Quantitative and Compositional MRI of the Articular Cartilage: A Narrative Review
Abstract
1. Introduction
2. MRI of the Articular Cartilage
2.1. Conventional MRI
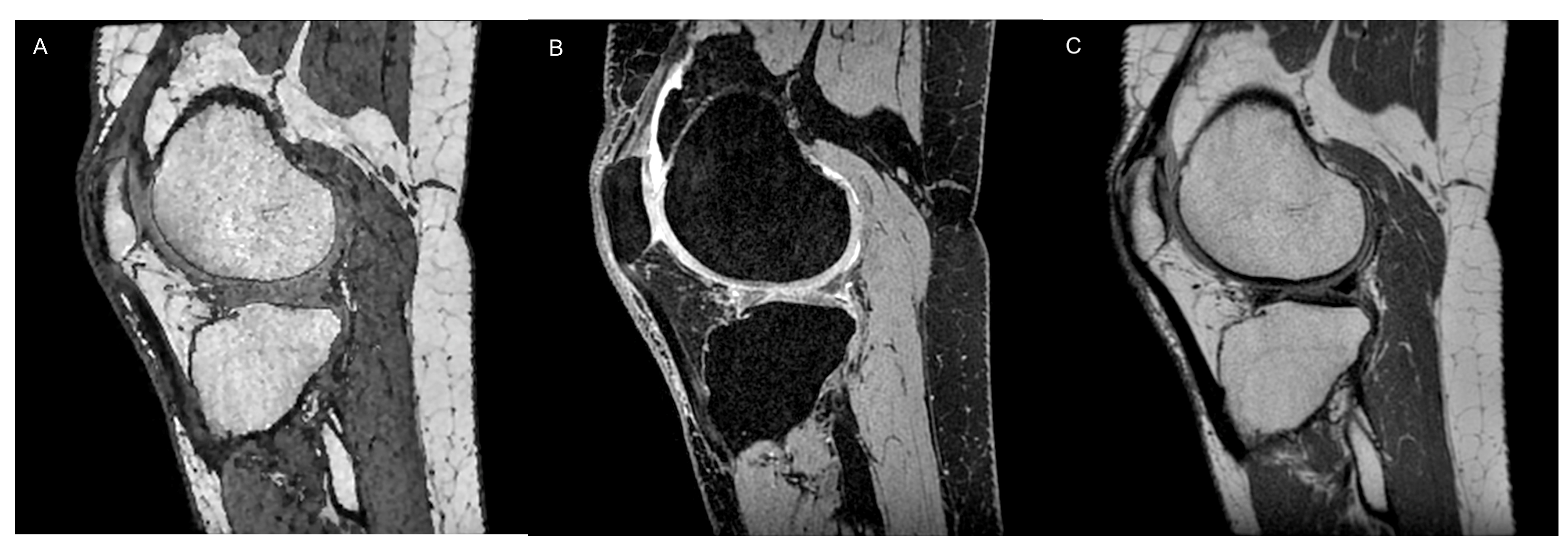
2.2. Cartilage Morphometry
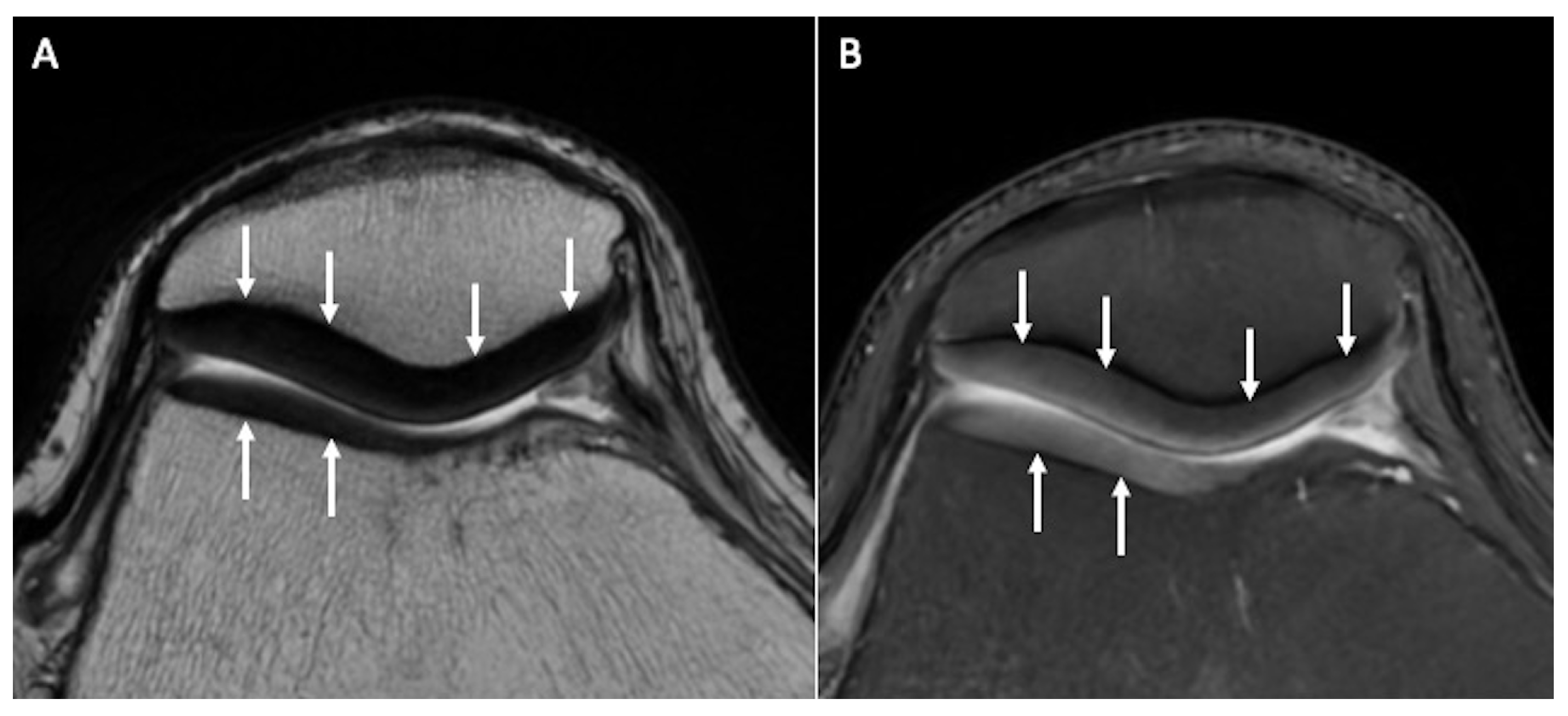
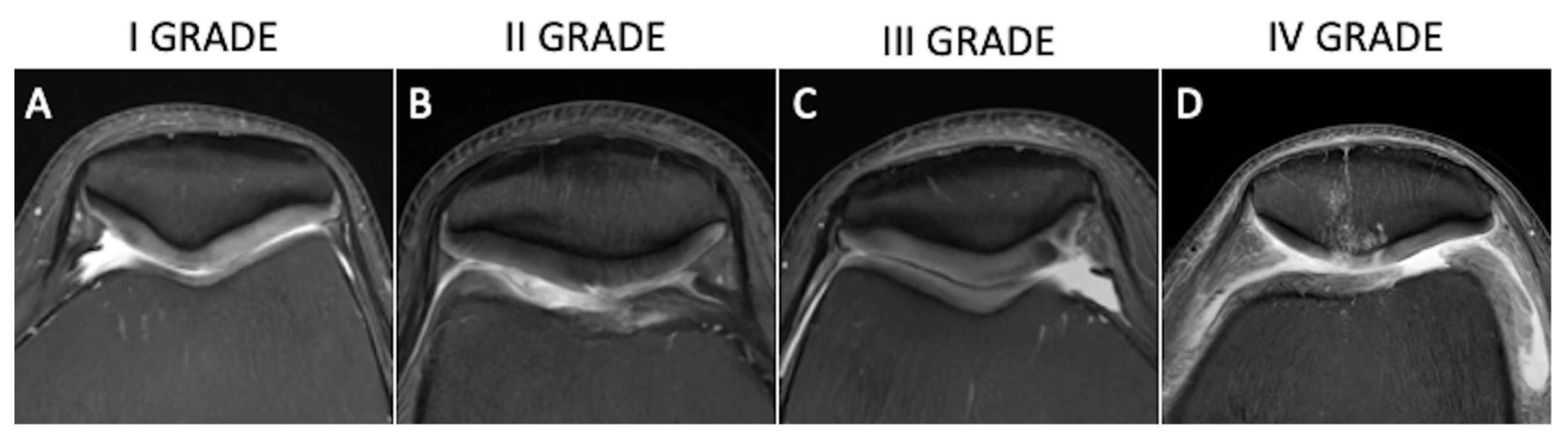
2.3. Semiquantitative (Morphological Changes and Lesion Appearance)
2.4. Compositional MRI
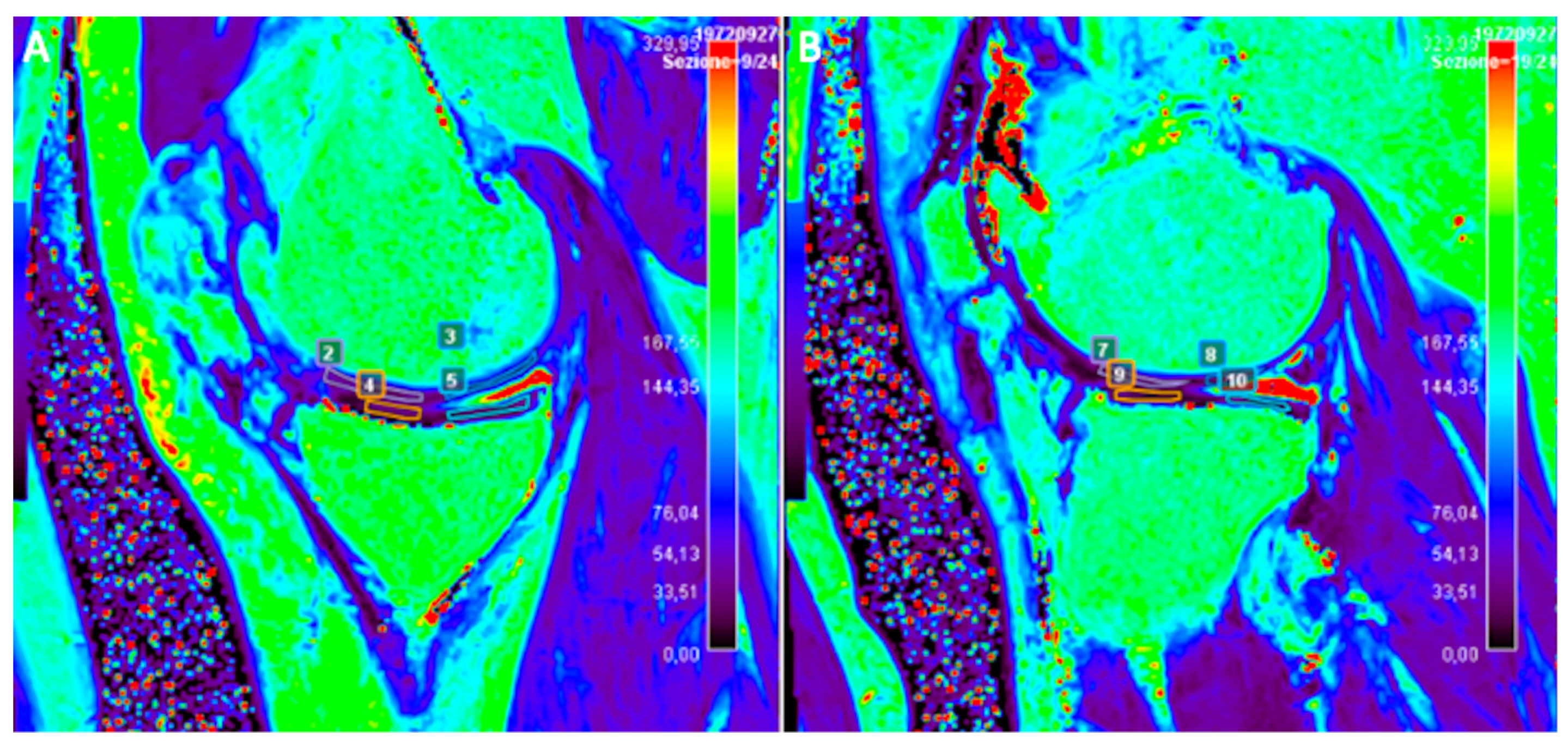
2.5. T2-Mapping
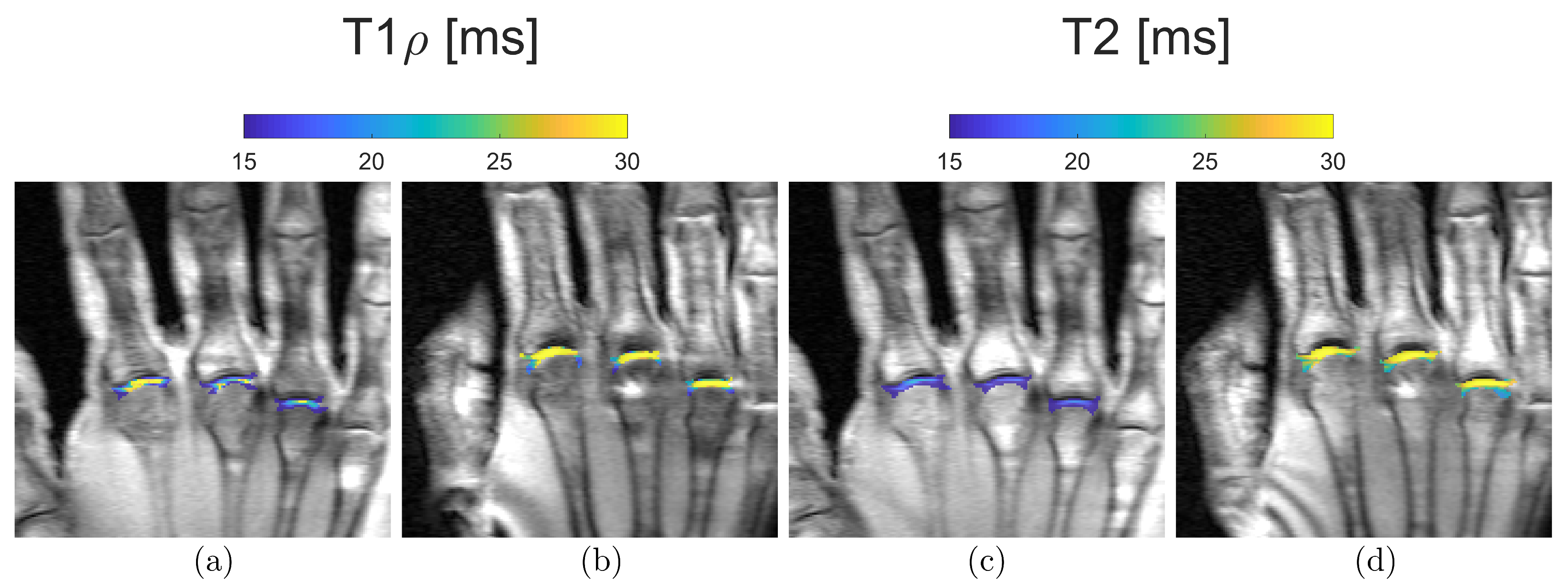
2.6. T1ρ
2.7. dGEMRIC—Delayed Gadolinium-Enhanced MRI
2.8. Sodium MRI
2.9. Diffusion-Weighted Imaging
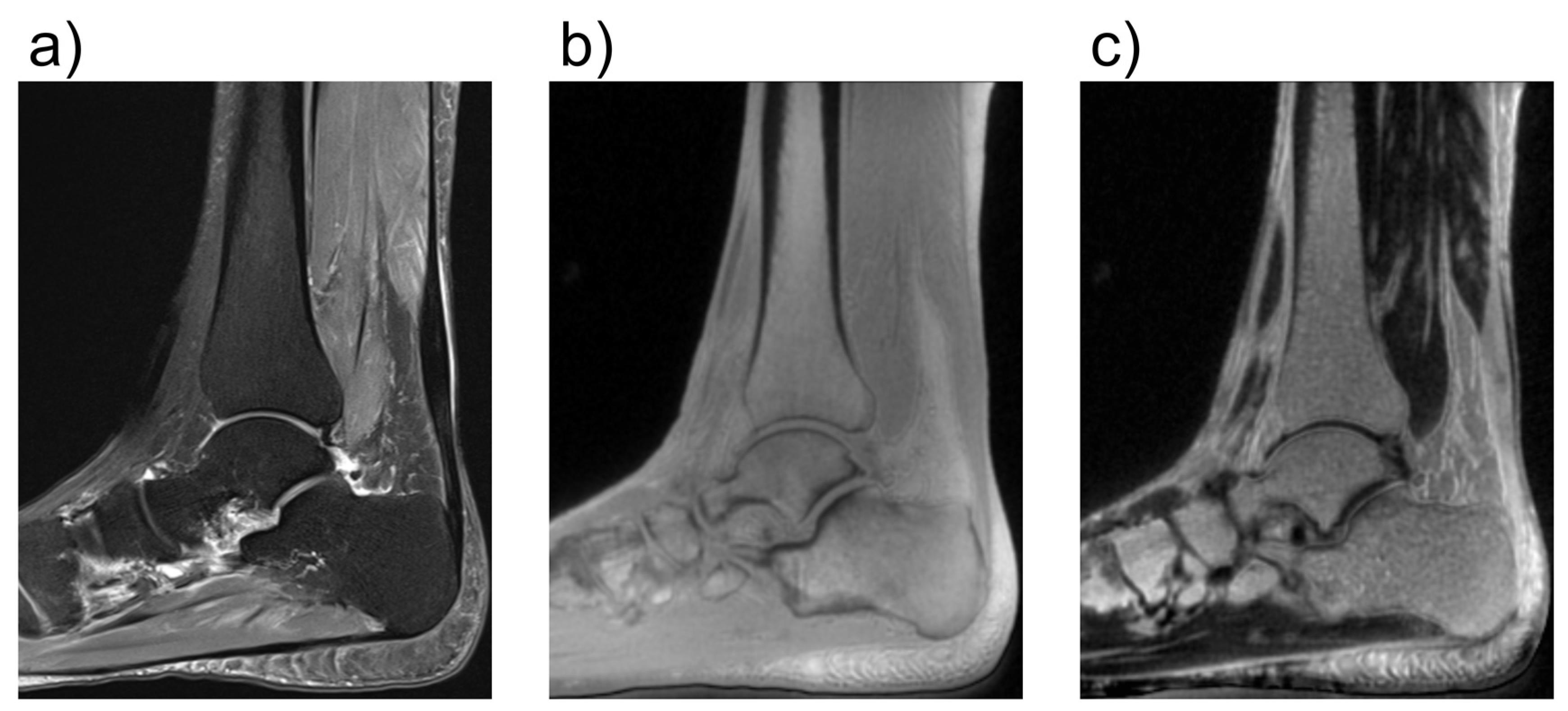
2.10. UTE Ultrashort-Time Echo MRI
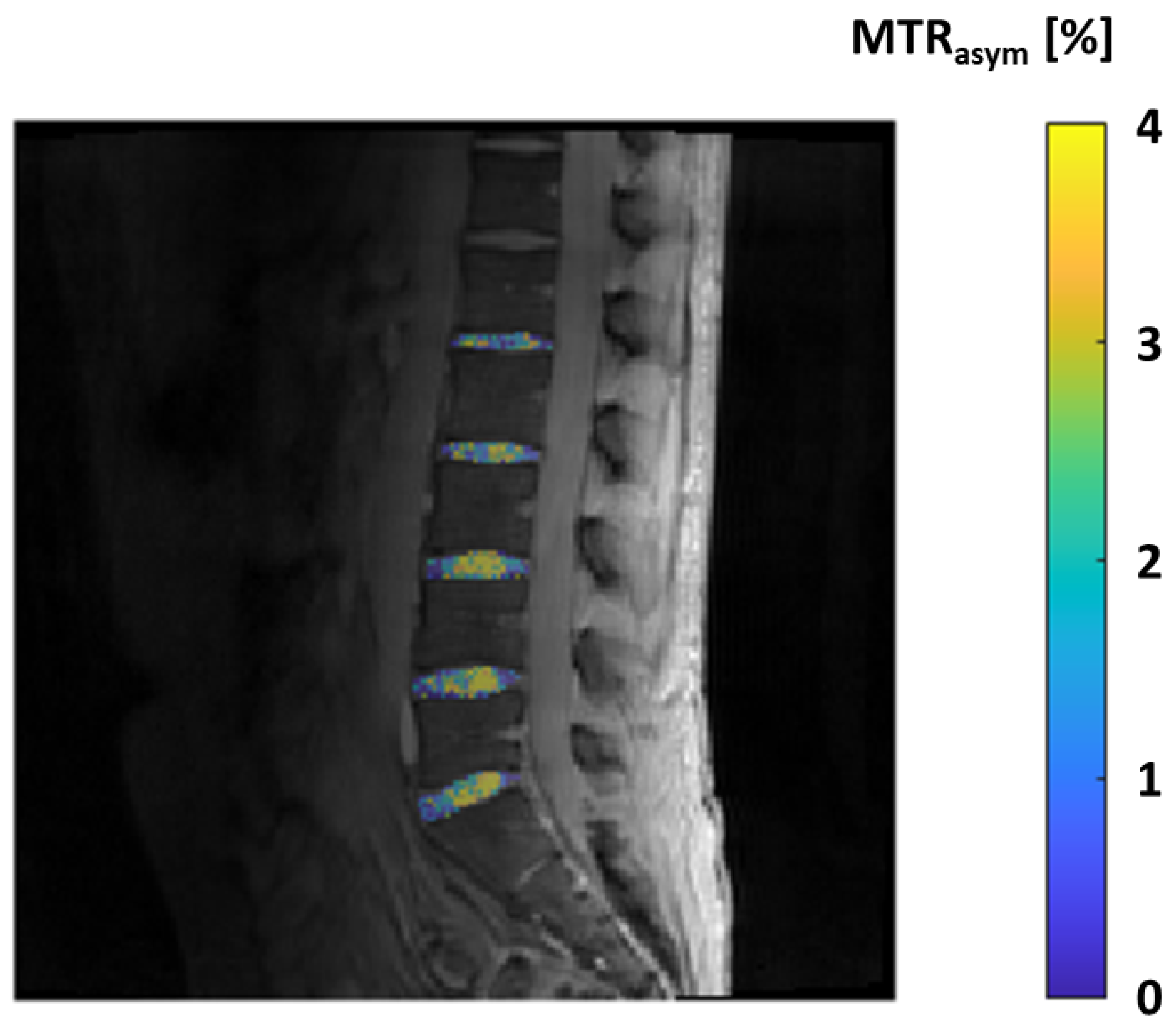
2.11. GAG-CEST—Glycosaminoglycan Chemical Exchange Saturation Transfer Imaging
3. Implementation and Significance for Clinical Practice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umlauf, D.; Frank, S.; Pap, T.; Bertrand, J. Cartilage biology, pathology, and repair. Cell. Mol. Life Sci. 2010, 67, 4197–4211. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, D.; Guermazi, A.; Kijowski, R.; Fritz, J.; Jalali-Farahani, S.; Mohajer, B.; Eng, J.; Demehri, S. Diagnostic Performance of Three-dimensional MRI for Depicting Cartilage Defects in the Knee: A Meta-Analysis. Radiology 2018, 289, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mosher, T.J.; Dardzinski, B.J.; Smith, M.B. Human Articular Cartilage: Influence of Aging and Early Symptomatic Degeneration on the Spatial Variation of T2—Preliminary Findings at 3 T. Radiology 2000, 214, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Omoumi, P.; Mourad, C.; Ledoux, J.-B.; Hilbert, T. Morphological assessment of cartilage and osteoarthritis in clinical practice and research: Intermediate-weighted fat-suppressed sequences and beyond. Skelet. Radiol. 