida colonization in denture wearers. J. Prosthodont. 2015, 24, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Yaragalla, S.; Bhavitha, K.B.; Athanassiou, A. A review on graphene-based materials and their antimicrobial properties. Coatings 2021, 11, 1197. [Google Scholar] [CrossRef]
- Dasari Shareena, T.P.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Chaves-Lopez, C.; Oliveira, R.C.; Paparella, A.; Rodrigues, D.F. Cellular and metabolic approaches to investigate the effects of graphene and graphene oxide in the fungi Aspergillus flavus and Aspergillus niger. Carbon 2019, 143, 419–429. [Google Scholar] [CrossRef]
- Farzanegan, A.; Roudbary, M.; Falahati, M.; Khoobi, M.; Gholibegloo, E.; Farahyar, S.; Karimi, P.; Khanmohammadi, M. Synthesis, characterization and antifungal activity of a novel formulated nanocomposite containing Indolicidin and Graphene oxide against disseminated candidiasis. J. Mycol. Med. 2018, 28, 628–636. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Diez-Orejas, R.; Feito, M.J.; Cicuéndez, M.; Casarrubios, L.; Rojo, J.M.; Portolés, M.T. Graphene oxide nanosheets increase Candida albicans killing by pro-inflammatory and reparative peritoneal macrophages. Colloids Surf. B Biointerfaces 2018, 171, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Sangsri, T.; Siwayaprahm, P. Synthesis and antifungal activity of reduced graphene oxide nanosheets. Carbon 2012, 50, 5156–5161. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, M.R.; Kim, J.H. Antibacterial activity of dithiothreitol reduced graphene oxide. J. Ind. Eng. Chem. 2012, 18, 182–186. [Google Scholar] [CrossRef]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Streitburger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Kemper, F.H.; Winkelmann, W.; Von Eiff, C. Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Alimardani, V.; Abolmaali, S.S.; Borandeh, S. Antifungal and antibacterial properties of graphene-based nanomaterials: A mini-review. J. Nanostruct. 2019, 9, 402–413. [Google Scholar]
