Adaptive Adjustments in Lower Limb Muscle Coordination during Single-Leg Landing Tasks in Latin Dancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
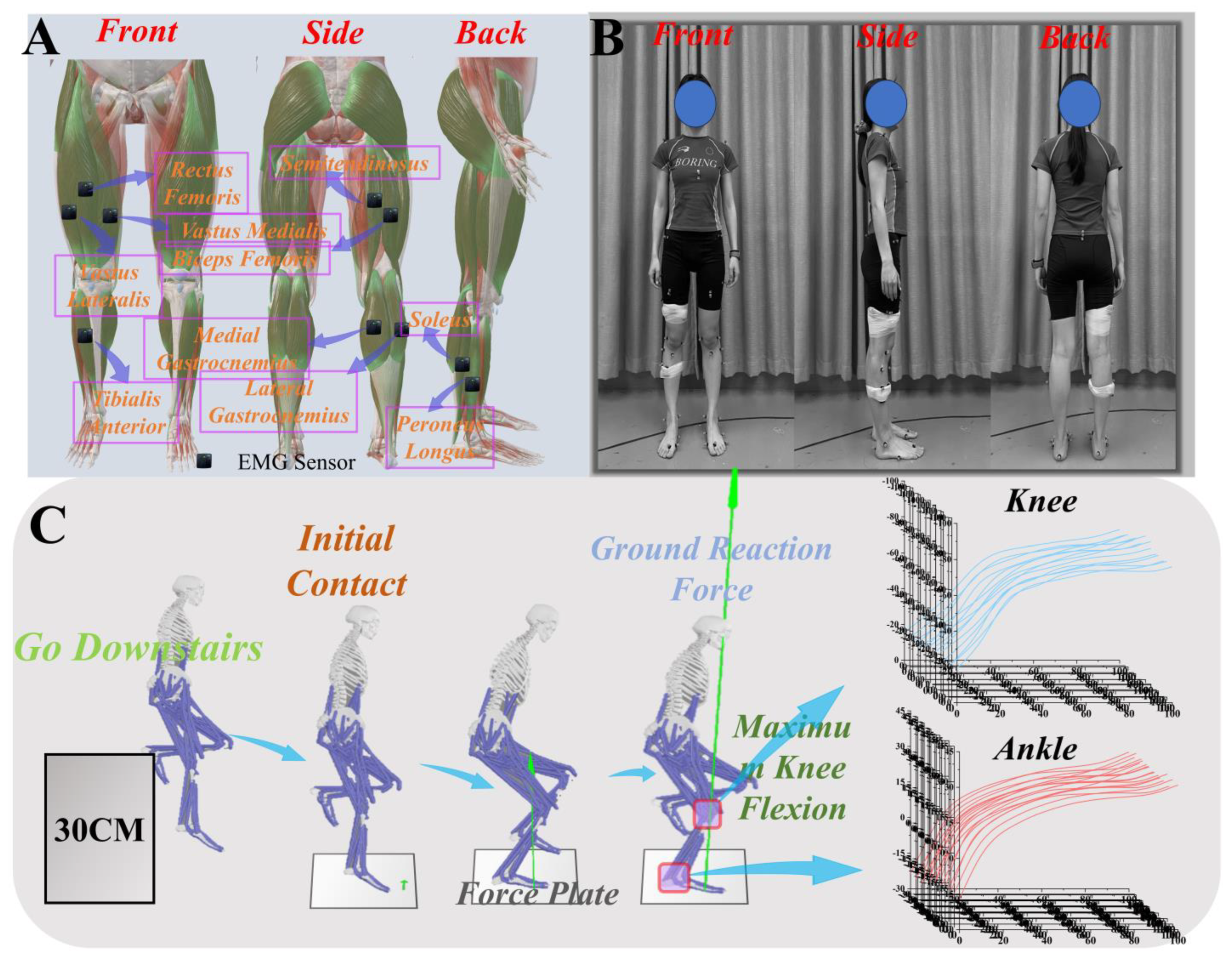
2.2. Experimental Procedure
2.3. Data Processing
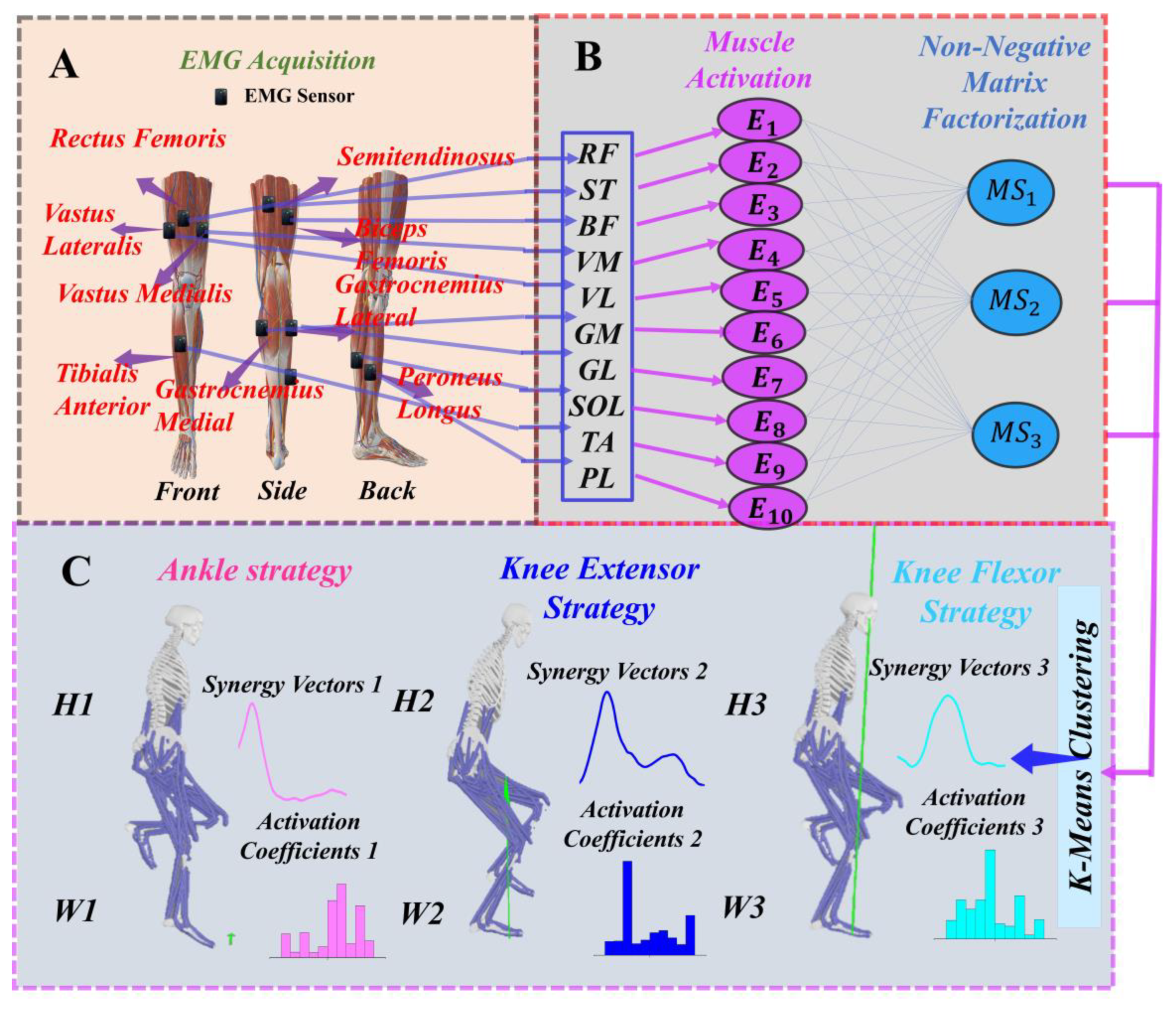
2.4. Extraction of Muscle Synergies
2.5. Statistical Analysis
3. Results
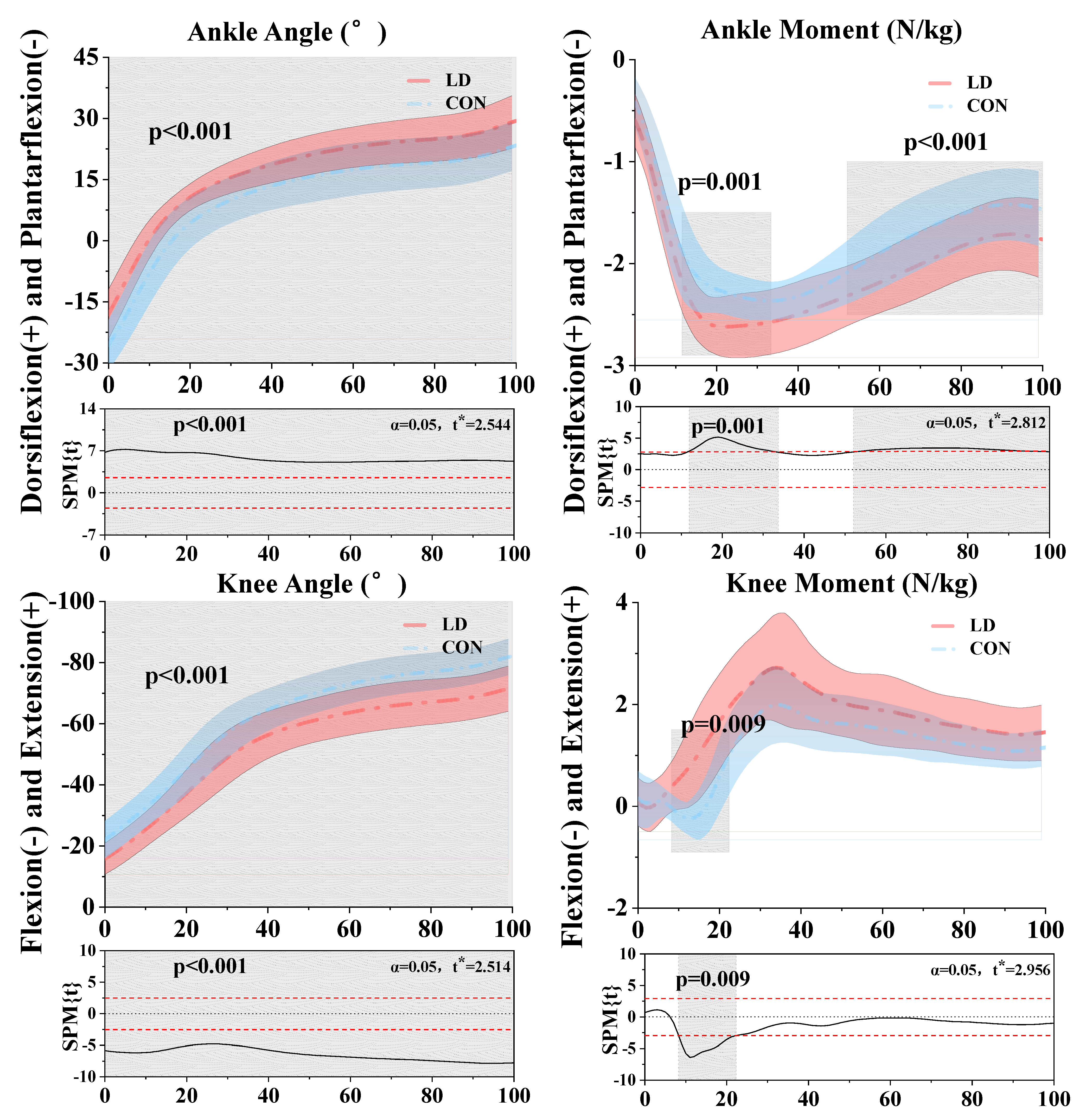
3.1. Kinematics and Kinetics
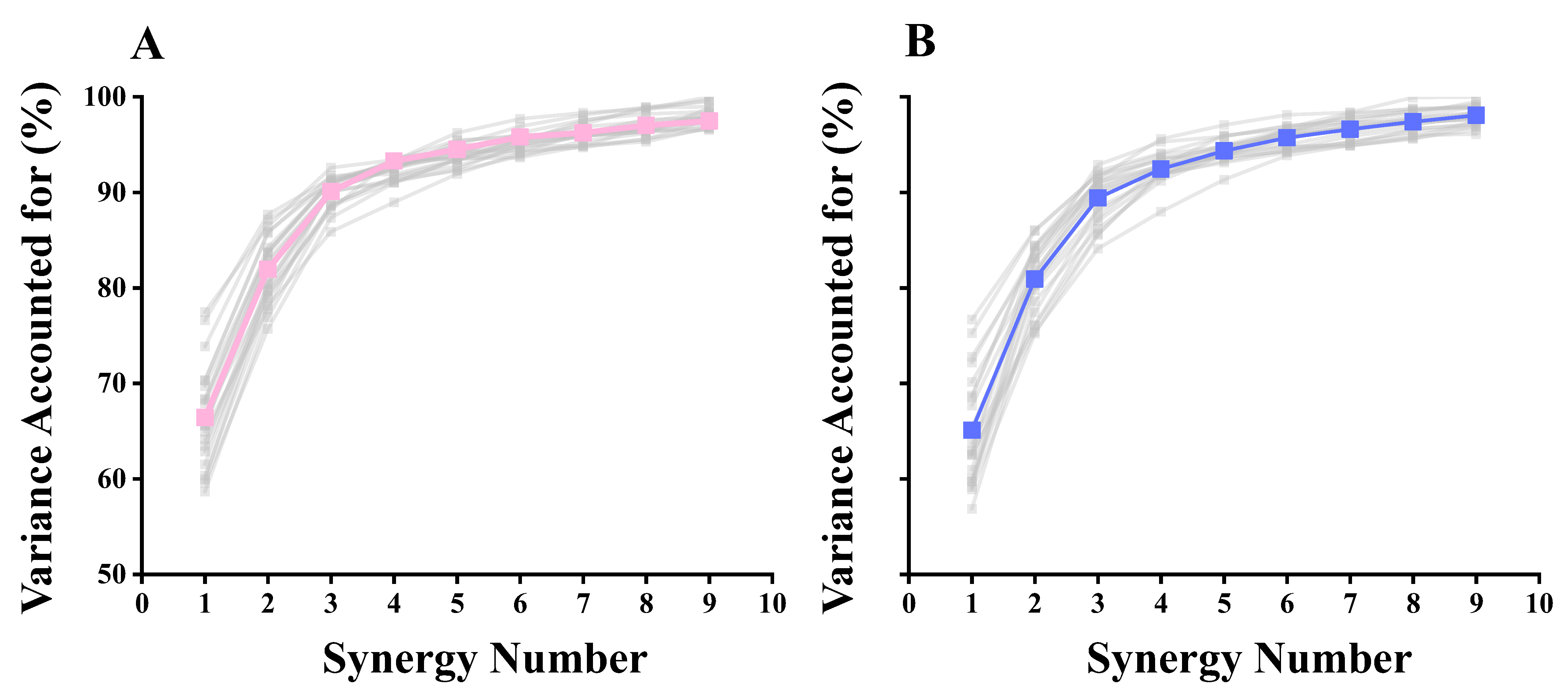
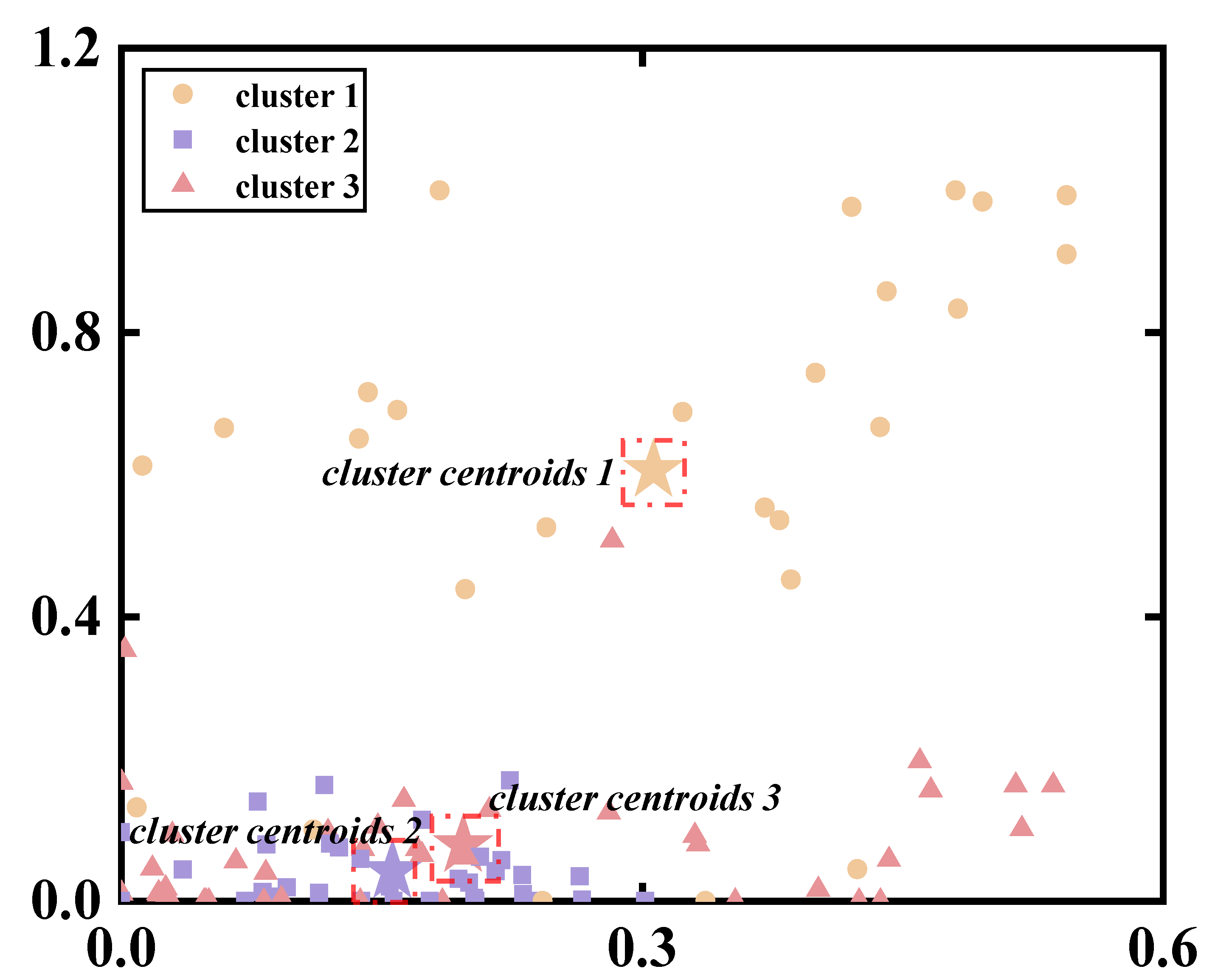
3.2. Selection of the Number of Synergies
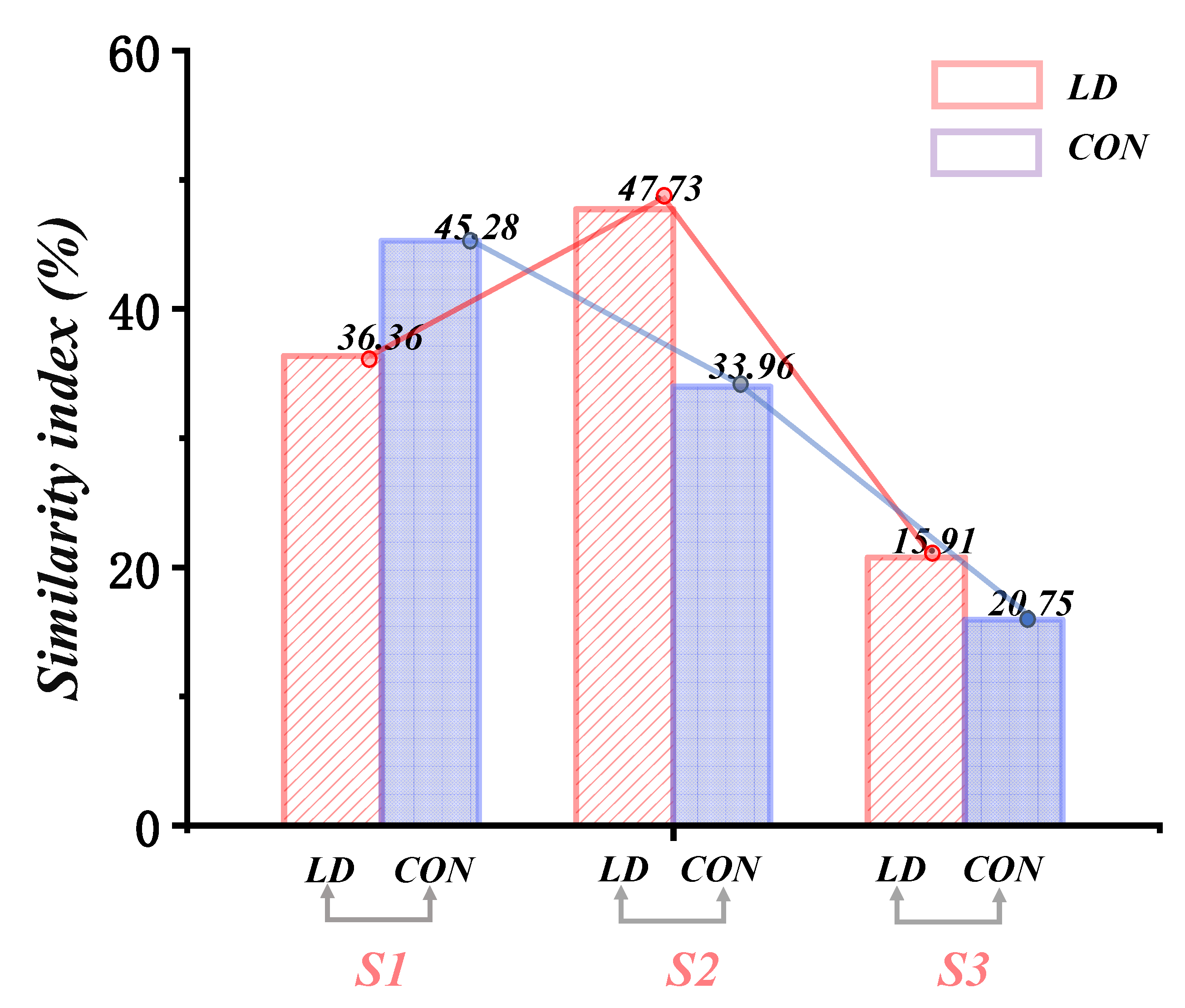
3.3. Similarity of Muscle Synergies
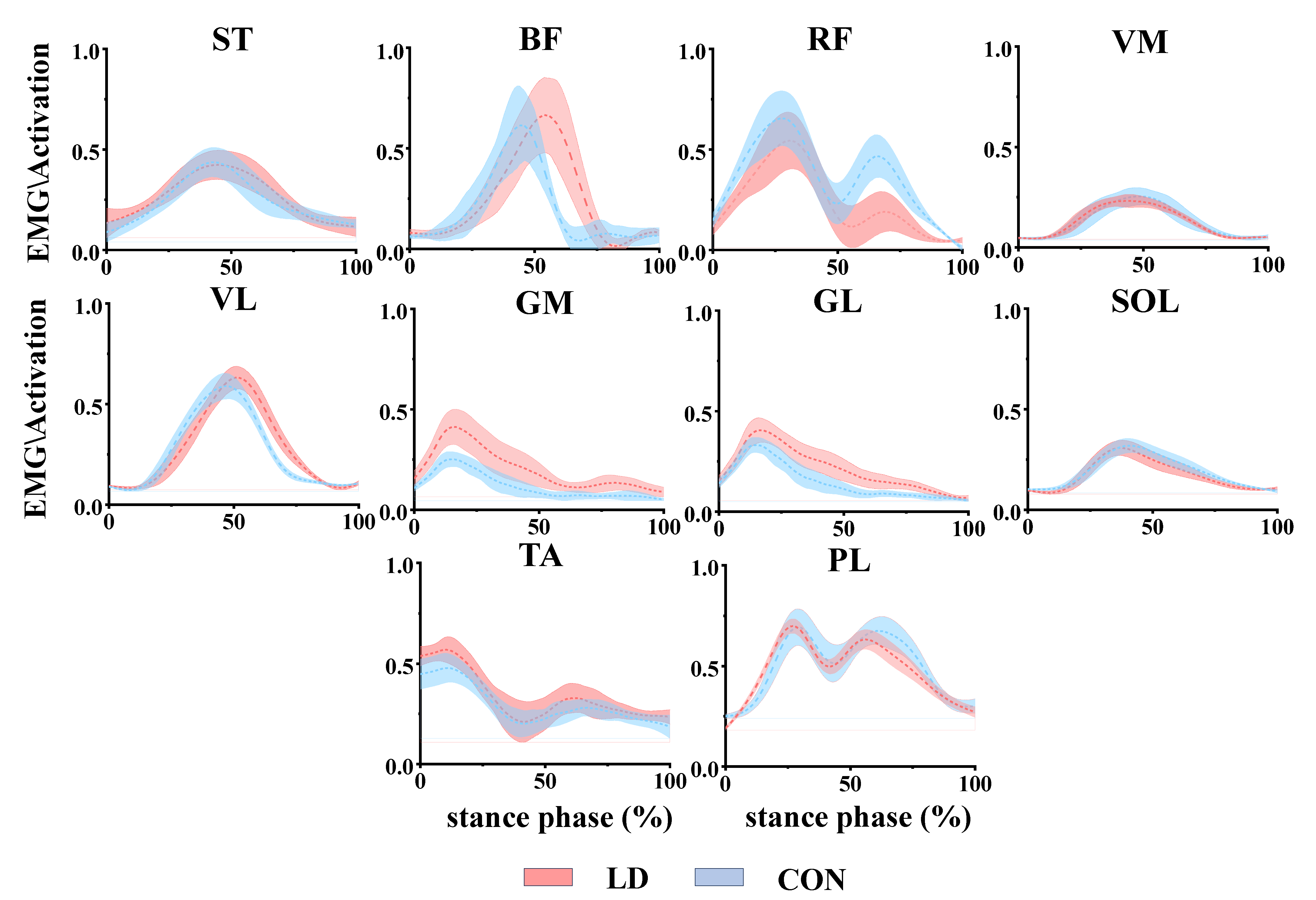
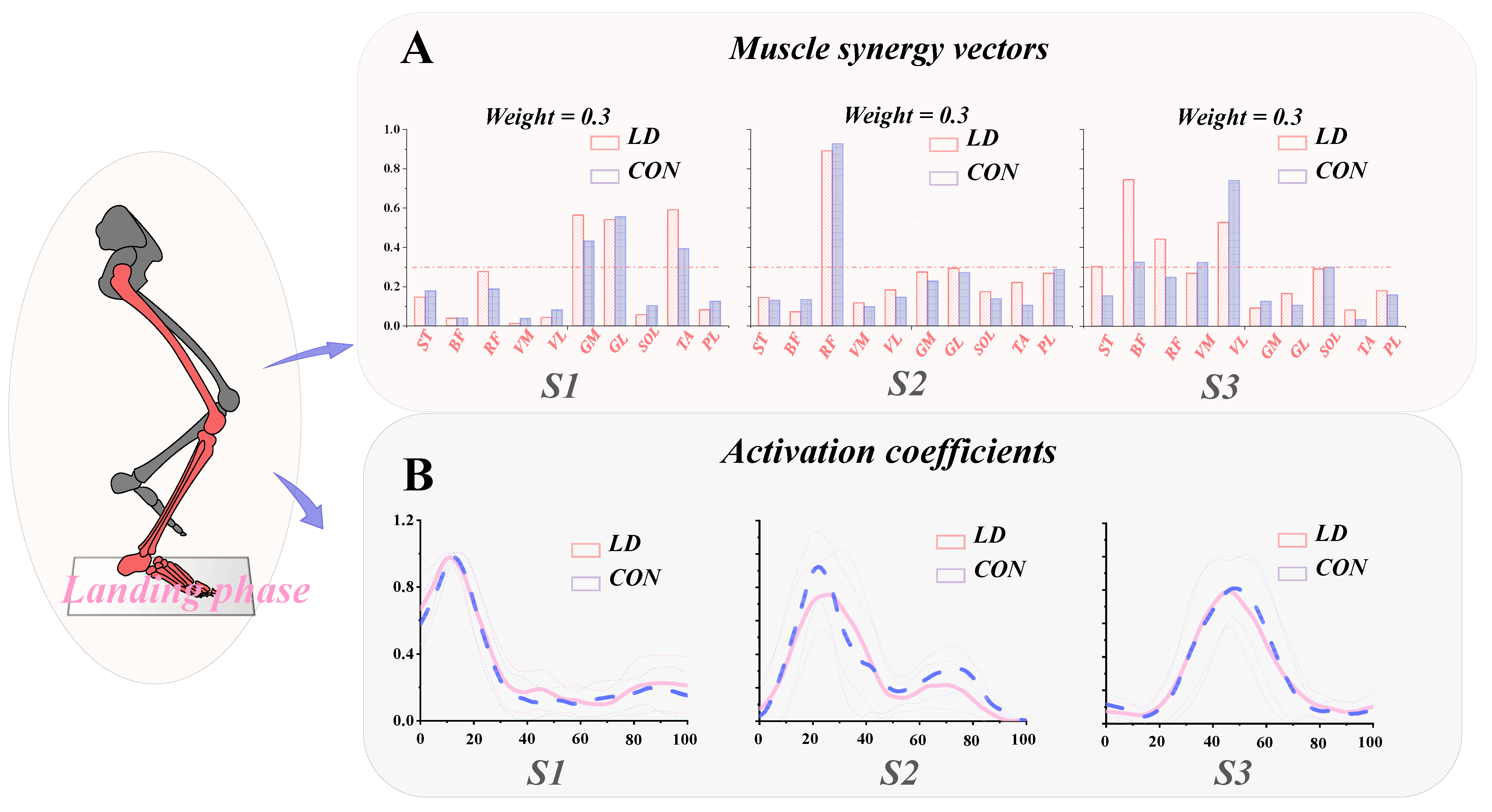
3.4. Functional Interpretation of Muscle Synergies
3.5. General Characteristics of Muscle Synergies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aquino, J.; Amasay, T.; Shapiro, S.; Kuo, Y.-T.; Ambegaonkar, J.P. Lower extremity biomechanics and muscle activity differ between ‘new’ and ‘dead’ pointe shoes in professional ballet dancers. Sports Biomech. 2021, 20, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Watanabe, K.; Asaka, T. Effect of dance on multi-muscle synergies in older adults: A cross-sectional study. BMC Geriatr. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, D.; Li, F.; Baker, J.S.; Li, J.; Gu, Y. Biomechanical Analysis of Latin Dancers’ Lower Limb during Normal Walking. Bioengineering 2023, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Hansberger, B.L.; Acocello, S.; Slater, L.V.; Hart, J.M.; Ambegaonkar, J.P. Peak lower extremity landing kinematics in dancers and nondancers. J. Athl. Train. 2018, 53, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Park, Y.S.; Park, D.W.; Shim, J.K. Dance training improves the CNS’s ability to utilize the redundant degrees of freedom of the whole body. Sci. Rep. 2020, 10, 22197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Lin, A.-C.; Chen, S.-F.; Shih, C.-J.; Kuo, T.-Y.; Wang, F.-C.; Lee, P.-H.; Lee, A.P. Superior gait performance and balance ability in Latin dancers. Front. Med. 2022, 9, 834497. [Google Scholar] [CrossRef]