2023, 52, 2185–2198. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Cao, Y.; Xu, Y.; Wang, H.; Wen, L.; Meng, Z.; Liu, H.; Wang, R.; Li, X. Efficacy of magnetic resonance imaging with an SPGR sequence for the early evaluation of knee cartilage degeneration and the relationship between cartilage and other tissues. J. Orthop. Surg. Res. 2019, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Gold, G.E.; Burstein, D.; Dardzinski, B.; Lang, P.; Boada, F.; Mosher, T. MRI of articular cartilage in OA: Novel pulse sequences and compositional/functional markers. Osteoarthr. Cartil. 2006, 14 (Suppl. S1), 76–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Disler, D.G.; McCauley, T.R.; Kelman, C.G.; Fuchs, M.D.; Ratner, L.M.; Wirth, C.R.; Hospodar, P.P. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: Comparison with standard MR imaging and arthroscopy. Am. J. Roentgenol. 1996, 167, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Allmann, K.H.; Ihling, C.; Hauer, M.P.; Conca, W.; Langer, M. Cartilage destruction in small joints by rheumatoid arthritis: Assessment of fat-suppressed three-dimensional gradient-echo MR pulse sequences in vitro. Skelet. Radiol. 1998, 27, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Fritz, B.; Bensler, S.; Thawait, G.K.; Raithel, E.; Stern, S.E.; Fritz, J. CAIPIRINHA-accelerated 10-min 3D TSE MRI of the ankle for the diagnosis of painful ankle conditions: Performance evaluation in 70 patients. Eur. Radiol. 2019, 29, 609–619. [Google Scholar] [CrossRef]
- Kijowski, R. 3D MRI of Articular Cartilage. Semin. Musculoskelet. Radiol. 2021, 25, 397–408. [Google Scholar] [CrossRef]
- Hargreaves, B.A.; Gold, G.E.; Lang, P.K.; Conolly, S.M.; Pauly, J.M.; Bergman, G.; Vandevenne, J.; Nishimura, D.G. MR imaging of articular cartilage using driven equilibrium. Magn. Reson. Med. 1999, 42, 695–703. [Google Scholar] [CrossRef]