- Cacaci, M.; Martini, C.; Guarino, C.; Torelli, R.; Bugli, F.; Sanguinetti, M. Graphene oxide coatings as tools to prevent microbial biofilm formation on medical devices. Adv. Microbiol. Infect. Dis. Public Health 2020, 14, 21–35. [Google Scholar]
- Palmieri, V.; Bugli, F.; Cacaci, M.; Perini, G.; Maio, F.D.; Delogu, G.; Torelli, R.; Conti, C.; Sanguinetti, M.; Spirito, M.D.; et al. Graphene oxide coatings prevent Candida albicans biofilm formation with a controlled release of curcumin-loaded nanocomposites. Nanomedicine 2018, 13, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and-resistant human pathogenic Candida species. BMC Infect. Dis. 2006, 6, 158. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Thu Ha, T.T. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 2020, 11, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Qingzhi, W.; Zou, S.; Wang, Q.; Chen, L.; Yan, X.; Gao, L. Catalytic defense against fungal pathogens using nanozymes. Nanotechnol. Rev. 2021, 10, 1277–1292. [Google Scholar] [CrossRef]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Gupta, R.D. Chitinases from bacteria to human: Properties, applications, and future perspectives. Enzym. Res. 2015, 2015, 791907. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Imazato, K.; Ono, H. Human lysozyme possesses novel antimicrobial peptides within its N-terminal domain that target bacterial respiration. J. Agric. Food Chem. 2011, 59, 10336–10345. [Google Scholar] [CrossRef]
- Sowa-Jasiłek, A.; Zdybicka-Barabas, A.; Stączek, S.; Wydrych, J.; Skrzypiec, K.; Mak, P.; Deryło, K.; Tchórzewski, M.; Cytryńska, M. Galleria mellonella lysozyme induces apoptotic changes in Candida albicans cells. Microbiol. Res. 2016, 193, 121–131. [Google Scholar] [CrossRef]