- Kiliç, M.; Nalbant, S.S. The effect of latin dance on dynamic balance. Gait Posture 2022, 92, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Fotaki, A.; Triantafyllou, A.; Koulouvaris, P.; Skouras, A.Z.; Stasinopoulos, D.; Gkrilias, P.; Kyriakidou, M.; Stasi, S.; Antonakis-Karamintzas, D.; Tsolakis, C.J.S. Excessive Knee Internal Rotation during Grand Plié in Classical Ballet Female Dancers. Sports 2024, 12, 54. [Google Scholar] [CrossRef]
- Janura, M.; Procházková, M.; Svoboda, Z.; Bizovská, L.; Jandová, S.; Konečný, P. Standing balance of professional ballet dancers and non-dancers under different conditions. PLoS ONE 2019, 14, e0224145. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Oliveira, R.; Vaz, J.R.; Cortes, N. Oxford foot model kinematics in landings: A comparison between professional dancers and non-dancers. J. Sci. Med. Sport 2020, 23, 347–352. [Google Scholar] [CrossRef]
- Krityakiarana, W.; Jongkamonwiwat, N. Comparison of balance performance between Thai classical dancers and non-dancers. J. Dance Med. Sci. 2016, 20, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kovács, B.; Csala, D.; Sebestyén, Ö.; Matlák, J.; Groszmann, Á.; Tihanyi, J.; Petridis, L.J. Arm swing during vertical jumps does not increase EMG activity of the lower limb muscles. Phys. Act. Health 2023, 7, 132–142. [Google Scholar] [CrossRef]
- Kim, H.; Palmieri-Smith, R.; Kipp, K. Muscle synergies in people with chronic ankle instability during anticipated and unanticipated landing-cutting tasks. J. Athl. Train. 2023, 58, 143–152. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Grasso, R.; Zago, M.; Molinari, M.; Scivoletto, G.; Castellano, V.; Macellari, V.; Lacquaniti, F. Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J. Neurophysiol. 2003, 90, 3555–3565. [Google Scholar] [CrossRef]
- Ting, L.H.; McKay, J.L. Neuromechanics of muscle synergies for posture and movement. Curr. Opin. Neurobiol. 2007, 17, 622–628. [Google Scholar] [CrossRef] [PubMed]
- d’Avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef]