- Welsch, G.H.; Trattnig, S.; Renner, N.; Lauer, L.; Paul, D.; Uder, M.; Schlechtweg, P.; Janka, R.; Schett, G. Morphological and biochemical magnetic resonance techniques for cartilage imaging in rheumatoid arthritis: Application and analysis. Int. J. Clin. Rheumatol. 2011, 6, 95–107. [Google Scholar] [CrossRef]
- Duerk, J.L.; Lewin, J.S.; Wendt, M.; Petersilge, C. Invited. Remember true FISP? a high SNR, near 1-second imaging method for T2-like contrast in interventional MRI at 2 T. J. Magn. Reson. Imaging 1998, 8, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kijowski, R.; Blankenbaker, D.G.; Klaers, J.L.; Shinki, K.; De Smet, A.A.; Block, W.F. Vastly Undersampled Isotropic Projection Steady-State Free Precession Imaging of the Knee: Diagnostic Performance Compared with Conventional MR. Radiology 2009, 251, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Graichen, H.; Eisenhart-Rothe, R.V.; Vogl, T.; Englmeier, K.; Eckstein, F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: Technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004, 50, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Cicuttini, F.; Raynauld, J.-P.; Waterton, J.; Peterfy, C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): Morphological assessment. Osteoarthr. Cartil. 2006, 14 (Suppl. S1), 46–75. [Google Scholar] [CrossRef] [PubMed]
- Joseph, G.B.; McCulloch, C.E.; Sohn, J.H.; Pedoia, V.; Majumdar, S.; Link, T.M. AI MSK clinical applications: Cartilage and osteoarthritis. Skelet. Radiol. 2022, 51, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Brett, A.; Bowes, M.; Conaghan, P.; Ladel, C.; Guehring, H.; Moreau, F.; Eckstein, F. Automated MRI assessment confirms cartilage thickness modification in patients with knee osteoarthritis: Post-hoc analysis from a phase II sprifermin study. Osteoarthr. Cartil. 