- Sebaa, S.; Hizette, N.; Boucherit-Otmani, Z.; Courtois, P. Dose-dependent effect of lysozyme upon Candida albicans biofilm. Mol. Med. Rep. 2017, 15, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09416. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Xiao, Q.; Fan, L.; Huang, D.; Chen, W.; He, W. Liposomes and liposome-like nanoparticles: From anti-fungal infection to the COVID-19 pandemic treatment. Asian J. Pharm. Sci. 2022, 17, 817–837. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Pinheiro, L. Lipid systems for the delivery of amphotericin B in antifungal therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Bouchelaghem, S. Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: A review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhang, Y.; Xu, M.; Wang, C.; Huang, J.; Zhang, H.; Zhao, Q.; Zhang, L.; Yu, D.; Wei, Q.; et al. Curcumin-silk fibroin nanoparticles for enhanced anti-Candida albicans activity in vitro and in vivo. J. Biomed. Nanotechnol. 2019, 15, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.C.; Ansary, R.H.; Biswas, P.; Haider, M.A. Silk fibroin hydrogel-assisted controlled release of antifungal drug ketoconazole. J. Drug Deliv. Ther. 2023, 13, 125–130. [Google Scholar] [CrossRef]
- Ul Haq, I.; Maryam, S.; Shyntum, D.Y.; Khan, T.A.; Li, F. Exploring the frontiers of therapeutic breadth of antifungal peptides: A new avenue in antifungal drugs. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae018. [Google Scholar] [CrossRef] [PubMed]
- Böttger, R.; Hoffmann, R.; Knappe, D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef]
- Konakbayeva, D.; Karlsson, A.J. Strategies and opportunities for engineering antifungal peptides for therapeutic applications. Curr. Opin. Biotechnol. 2023, 81, 102926. [Google Scholar] [CrossRef]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Drayton, M.; Kizhakkedathu, J.N.; Straus, S.K. Towards robust delivery of antimicrobial peptides to combat bacterial resistance. Molecules 2020, 25, 3048. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Egelund, P.H.; Johansson, H.; Le Quement, S.T.; Wojcik, F.; Pedersen, D.S. Greening the synthesis of peptide therapeutics: An industrial perspective. RSC Adv. 2020, 10, 42457–42492. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Kaigler, D.; Kim, K.; Mooney, D.J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 2004, 5, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug delivery strategies and biomedical significance of hydrogels: Translational considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Teng, Y.; Li, S.; Tang, H.; Tao, X.; Fan, Y.; Huang, Y. Medical applications of hydrogels in skin infections: A review. Infect. Drug Resist. 2023, 16, 391–401. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-based (Nano) materials for novel biomedical applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Thenissery, A.; Khupse, R.; Rajashekara, G. Strategies for the preparation of chitosan derivatives for antimicrobial, drug delivery, and agricultural applications: A review. Molecules 2023, 28, 7659. [Google Scholar] [CrossRef] [PubMed]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan nanoparticle toxicity: A comprehensive literature review of in vivo and in vitro assessments for medical applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Maheswaran, H.; Djearamane, S.; Dhanapal, A.C.T.A.; Shing, W.L. Cytotoxicity of green synthesized zinc oxide nanoparticles using Musa acuminata on vero cells. Heliyon 2024, 10, E31316. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Weber, K.; Schulz, B.; Ruhnke, M. Resveratrol and its antifungal activity against Candida species. Mycoses 2011, 54, 30–33. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Wang, K.; Wang, Y. Therapeutic activity of green tea epigallocatechin-3-gallate on metabolic diseases and non-alcoholic fatty liver diseases: The current updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.K.; Mei, L.; Zeng, X. Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Tiwari, S.; Purkait, M.K. A comprehensive review on graphene oxide-based nanocarriers: Synthesis, functionalization and biomedical applications. FlatChem 2023, 38, 100484. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Yusaf, T.; Mahamude, A.S.F.; Farhana, K.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Kamarulzaman, M.K.; Subramonian, S.; Hall, S.; Dhahad, H.A. A comprehensive review on graphene nanoparticles: Preparation, properties, and applications. Sustainability 2022, 14, 12336. [Google Scholar] [CrossRef]
- Rose Jørgensen, M.; Thestrup Rikvold, P.; Lichtenberg, M.; Østrup Jensen, P.; Kragelund, C.; Twetman, S. Lactobacillus rhamnosus strains of oral and vaginal origin show strong antifungal activity in vitro. J. Oral Microbiol. 2020, 12, 1832832. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Franceschini, S.A.; Patta, M.C.; Quintana, S.M.; Candido, R.C.; Ferreira, J.C.; De Martinis, E.C.P.; Reid, G. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett. Appl. Microbiol. 2009, 48, 269–274. [Google Scholar] [CrossRef]
- Petrariu, O.A.; Barbu, I.C.; Niculescu, A.G.; Constantin, M.; Grigore, G.A.; Cristian, R.E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 2024, 14, 1296447. [Google Scholar] [CrossRef] [PubMed]
- Mikucka, A.; Deptuła, A.; Bogiel, T.; Chmielarczyk, A.; Nurczyńska, E.; Gospodarek-Komkowska, E. Bacteraemia caused by probiotic strains of Lacticaseibacillus rhamnosus—Case studies highlighting the need for careful thought before using microbes for health benefits. Pathogens 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.Z.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; Britto, D.D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2016, 29, 55–67. [Google Scholar] [CrossRef]
