Ablative Management of Persistent Atrial Fibrillation (PeAF) with Posterior Wall Isolation (PWI): Where Do We Stand?
Abstract
:1. Introduction
2. Posterior Wall Isolation in Persistent Atrial Fibrillation
3. The Role of Surgical Ablation
4. The Advent of Hybrid Endocardial/Epicardial Ablation
5. Our Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N. Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Cha, T.J.; Zhang, L.; Chartier, D.; Melnyk, P.; Hohnloser, S.H.; Nattel, S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: Action potential and ionic current properties. J. Physiol. 2003, 551 Pt. 3, 801–813. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salih, M.; Darrat, Y.; Ibrahim, A.M.; Al-Akchar, M.; Bhattarai, M.; Do, C.K.; Ayan, M.; Labedi, M.; Elayi, C.S. Clinical outcomes of adjunctive posterior wall isolation in persistent atrial fibrillation: A meta-analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 1394–1402. [Google Scholar] [CrossRef]
- Ahn, J.; Shin, D.G.; Han, S.J.; Lim, H.E. Does isolation of the left atrial posterior wall using cryoballoon ablation improve clinical outcomes in patients with persistent atrial fibrillation? A prospective randomized controlled trial. Europace 2022, 24, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Cutler, M.J.; Johnson, J.; Abozguia, K.; Rowan, S.; Lewis, W.; Costantini, O.; Natale, A.; Ziv, O. Impact of voltage mapping to guide whether to perform ablation of the posterior wall in patients with persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2016, 27, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Di Biase, L.; Mohanty, P.; Trivedi, C.; Russo, A.D.; Themistoclakis, S.; Casella, M.; Santarelli, P.; Fassini, G.; Santangeli, P.; et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm 2016, 13, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Shin, S.Y.; Na, J.O.; Choi, C.U.; Kim, S.H.; Kim, E.J.; Rha, S.-W.; Park, C.G.; Seo, H.S.; Oh, D.J.; et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation? A prospective randomized clinical trial. Int. J. Cardiol. 2015, 181, 277–283. [Google Scholar] [CrossRef]
- O’Neill, L.; Hensey, M.; Nolan, W.; Keane, D. Clinical outcome when left atrial posterior wall box isolation is included as a catheter ablation strategy in patients with persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2015, 44, 63–70. [Google Scholar] [CrossRef]
- Kanitsoraphan, C.; Rattanawong, P.; Techorueangwiwat, C.; Kewcharoen, J.; Mekritthikrai, R.; Prasitlumkum, N.; Shah, P.; El Masry, H. The efficacy of posterior wall isolation in atrial fibrillation ablation: A systematic review and meta-analysis of randomized controlled trials. J. Arrhythm. 2022, 38, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liao, J.; Ling, Z.; Meyer, C.; Sommer, P.; Futyma, P.; Martinek, M.; Schratter, A.; Acou, W.-J.; Wang, J.; et al. Adjunctive Left Atrial Posterior Wall Isolation in Treating Atrial Fibrillation: Insight from a Large Secondary Analysis. JACC Clin. Electrophysiol. 2022, 8, 605–618. [Google Scholar] [CrossRef] [PubMed]
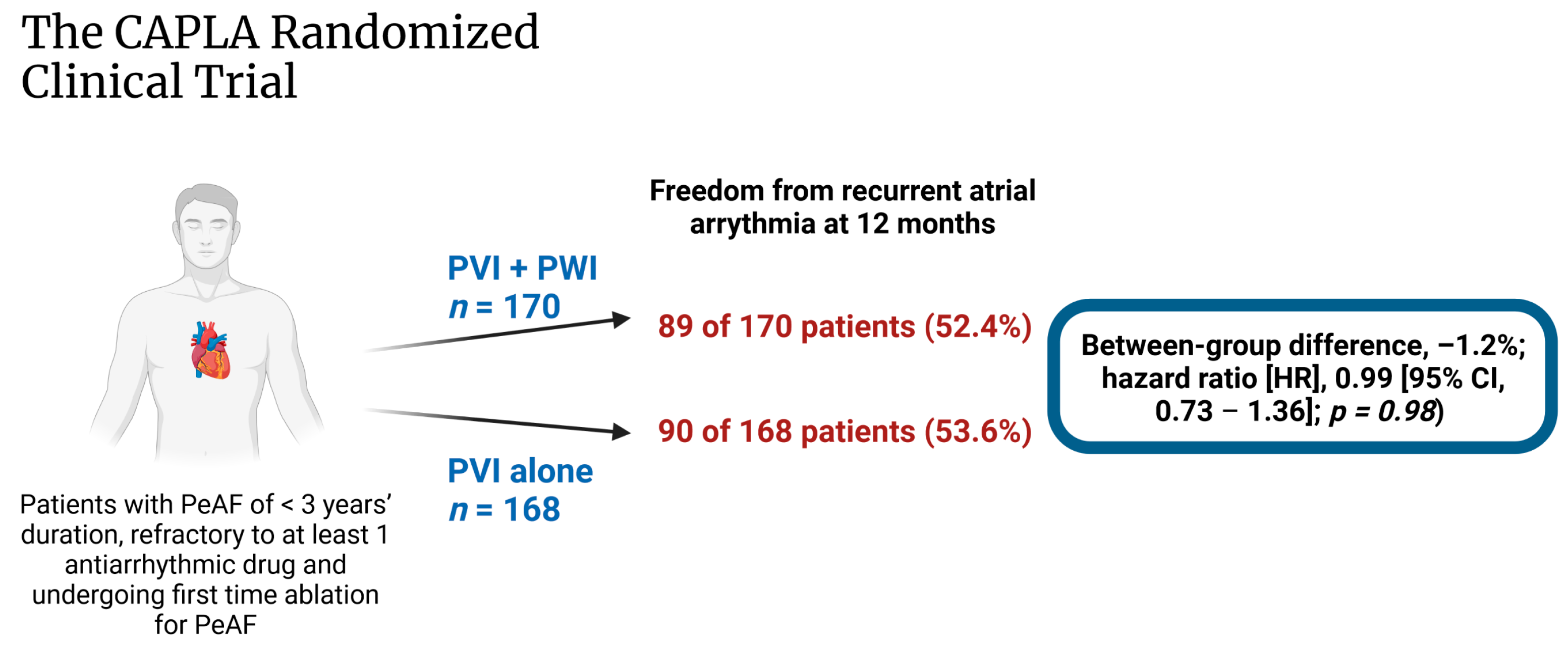