- Munoz-Martel, V.; Santuz, A.; Bohm, S.; Arampatzis, A. Proactive modulation in the spatiotemporal structure of muscle synergies minimizes reactive responses in perturbed landings. Front. Bioeng. Biotechnol. 2021, 9, 761766. [Google Scholar] [CrossRef]
- Torricelli, D.; Barroso, F.; Coscia, M.; Alessandro, C.; Lunardini, F.; Bravo Esteban, E.; d’Avella, A. Muscle synergies in clinical practice: Theoretical and practical implications. Emerg. Ther. Neurorehabil. 2016, 251–272. [Google Scholar]
- Tresch, M.C.; Saltiel, P.; Bizzi, E. The construction of movement by the spinal cord. Nat. Neurosci. 1999, 2, 162–167. [Google Scholar] [CrossRef]
- d’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Ting, L.H. Muscle synergies characterizing human postural responses. J. Neurophysiol. 2007, 98, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Hug, F. Can muscle coordination be precisely studied by surface electromyography? J. Electromyogr. Kinesiol. 2011, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.B.; Cen, X.Z. Can running technique modification benefit patellofemoral pain improvement in runners? A systematic review and meta-analysis. Int. J. Biomed. Eng. Technol. 2024, 45, 83–101. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Quan, W.; Gusztav, F.; Baker, J.S.; Gu, Y. Adaptive neuro-fuzzy inference system model driven by the non-negative matrix factorization-extracted muscle synergy patterns to estimate lower limb joint movements. Comput. Methods Programs Biomed. 2023, 242, 107848. [Google Scholar] [CrossRef]
- Jie, T.; Xu, D.; Zhang, Z.; Teo, E.-C.; Baker, J.S.; Zhou, H.; Gu, Y. Structural and Organizational Strategies of Locomotor Modules during Landing in Patients with Chronic Ankle Instability. Bioengineering 2024, 11, 518. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor patterns in human walking and running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef]