- Assoni, L.; Milani, B.; Carvalho, M.R.; Nepomuceno, L.N.; Waz, N.T.; Guerra, M.E.S.; Converso, T.R.; Darrieux, M. Resistance mechanisms to antimicrobial peptides in gram-positive bacteria. Front. Microbiol. 2020, 11, 593215. [Google Scholar] [CrossRef]
- Yaraghi, N.A.; Kisailus, D. Biomimetic structural materials: Inspiration from design and assembly. Annu. Rev. Phys. Chem. 2018, 69, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.M.; Monteiro, G.A.; Prazeres, D.M.F. General aspects of biomimetic materials. In Biotechnologies and Biomimetics for Civil Engineering; Springer: Berlin, Germany, 2015; pp. 57–79. [Google Scholar]
- Khalifa, H.O.; Oreiby, A.F.; Abd El-Hafeez, A.A.; Okanda, T.; Haque, A.; Anwar, K.S.; Tanaka, M.; Miyako, K.; Tsuji, S.; Kato, Y.; et al. First report of multidrug-resistant carbapenemase-producing bacteria coharboring mcr-9 associated with respiratory disease complex in pets: Potential of animal-human transmission. Antimicrob. Agents Chemother. 2020, 65, e01890-20. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Oreiby, A.; Abd El-Hafeez, A.A.; Abd El Latif, A.; Okanda, T.; Kato, Y.; Matsumoto, T. High β-lactam and quinolone resistance of Enterobacteriaceae from the respiratory tract of sheep and goat with respiratory disease. Animals 2021, 11, 2258. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Oreiby, A.F.; Okanda, T.; Kato, Y.; Matsumoto, T. High β-lactam resistance in Gram-negative bacteria associated with kennel cough and cat flu in Egypt. Sci. Rep. 2021, 11, 3347. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Shikoray, L.; Mohamed, M.-Y.I.; Habib, I.; Matsumoto, T. Veterinary drug residues in the food chain as an emerging public health threat: Sources, analytical methods, health impacts, and preventive measures. Foods 2024, 13, 1629. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Okanda, T.; Yang, Y.; Khalifa, H.O.; Haque, A.; Takemura, H.; Matsumoto, T. High-speed quenching probe-polymerase chain reaction assay for the rapid detection of carbapenemase-producing gene using GENECUBE: A fully automatic gene analyzer. Mol. Diagn. Ther. 2021, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.; Elbediwi, M.; Mohteshamuddin, K.; Mohamed, M.Y.I.; Lakshmi, G.B.; Abdalla, A.; Anes, F.; Ghazawi, A.; Khan, M.; Khalifa, H. Genomic profiling of extended-spectrum β-lactamase-producing Escherichia coli from pets in the United Arab Emirates: Unveiling colistin resistance mediated by mcr-1.1 and its probable transmission from chicken meat—A One Health perspective. J. Infect. Public Health 2023, 16, 163–171. [Google Scholar]
- Khalifa, H.O.; Al Ramahi, Y.M. After the Hurricane: Anti-COVID-19 drugs development, molecular mechanisms of action and future perspectives. Int. J. Mol. Sci. 2024, 25, 739. [Google Scholar] [CrossRef]
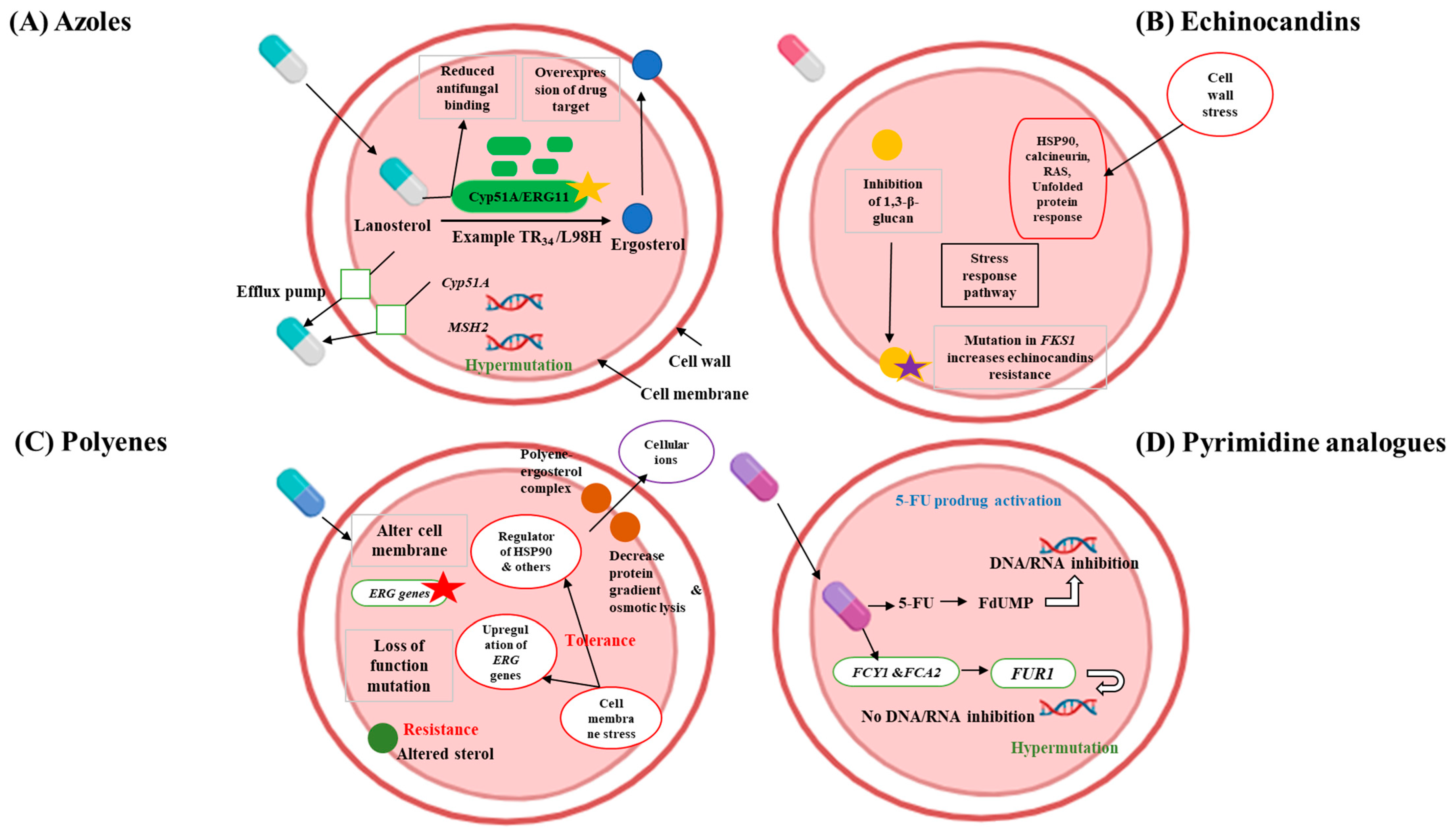
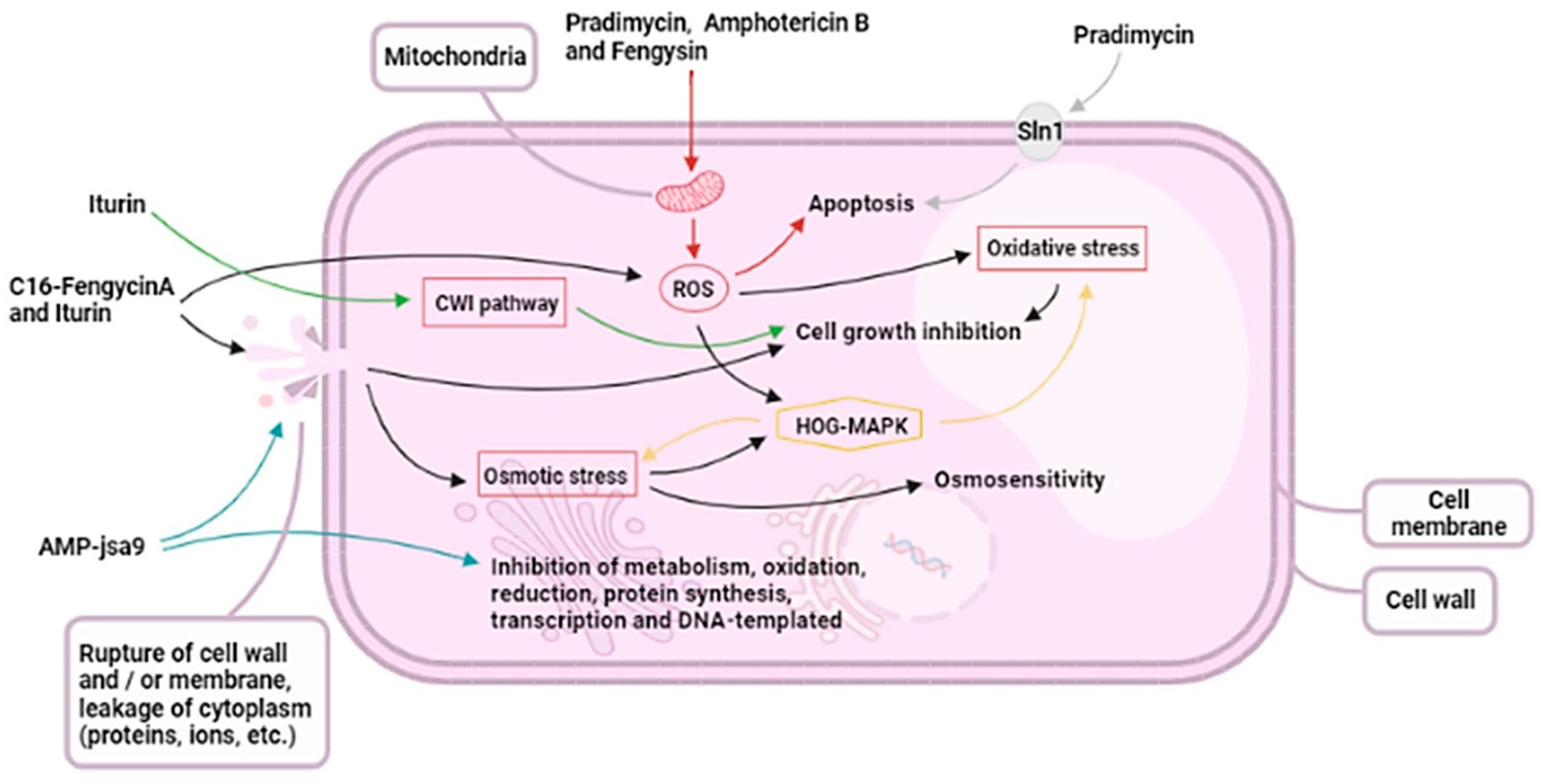
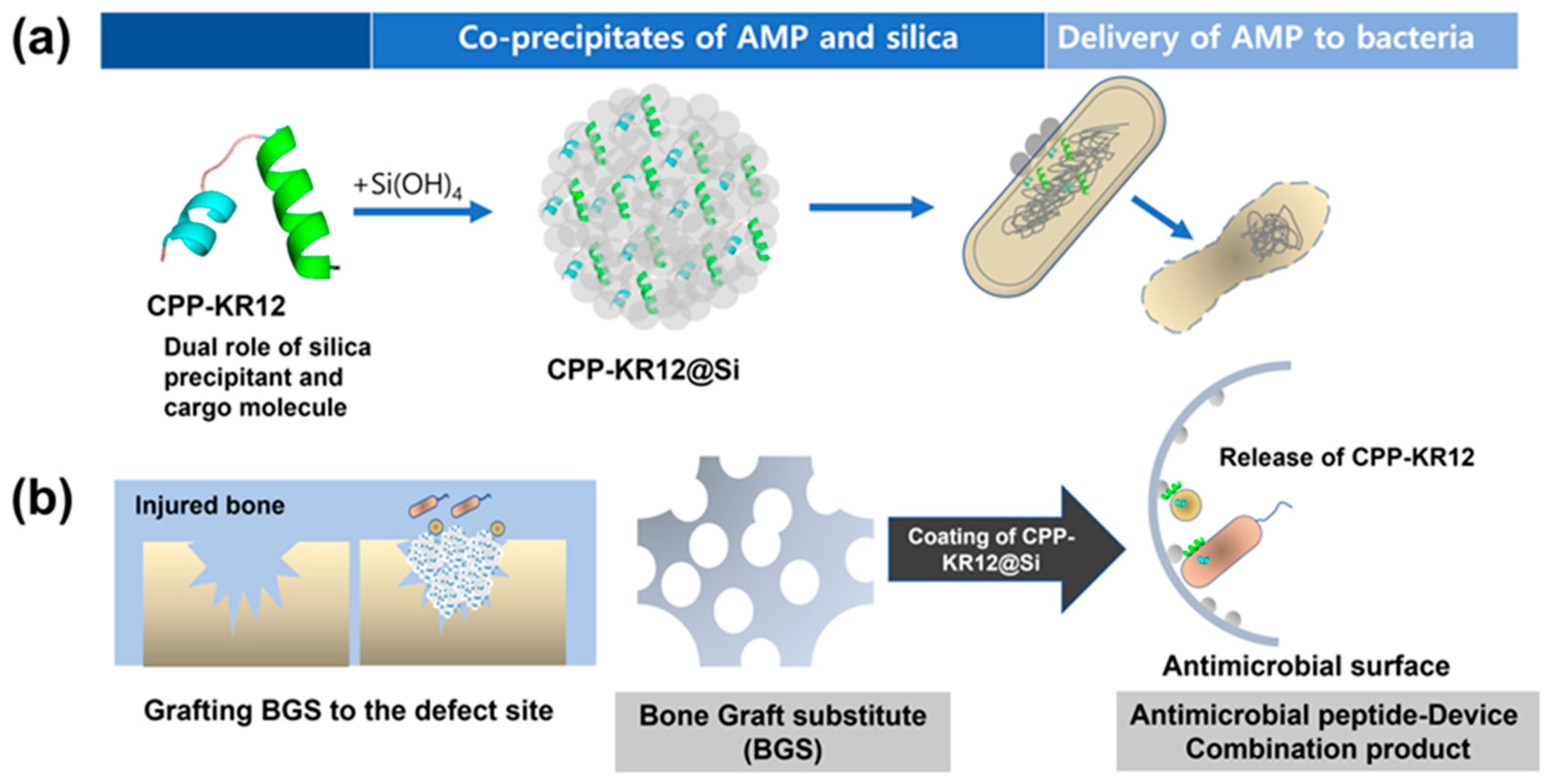
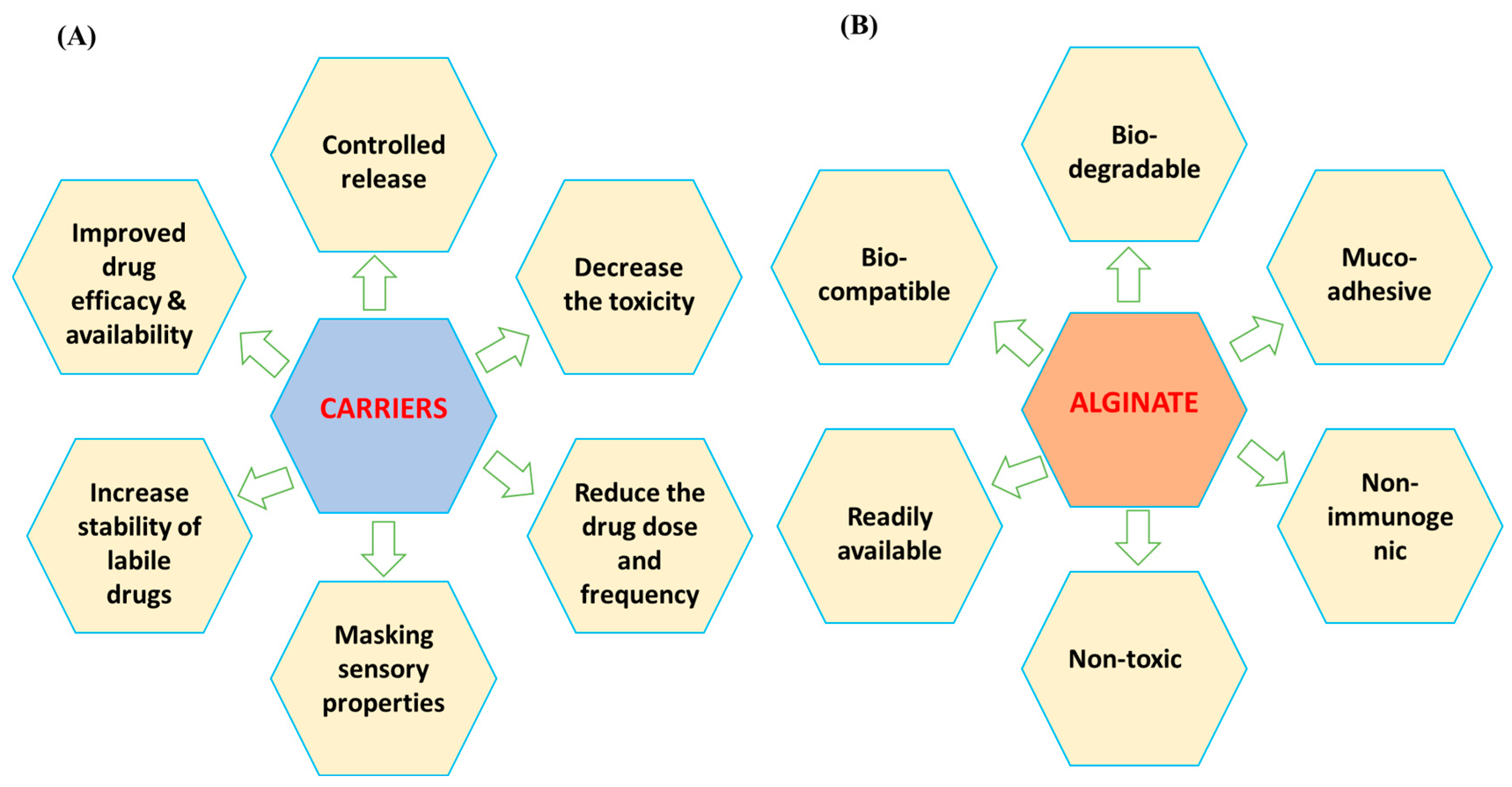

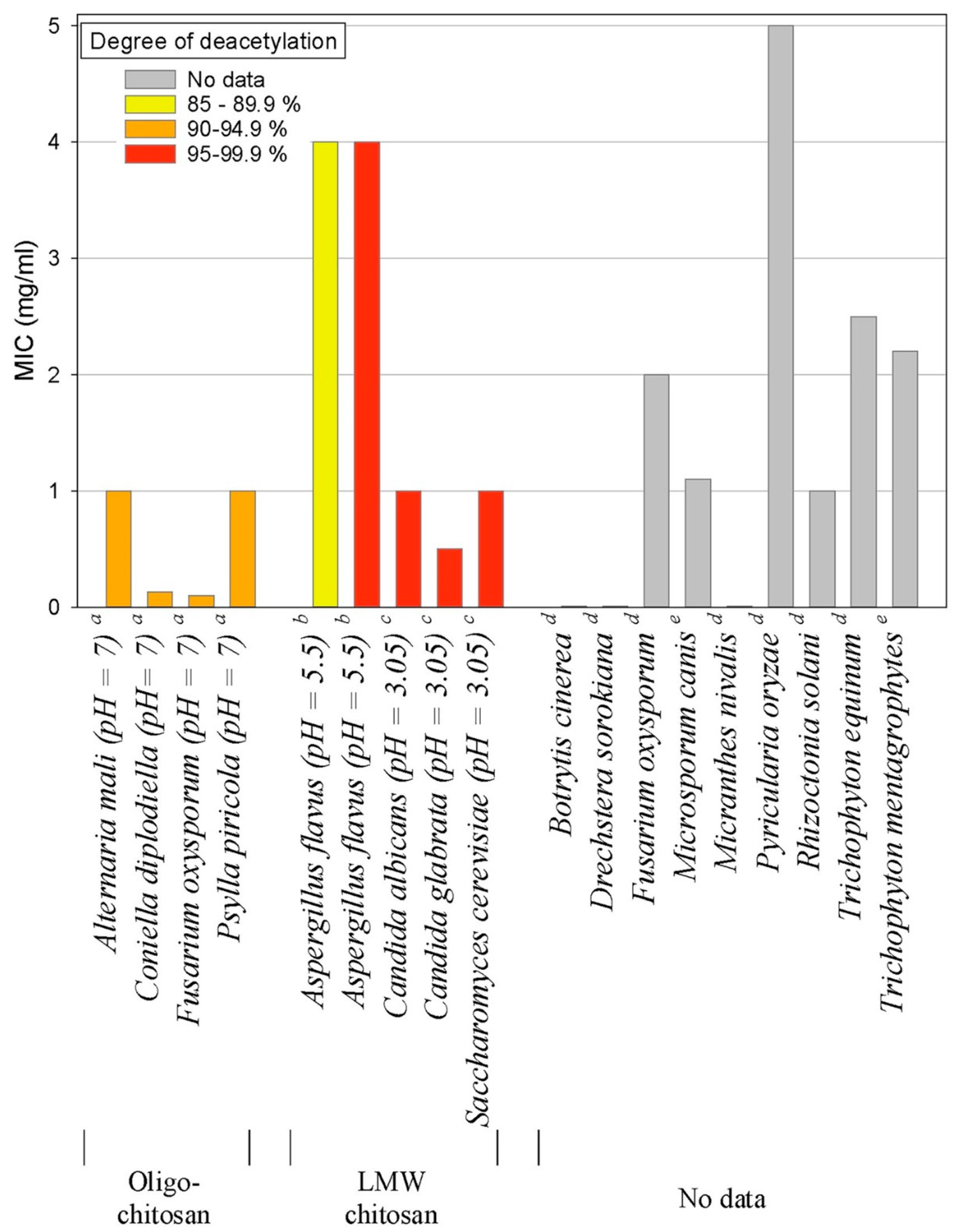
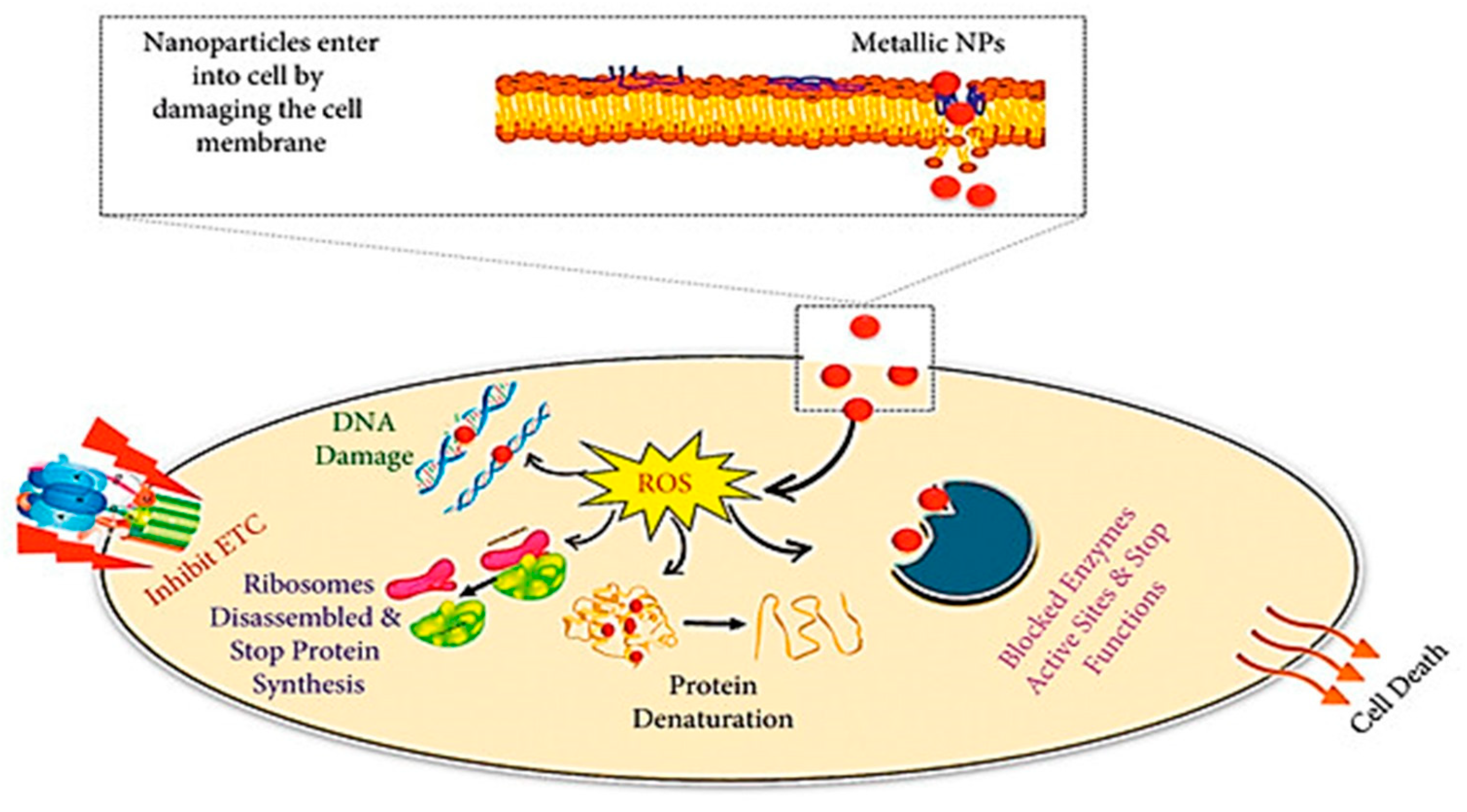
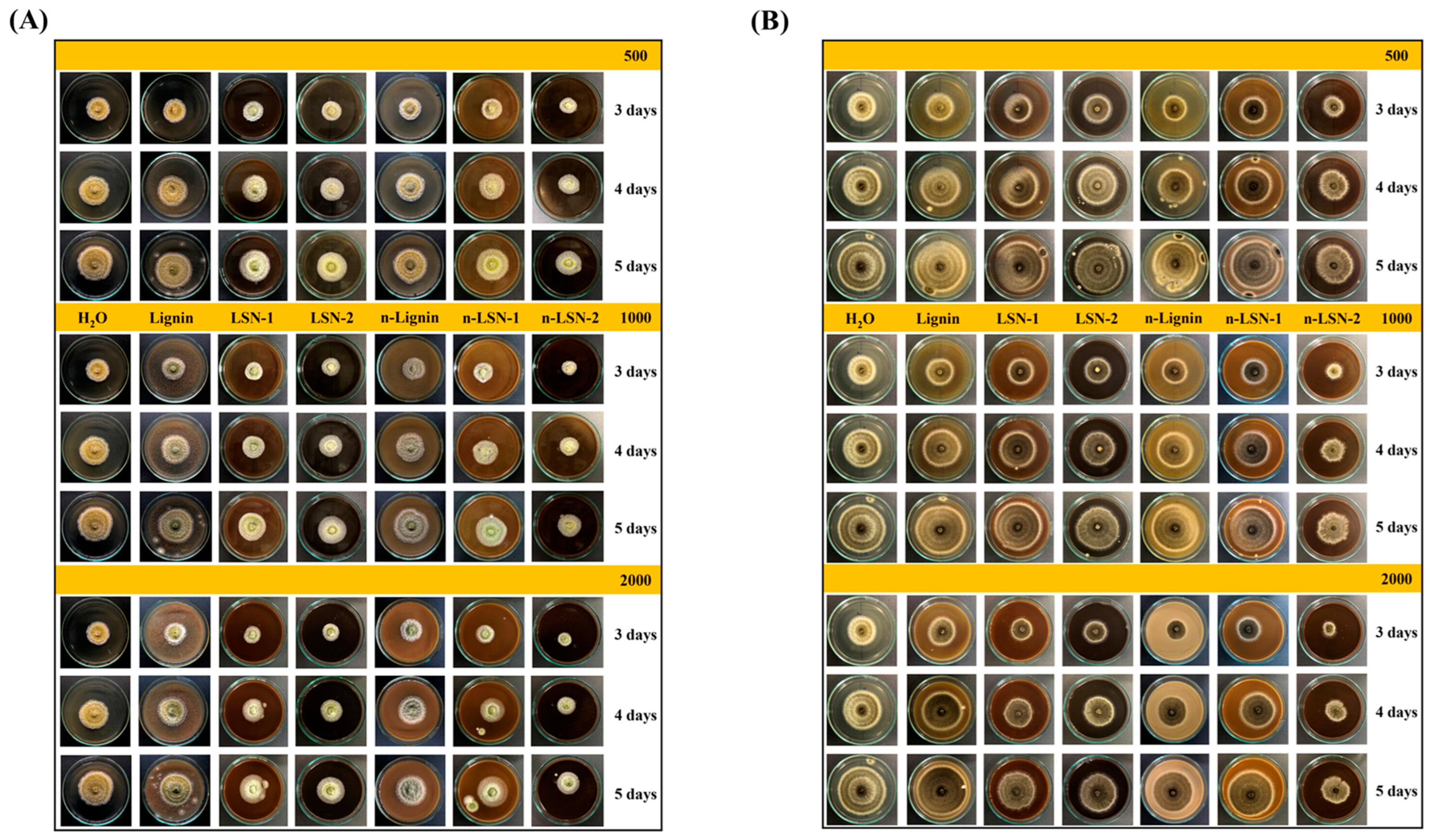
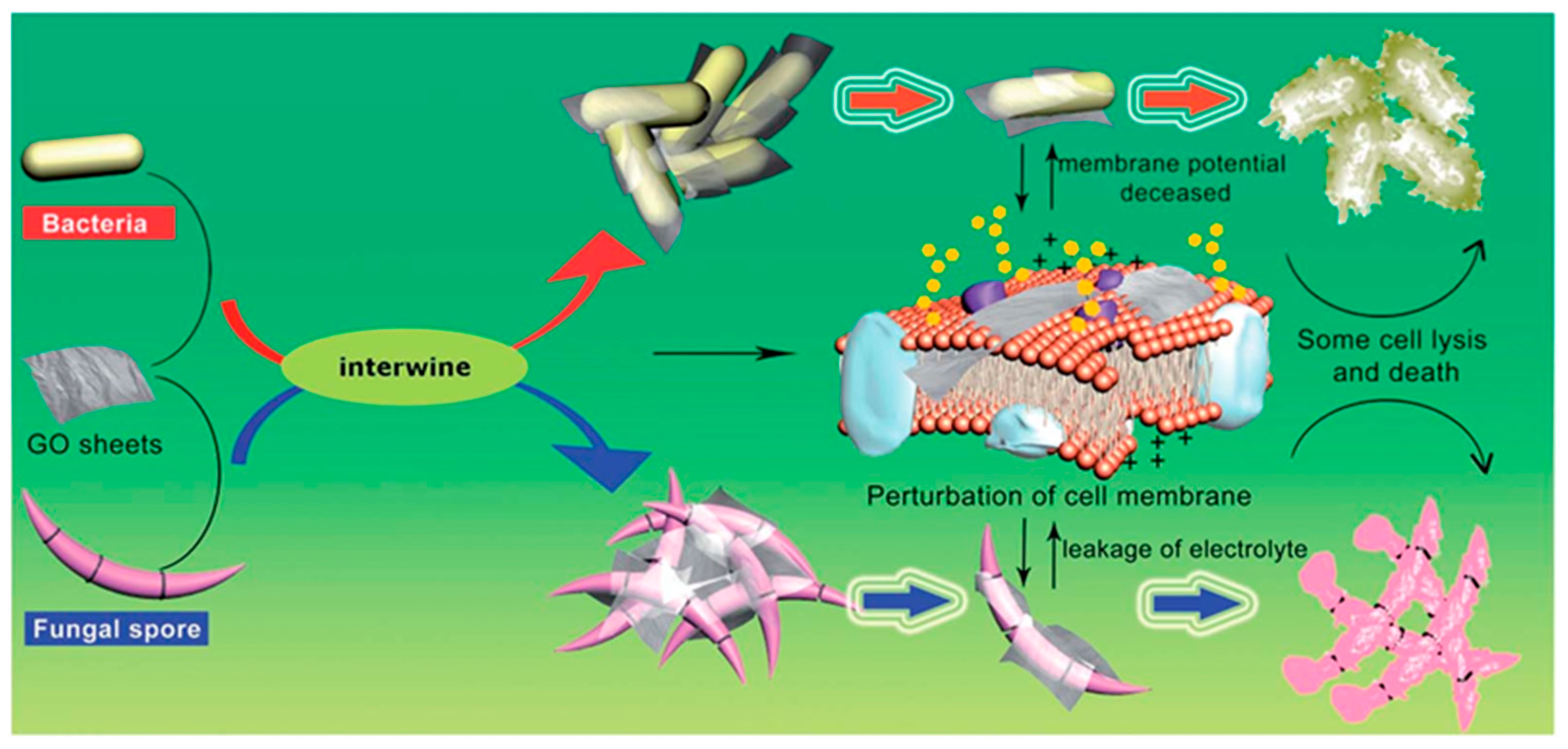

| Biomimetic Name | Major Challenges | References |
|---|---|---|
| Antifungal peptides | Susceptibility to proteolytic degradation, cytotoxicity, development of resistance, delivery to the site of infection, and cost of production | [203,204,205,206,207,208] |
| Alginate-based hydrogels | Poor mechanical strength, controlled drug release issues, potential immune response and biocompatibility issues, biofilm penetration, scalability, and cost | [209,210,211,212,213,214,215] |
| Chitosan and chitosan derivatives | Variability in antifungal efficacy, solubility limitations, unclear mechanism of action, potential cytotoxicity, and production scalability | [113,121,216,217,218,219] |