2020, 28, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Hochberg, M.C.; Guehring, H.; Moreau, F.; Ona, V.; Bihlet, A.R.; Byrjalsen, I.; Andersen, J.R.; Daelken, B.; Guenther, O.; et al. Long-term structural and symptomatic effects of intra-articular sprifermin in patients with knee osteoarthritis: 5-year results from the FORWARD study. Ann. Rheum. Dis. 2021, 80, 1062–1069. [Google Scholar] [CrossRef]
- Gandy, S.J.; Brett, A.D.; A Dieppe, P.; Keen, M.C.; A Maciewicz, R.; Taylor, C.J.; Waterton, J.C.; Watt, I. Measurement of cartilage volumes in rheumatoid arthritis using MRI. Br. J. Radiol. 2005, 78, 39–45. [Google Scholar] [CrossRef]
- Outerbridge, R.E. The etiology of chondromalacia patellae. J. Bone Jt. Surgery. Br. Vol. 1961, 43, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Colak, C.; Polster, J.M.; Obuchowski, N.A.; Jones, M.H.; Strnad, G.; Gyftopoulos, S.; Spindler, K.P.; Subhas, N. Comparison of Clinical and Semiquantitative Cartilage Grading Systems in Predicting Outcomes After Arthroscopic Partial Meniscectomy. Am. J. Roentgenol. 2020, 215, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.M.; Raudner, M.; Marlovits, S.; Bohndorf, K.; Weber, M.; Zalaudek, M.; Röhrich, S.; Szomolanyi, P.; Filardo, G.; Windhager, R.; et al. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 Knee Score and Atlas. Cartilage 2021, 13 (Suppl. S1), 571S–587S. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Martinelli, N.; Bianchi, A.; Giacalone, A.; Sconfienza, L.M. Evaluation of reproducibility of the MOCART score in patients with osteochondral lesions of the talus repaired using the autologous matrix-induced chondrogenesis technique. Radiol. Med. 2017, 122, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Martinelli, N.; Bianchi, A.; Messina, C.; Malerba, F.; Sconfienza, L.M. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet. Disord. 2017, 18, 306. [Google Scholar] [CrossRef] [PubMed]