- Abt, J.P.; Sell, T.C.; Laudner, K.G.; McCrory, J.L.; Loucks, T.L.; Berga, S.L.; Lephart, S.M. Neuromuscular and biomechanical characteristics do not vary across the menstrual cycle. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ageberg, E.; Roberts, D.; Holmström, E.; Fridén, T. Balance in single-limb stance in patients with anterior cruciate ligament injury: Relation to knee laxity, proprioception, muscle strength, and subjective function. Am. J. Sports Med. 2005, 33, 1527–1537. [Google Scholar] [CrossRef]
- Sell, T.C.; Ferris, C.M.; Abt, J.P.; Tsai, Y.S.; Myers, J.B.; Fu, F.H.; Lephart, S.M. Predictors of proximal tibia anterior shear force during a vertical stop-jump. J. Orthop. Res. 2007, 25, 1589–1597. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Quan, W.; Ma, X.; Chon, T.-E.; Fernandez, J.; Gusztav, F.; Kovács, A.; Baker, J.S.; Gu, Y. New insights optimize landing strategies to reduce lower limb injury risk. Cyborg Bionic. Syst. 2024, 5, 0126. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Quan, W.; Jiang, X.; Liang, M.; Li, S.; Ugbolue, U.C.; Baker, J.S.; Gusztav, F.; Ma, X. A new method proposed for realizing human gait pattern recognition: Inspirations for the application of sports and clinical gait analysis. Gait Posture 2024, 107, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ugbolue, U.C. Biomechanical Analysis of Lower Limbs Based on Unstable Condition Sports Footwear: A Systematic Review. Phys. Act. Health 2024, 24, 93–104. [Google Scholar] [CrossRef]
- Pan, J.W.; Ho, M.Y.M.; Loh, R.B.C.; Iskandar, M.N.S.; Kong, P. Foot morphology and running gait pattern between the left and right limbs in recreational runners. Phys. Act. Health 2023, 7, 43–52. [Google Scholar] [CrossRef]
- Kang, Z.H.; Jiang, X.Y. The effect of running experience on muscle forces and knee joint reaction forces during running. Int. J. Biomed. Eng. Technol. 2024, 45, 183–197. [Google Scholar] [CrossRef]
- Molnár, C.; Pálya, Z.; Kiss, R.M.J.A.S. Static Balancing Ability and Lower Body Kinematics Examination of Hungarian Folk Dancers: A Pilot Study Investigating the “Kalocsai Mars” Dance Sequence. Appl. Sci. 2021, 11, 8789. [Google Scholar] [CrossRef]