- Kistler, P.M.; Chieng, D.; Sugumar, H.; Ling, L.-H.; Segan, L.; Azzopardi, S.; Al-Kaisey, A.; Parameswaran, R.; Anderson, R.D.; Hawson, J.; et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: The CAPLA randomized clinical trial. JAMA 2023, 329, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.-Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008, 37, 1148–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherr, D.; Khairy, P.; Miyazaki, S.; Aurillac-Lavignolle, V.; Pascale, P.; Wilton, S.B.; Ramoul, K.; Komatsu, Y.; Roten, L.; Jadidi, A.; et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ. Arrhythm. Electrophysiol. 2015, 8, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilz, R.R.; Rillig, A.; Thum, A.-M.; Arya, A.; Wohlmuth, P.; Metzner, A.; Mathew, S.; Yoshiga, Y.; Wissner, E.; Kuck, K.-H.; et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J. Am. Coll. Cardiol. 2012, 60, 1921–1929. [Google Scholar] [CrossRef] [Green Version]
- Damiano, R.J., Jr.; Bailey, M. The Cox-Maze IV procedure for lone atrial fibrillation. Multimed. Man. Cardiothorac. Surg. 2007, 2007, mmcts.2007.002758. [Google Scholar] [CrossRef]
- Cox, J.L.; Ad, N. New surgical and catheter-based modifications of the Maze procedure. Semin. Thorac. Cardiovasc. Surg. 2000, 12, 68–73. [Google Scholar] [CrossRef]
- Yonas, E.; Pranata, R.; Siswanto, B.B.; Abdulgani, H.B. Comparison between surgical and catheter based ablation in atrial fibrillation, should surgical based ablation be implemented as first line? A meta-analysis of studies. Indian Pacing Electrophysiol. J. 2020, 20, 14–20. [Google Scholar] [CrossRef]
- Rattanawong, P.; Kanitsoraphan, C.; Kewcharoen, J.; Sriramoju, A.; Shanbhag, A.; Ko, N.L.K.; Barry, T.; Vutthikraivit, W.; Shen, W. Surgical versus catheter ablation in atrial fibrillation: A systematic review and meta-analysis of randomized controlled trials. J. Cardiovasc. Electrophysiol. 2022, 33, 2152–2163. [Google Scholar] [CrossRef]
- Berger, W.R.; Meulendijks, E.R.; Limpens, J.; Berg, N.W.V.D.; Neefs, J.; Driessen, A.H.; Krul, S.P.; van Boven, W.J.P.; de Groot, J.R. Persistent atrial fibrillation: A systematic review and meta-analysis of invasive strategies. Int. J. Cardiol. 2019, 278, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldar, S.; Khan, H.R.; Boyalla, V.; Kralj-Hans, I.; Jones, S.; Lord, J.; Onyimadu, O.; Satishkumar, A.; Bahrami, T.; De Souza, A.; et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. Eur. Heart J. 2020, 41, 4471–4480. [Google Scholar] [CrossRef] [PubMed]
- Adiyaman, A.; Buist, T.J.; Beukema, R.J.; Smit, J.J.J.; Delnoy, P.P.H.; Hemels, M.E.; Sie, H.T.; Misier, A.R.R.; Elvan, A. Randomized Controlled Trial of Surgical Versus Catheter Ablation for Paroxysmal and Early Persistent Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006182. [Google Scholar] [CrossRef]
- Nault, I.; Miyazaki, S.; Forclaz, A.; Wright, M.; Jadidi, A.; Jaïs, P.; Hocini, M.; Haïssaguerre, M. Drugs vs. ablation for the treatment of atrial fibrillation: The evidence supporting catheter ablation. Eur. Heart J. 2010, 31, 1046–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronis, K.N.; Trayanova, N.A. Endocardial-Epicardial Dissociation in Persistent Atrial Fibrillation: Driver or Bystander Activation Pattern? Circ. Arrhythm. Electrophysiol. 2020, 13, e009110. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, R.; Kalman, J.M.; Royse, A.; Goldblatt, J.; Larobina, M.; Watts, T.; Walters, T.E.; Nalliah, C.J.; Wong, G.; Al-Kaisey, A.; et al. Endocardial-Epicardial Phase Mapping of Prolonged Persistent Atrial Fibrillation Recordings: High Prevalence of Dissociated Activation Patterns. Circ. Arrhythm. Electrophysiol. 2020, 13, e008512. [Google Scholar] [CrossRef]
- Delurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ. Arrhythm. Electrophysiol. 2020, 13, e009288. [Google Scholar] [CrossRef]
- Van der Heijden, C.A.; Weberndörfer, V.; Vroomen, M.; Luermans, J.G.; Chaldoupi, S.-M.; Bidar, E.; Vernooy, K.; Maessen, J.G.; Pison, L.; van Kuijk, S.M.; et al. Hybrid Ablation Versus Repeated Catheter Ablation in Persistent Atrial Fibrillation: A Randomized Controlled Trial. JACC Clin. Electrophysiol 2023. published online ahead of print. [Google Scholar] [CrossRef]
- Shrestha, S.; Plasseraud, K.M.; Makati, K.; Sood, N.; Killu, A.M.; Contractor, T.; Ahsan, S.; De Lurgio, D.B.; Shults, C.C.; Eldadah, Z.A.; et al. Hybrid Convergent ablation for atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm O2 2022, 3, 396–404. [Google Scholar] [CrossRef]
- Huo, Y.; Gaspar, T.; Schönbauer, R.; Wójcik, M.; Fiedler, L.; Roithinger, F.X.; Martinek, M.; Pürerfellner, H.; Kirstein, B.; Richter, U.; et al. Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evid. 2022, 1. [Google Scholar] [CrossRef]
- Lin, W.-S.; Tai, C.-T.; Hsieh, M.-H.; Tsai, C.-F.; Lin, Y.-K.; Tsao, H.-M.; Huang, J.-L.; Yu, W.-C.; Yang, S.-P.; Ding, Y.-A.; et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003, 107, 3176–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markides, V.; Schilling, R.J.; Ho, S.Y.; Chow, A.W.; Davies, D.W.; Peters, N.S. Characterization of left atrial activation in the intact human heart. Circulation 2003, 107, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Thomson, K.C.; Stevenson, I.H.; Kistler, P.M.; Haqqani, H.M.; Goldblatt, J.C.; Sanders, P.; Kalman, J.M. Anatomically determined functional conduction delay in the posterior left atrium relationship to structural heart disease. J. Am. Coll. Cardiol. 2008, 51, 856–862. [Google Scholar] [CrossRef]
- Sugumar, H.; Thomas, S.P.; Prabhu, S.; Voskoboinik, A.; Kistler, P.M. How to perform posterior wall isolation in catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 345–352. [Google Scholar] [CrossRef]
- Pambrun, T.; Duchateau, J.; Delgove, A.; Denis, A.; Constantin, M.; Ramirez, F.D.; Chauvel, R.; Tixier, R.; Welte, N.; André, C.; et al. Epicardial course of the septopulmonary bundle: Anatomical considerations and clinical implications for roof line completion. Heart Rhythm 2021, 18, 349–357. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baqal, O.; El Masry, H.Z. Ablative Management of Persistent Atrial Fibrillation (PeAF) with Posterior Wall Isolation (PWI): Where Do We Stand? J. Cardiovasc. Dev. Dis. 2023, 10, 273. https://doi.org/10.3390/jcdd10070273
Baqal O, El Masry HZ. Ablative Management of Persistent Atrial Fibrillation (PeAF) with Posterior Wall Isolation (PWI): Where Do We Stand? Journal of Cardiovascular Development and Disease. 2023; 10(7):273. https://doi.org/10.3390/jcdd10070273
Chicago/Turabian StyleBaqal, Omar, and Hicham Z. El Masry. 2023. "Ablative Management of Persistent Atrial Fibrillation (PeAF) with Posterior Wall Isolation (PWI): Where Do We Stand?" Journal of Cardiovascular Development and Disease 10, no. 7: 273. https://doi.org/10.3390/jcdd10070273
APA StyleBaqal, O., & El Masry, H. Z. (2023). Ablative Management of Persistent Atrial Fibrillation (PeAF) with Posterior Wall Isolation (PWI): Where Do We Stand? Journal of Cardiovascular Development and Disease, 10(7), 273. https://doi.org/10.3390/jcdd10070273