- Peterfy, C.; Guermazi, A.; Zaim, S.; Tirman, P.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Kornaat, P.R.; Ceulemans, R.Y.T.; Kroon, H.M.; Riyazi, N.; Kloppenburg, M.; Carter, W.O.; Woodworth, T.G.; Bloem, J.L. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)? inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skelet. Radiol. 2005, 34, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Lo, G.H.; Gale, D.; Grainger, A.J.; Guermazi, A.; Conaghan, P.G. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston–Leeds Osteoarthritis Knee Score). Ann. Rheum. Dis. 2008, 67, 206–211. [Google Scholar] [CrossRef]
- Felson, D.; Lynch, J.; Guermazi, A.; Roemer, F.; Niu, J.; McAlindon, T.; Nevitt, M. Comparison of BLOKS and WORMS scoring systems part II. Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2010, 18, 1402–1407. [Google Scholar] [CrossRef]
- Hunter, D.; Guermazi, A.; Lo, G.; Grainger, A.; Conaghan, P.; Boudreau, R.; Roemer, F. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 2011, 19, 990–1002. [Google Scholar] [CrossRef]
- Roemer, F.; Collins, J.; Kwoh, C.; Hannon, M.; Neogi, T.; Felson, D.; Hunter, D.; Lynch, J.; Guermazi, A. MRI-based screening for structural definition of eligibility in clinical DMOAD trials: Rapid OsteoArthritis MRI Eligibility Score (ROAMES). Osteoarthr. Cartil. 2020, 28, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Held, A. Role of proteoglycans in osteoarthritis. In Cartilage: Volume 2: Pathophysiology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Bellelli, A.; Silvestri, E.; Barile, A.; Albano, D.; Aliprandi, A.; Caudana, R.; Chianca, V.; Di Pietto, F.; Faletti, C.; Genovese, E.; et