- Dominici, N.; Ivanenko, Y.P.; Cappellini, G.; d’Avella, A.; Mondì, V.; Cicchese, M.; Fabiano, A.; Silei, T.; Di Paolo, A.; Giannini, C. Locomotor primitives in newborn babies and their development. Science 2011, 334, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, L.; Nielsen, J.F.; Felici, F.; Ivanenko, Y.P.; Farina, D. Impulses of activation but not motor modules are preserved in the locomotion of subacute stroke patients. J. Neurophysiol. 2011, 106, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. On the methodological implications of extracting muscle synergies from human locomotion. Int. J. Neural Syst. 2017, 27, 1750007. [Google Scholar] [CrossRef]
- Chvatal, S.A.; Ting, L.H. Common muscle synergies for balance and walking. Front. Comput. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef]
- Hug, F.; Turpin, N.A.; Couturier, A.; Dorel, S. Consistency of muscle synergies during pedaling across different mechanical constraints. J. Neurophysiol. 2011, 106, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.A.; Vanrenterghem, J.; Pataky, T.C. Sample size estimation for biomechanical waveforms: Current practice, recommendations and a comparison to discrete power analysis. J. Biomech. 2021, 122, 110451. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 2010, 43, 1976–1982. [Google Scholar] [CrossRef]
- Pataky, T.; Robinson, M.; Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 2015, 48, 190–192. [Google Scholar] [CrossRef]
- Ebig, M.; Lephart, S.M.; Burdett, R.C.; Miller, M.C.; Pincivero, D.M. The effect of sudden inversion stress on EMG activity of the peroneal and tibialis anterior muscles in the chronically unstable ankle. J. Orthop. Sports Phys. Ther. 1997, 26, 73–77. [Google Scholar] [CrossRef]
- Pinney, S.J.; Hansen, S.T., Jr.; Sangeorzan, B.J., Jr. The effect on ankle dorsiflexion of gastrocnemius recession. Foot Ankle Int. 2002, 23, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Feger, M.A.; Donovan, L.; Hart, J.M.; Hertel, J. Lower extremity muscle activation in patients with or without chronic ankle instability during walking. J. Athl. Train. 2015, 50, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Feger, M.A.; Donovan, L.; Hart, J.M.; Hertel, J. Lower extremity muscle activation during functional exercises in patients with and without chronic ankle instability. PMR 2014, 6, 602–611. [Google Scholar] [CrossRef]