| Nanoparticles | Potential cytotoxicity, stability in biological environments issues, production scalability, and regulatory and environmental concerns | [220,221,222,223,224,225] |
| Plant-derived polyphenols | Poor bioavailability, variable in its antifungal efficacy, potential toxicity, unclear mechanisms of action, and delivery to the site of infection challenges | [226,227,228,229,230,231] |
| Graphene-based materials | Potential cytotoxicity, functionalization affecting properties and stability, environmental impact, and high production cost | [232,233,234,235,236] |
| Probiotics | Strain-specific efficacy, gastrointestinal survival, optimal dosage, unclear mechanisms of action, potential risks for immunocompromised individuals, interaction with host microbiota, and regulatory frameworks | [237,238,239,240,241,242,243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, H.O.; Oreiby, A.; Abdelhamid, M.A.A.; Ki, M.-R.; Pack, S.P. Biomimetic Antifungal Materials: Countering the Challenge of Multidrug-Resistant Fungi. Biomimetics 2024, 9, 425. https://doi.org/10.3390/biomimetics9070425
Khalifa HO, Oreiby A, Abdelhamid MAA, Ki M-R, Pack SP. Biomimetic Antifungal Materials: Countering the Challenge of Multidrug-Resistant Fungi. Biomimetics. 2024; 9(7):425. https://doi.org/10.3390/biomimetics9070425
Chicago/Turabian StyleKhalifa, Hazim O., Atef Oreiby, Mohamed A. A. Abdelhamid, Mi-Ran Ki, and Seung Pil Pack. 2024. "Biomimetic Antifungal Materials: Countering the Challenge of Multidrug-Resistant Fungi" Biomimetics 9, no. 7: 425. https://doi.org/10.3390/biomimetics9070425
APA StyleKhalifa, H. O., Oreiby, A., Abdelhamid, M. A. A., Ki, M.-R., & Pack, S. P. (2024). Biomimetic Antifungal Materials: Countering the Challenge of Multidrug-Resistant Fungi. Biomimetics, 9(7), 425. https://doi.org/10.3390/biomimetics9070425