al. Position paper on magnetic resonance imaging protocols in the musculoskeletal system (excluding the spine) by the Italian College of Musculoskeletal Radiology. Radiol. Med. 2019, 124, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F.; Cina, A.; Panico, M.; Albano, D.; Messina, C. Image-based biomechanical models of the musculoskeletal system. Eur. Radiol. Exp. 2020, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Albano, D.; Cuocolo, R.; Messina, C.; Gitto, S.; Brunetti, A.; Sconfienza, L.M. T2 mapping of the trapeziometacarpal joint and triangular fibrocartilage complex: A feasibility and reproducibility study at 1.5 T. Radiol. Med. 2019, 125, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Chianca, V.; Cuocolo, R.; Bignone, R.; Ciccia, F.; Sconfienza, L.M.; Midiri, M.; Brunetti, A.; Lagalla, R.; Galia, M. T2-mapping of the sacroiliac joints at 1.5 Tesla: A feasibility and reproducibility study. Skelet. Radiol. 2018, 47, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Herz, B.; Albrecht, A.; Englbrecht, M.; Welsch, G.H.; Uder, M.; Renner, N.; Schlechtweg, P.; Paul, D.; Lauer, L.; Engelke, K.; et al. Osteitis and synovitis, but not bone erosion, is associated with proteoglycan loss and microstructure damage in the cartilage of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Bignone, R.; Chianca, V.; Cuocolo, R.; Messina, C.; Sconfienza, L.M.; Ciccia, F.; Brunetti, A.; Midiri, M.; Galia, M. T2 mapping of the sacroiliac joints in patients with axial spondyloarthritis. Eur. J. Radiol. 2020, 131, 109246. [Google Scholar] [CrossRef] [PubMed]
- Staroswiecki, E.; Granlund, K.L.; Alley, M.T.; Gold, G.E.; Hargreaves, B.A. Simultaneous estimation of T2 and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn. Reson. Med. 2012, 67, 1086–1096. [Google Scholar] [CrossRef]