- Caulfield, B.; Crammond, T.; O’Sullivan, A.; Reynolds, S.; Ward, T. Altered ankle-muscle activation during jump landing in participants with functional instability of the ankle joint. J. Sport. Rehabil. 2004, 13, 189–200. [Google Scholar] [CrossRef]
- Wright, I.; Neptune, R.; van den Bogert, A.J.; Nigg, B. The influence of foot positioning on ankle sprains. J. Biomech. 2000, 33, 513–519. [Google Scholar] [CrossRef]
- Matsunaga, N.; Imai, A.; Kaneoka, K. Comparison of muscle synergies before and after 10 minutes of running. J. Phys. Ther. Sci. 2017, 29, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.M.; Watt, D. Observations on the control of stepping and hopping movements in man. J. Physiol. 1971, 219, 709–727. [Google Scholar] [PubMed]
- Neptune, R.R.; Wright, I.C.; Van Den Bogert, A.J. Muscle coordination and function during cutting movements. Med. Sci. Sports Exerc. 1999, 31, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kawada, M.; Takeshita, Y.; Miyazaki, T.; Nakai, Y.; Hata, K.; Nakatsuji, S.; Kiyama, R. Contribution of hip and knee muscles to lateral knee stability during gait. J. Phys. Ther. Sci. 2020, 32, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Fernandez, J. Alterations in Lower Limb Biomechanical Characteristics during the Cutting Manoeuvre in Chronic Ankle Instability Population and Copers. Phys. Act. Health 2024, 8, 148–156. [Google Scholar] [CrossRef]
- Ortiz, A.; Olson, S.L.; Etnyre, B.; Trudelle-Jackson, E.E.; Bartlett, W.; Venegas-Rios, H.L. Fatigue effects on knee joint stability during two jump tasks in women. J. Strength. Cond. Res. 2010, 24, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Quan, W.; Zhou, H.; Sun, D.; Baker, J.S.; Gu, Y. Explaining the differences of gait patterns between high and low-mileage runners with machine learning. Sci. Rep. 2022, 12, 2981. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, H.; Quan, W.; Gusztav, F.; Wang, M.; Baker, J.S.; Gu, Y. Accurately and effectively predict the ACL force: Utilizing biomechanical landing pattern before and after-fatigue. Comput. Methods Programs Biomed. 2023, 241, 107761. [Google Scholar] [CrossRef]
- Xu, D.; Lu, J.; Baker, J.S.; Fekete, G.; Gu, Y. Temporal kinematic and kinetics differences throughout different landing ways following volleyball spike shots. P. I Mech. Eng. P-J. SPO 2022, 236, 200–208. [Google Scholar] [CrossRef]
- Shultz, S.J.; Carcia, C.R.; Perrin, D.H. Knee joint laxity affects muscle activation patterns in the healthy knee. J. Electromyogr. Kinesiol. 2004, 14, 475–483. [Google Scholar] [CrossRef]
- Andrish, J. The biomechanics of patellofemoral stability. J. Knee Surg. 2004, 17, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, T.L.; Rudolph, K.S.; Snyder-Mackler, L. Development of dynamic knee stability after acute ACL injury. J. Electromyogr. Kinesiol. 2002, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lephart, S.M.; Ferris, C.M.; Riemann, B.L.; Myers, J.B.; Fu, F.H. Gender differences in strength and lower extremity kinematics during landing. Clin. Orthop. Relat. Res. 2002, 401, 162–169. [Google Scholar] [CrossRef]
| LD Mean (SD) | CON Mean (SD) | p-Value | |
|---|---|---|---|
| 3.43 (0.51) | 3.31 (0.57) | 0.506 | |
| Global VAF (%) | |||
| With two synergies | 81.03 (3.45) | 81.94 (3.47) | 0.393 |
| With three synergies | 93.61 (2.57) | 90.41 (2.56) | 0.303 |
| With four synergies | 92.55 (1.62) | 92.31 (1.12) | 0.581 |
| Local VAFs with three synergies (%) | |||
| ST | 88.91 (5.42) | 90.41 (5.97) | 0.394 |
| BF | 90.50 (4.85) | 91.41 (4.57) | 0.527 |
| RF | 94.33 (4.32) | 96.36 (2.10) | 0.056 |
| VM | 89.93 (5.19) | 89.72 (4.49) | 0.886 |
| VL | 92.66 (3.53) | 94.45 (3.25) | 0.092 |
| GM | 91.56 (3.46) | 92.20 (2.58) | 0.496 |
| GL | 91.47 (3.28) | 93.02 (1.53) | 0.059 |
| SOL | 92.51 (3.78) | 92.46 (3.14) | 0.964 |
| TA | 91.48 (2.65) | 89.71 (3.41) | 0.065 |
| PL | 92.93 (2.44) | 91.32 (4.08) | 0.122 |