- Park, S.; Kwack, K.-S.; Lee, Y.J.; Gho, S.-M.; Lee, H.Y. Initial experience with synthetic MRI of the knee at 3T: Comparison with conventional T1 weighted imaging and T2 mapping. Br. J. Radiol. 2017, 90, 20170350. [Google Scholar] [CrossRef]
- Nischal, N.; Iyengar, K.P.; Herlekar, D.; Botchu, R. Imaging of Cartilage and Chondral Defects: An Overview. Life 2023, 13, 363. [Google Scholar] [CrossRef]
- Gatehouse, P.; Bydder, G. Magnetic Resonance Imaging of Short T2 Components in Tissue. Clin. Radiol. 2003, 58, 1–19. [Google Scholar] [CrossRef]
- Bittersohl, B.; Miese, F.; Hosalkar, H.; Herten, M.; Antoch, G.; Krauspe, R.; Zilkens, C. T2∗ mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthr. Cartil. 2012, 20, 653–660. [Google Scholar] [CrossRef]
- Williams, A.; Qian, Y.; Chu, C. UTE-T2∗ mapping of human articular cartilage in vivo: A repeatability assessment. Osteoarthr. Cartil. 2011, 19, 84–88. [Google Scholar] [CrossRef]
- Baum, T.; Joseph, G.B.; Nardo, L.; Virayavanich, W.; Arulanandan, A.; Alizai, H.; Carballido-Gamio, J.; Nevitt, M.C.; Lynch, J.; McCulloch, C.E.; et al. Correlation of magnetic resonance imaging–based knee cartilage T2 measurements and focal knee lesions with body mass index: Thirty-six–month followup data from a longitudinal, observational multicenter study. Arthritis Care Res. 2013, 65, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Carballido-Gamio, J.; Joseph, G.B.; Lynch, J.A.; Link, T.M.; Majumdar, S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: A texture approach. Magn. Reson. Med. 2011, 65, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Mosher, T.J.; Liu, Y.; Yang, Q.X.; Yao, J.; Smith, R.; Dardzinski, B.J.; Smith, M.B. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004, 50, 2820–2828. [Google Scholar] [CrossRef]