| Synergy Vectors | Muscle Synergy 1 | Muscle Synergy 2 | Muscle Synergy 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | LD | CON | p-Value | LD | CON | p-Value | LD | CON | p-Value |
| ST | 0.15 (0.08) | 0.18 (0.11) | 0.288 | 0.14 (0.12) | 0.14 (0.18) | 0.848 | 0.30 (0.18) | 0.15 (0.17) | 0.046 * |
| BF | 0.04 (0.06) | 0.04 (0.06) | 0.946 | 0.07 (0.13) | 0.12 (0.13) | 0.31 | 0.75 (0.20) | 0.32 (0.21) | <0.001 * |
| RF | 0.28 (0.29) | 0.19 (0.24) | 0.291 | 0.84 (0.28) | 0.91 (0.15) | 0.283 | 0.44 (0.31) | 0.25 (0.16) | 0.076 |
| VM | 0.01 (0.02) | 0.04 (0.04) | 0.010 * | 0.12 (0.11) | 0.09 (0.08) | 0.329 | 0.27 (0.12) | 0.32 (0.09) | 0.204 |
| VL | 0.04 (0.04) | 0.08 (0.16) | 0.282 | 0.19 (0.23) | 0.13 (0.14) | 0.372 | 0.53 (0.27) | 0.74 (0.20) | 0.039 * |
| GM | 0.56 (0.20) | 0.43 (0.16) | 0.024 * | 0.23 (0.23) | 0.26 (0.17) | 0.643 | 0.09 (0.08) | 0.13 (0.07) | 0.307 |
| GL | 0.54 (0.15) | 0.56 (0.18) | 0.771 | 0.26 (0.17) | 0.29 (0.17) | 0.549 | 0.17 (0.10) | 0.11 (0.08) | 0.119 |
| SOL | 0.06 (0.04) | 0.10 (0.06) | 0.002 * | 0.17 (0.11) | 0.13 (0.08) | 0.211 | 0.29 (0.10) | 0.30 (0.05) | 0.797 |
| TA | 0.59 (0.19) | 0.39 (0.12) | <0.001 * | 0.20 (0.15) | 0.13 (0.16) | 0.112 | 0.08 (0.08) | 0.03 (0.03) | 0.093 |
| PL | 0.08 (0.11) | 0.13 (0.15) | 0.298 | 0.25 (0.16) | 0.29 (0.18) | 0.476 | 0.18 (0.15) | 0.16 (0.08) | 0.683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Jie, T.; Xu, D.; Gál, J.; Fekete, G.; Liang, M.; Gu, Y. Adaptive Adjustments in Lower Limb Muscle Coordination during Single-Leg Landing Tasks in Latin Dancers. Biomimetics 2024, 9, 489. https://doi.org/10.3390/biomimetics9080489
Gao X, Jie T, Xu D, Gál J, Fekete G, Liang M, Gu Y. Adaptive Adjustments in Lower Limb Muscle Coordination during Single-Leg Landing Tasks in Latin Dancers. Biomimetics. 2024; 9(8):489. https://doi.org/10.3390/biomimetics9080489
Chicago/Turabian StyleGao, Xiangli, Tianle Jie, Datao Xu, János Gál, Gusztáv Fekete, Minjun Liang, and Yaodong Gu. 2024. "Adaptive Adjustments in Lower Limb Muscle Coordination during Single-Leg Landing Tasks in Latin Dancers" Biomimetics 9, no. 8: 489. https://doi.org/10.3390/biomimetics9080489