- Friedrich, K.M.; Shepard, T.; Chang, G.; Wang, L.; Babb, J.S.; Schweitzer, M.; Regatte, R. Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur. Radiol. 2010, 20, 1532–1538. [Google Scholar] [CrossRef]
- Chen, M.; Qiu, L.; Shen, S.; Wang, F.; Zhang, J.; Zhang, C.; Liu, S. The influences of walking, running and stair activity on knee articular cartilage: Quantitative MRI using T1 rho and T2 mapping. PLoS ONE 2017, 12, e0187008. [Google Scholar] [CrossRef] [PubMed]
- Wáng, Y.-X.J.; Zhang, Q.; Li, X.; Chen, W.; Ahuja, A.; Yuan, J. T1ρ magnetic resonance: Basic physics principles and applications in knee and intervertebral disc imaging. Quant. Imaging Med. Surg. 2015, 5, 858–885. [Google Scholar] [CrossRef]
- Chen, W.; Takahashi, A.; Han, E. 3D Quantitative Imaging of T1rho & T2. Available online: https://archive.ismrm.org/2011/0231.html (accessed on 20 April 2024).
- Duvvuri, U.; Charagundla, S.R.; Kudchodkar, S.B.; Kaufman, J.H.; Kneeland, J.B.; Rizi, R.; Leigh, J.S.; Reddy, R. Human Knee: In Vivo T1ρ-weighted MR Imaging at 1.5 T—Preliminary Experience. Radiology 2001, 220, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Witschey, W.R.; Borthakur, A.; Elliott, M.A.; Fenty, M.; Sochor, M.A.; Wang, C.; Reddy, R. T1ρ-prepared balanced gradient echo for rapid 3D T1ρ MRI. J. Magn. Reson. Imaging 2008, 28, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Akella, S.V.; Regatte, R.R.; Gougoutas, A.J.; Borthakur, A.; Shapiro, E.M.; Kneeland, J.B.; Leigh, J.S. Proteoglycan-induced changes in T1ρ-relaxation of articular cartilage at 4T. Magn. Reson. Med. 2001, 46, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Akella, S.V.; Regatte, R.R.; Wheaton, A.J.; Borthakur, A. Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn. Reson. Med. 2004, 52, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, A.J.; Dodge, G.R.; Elliott, D.M.; Nicoll, S.B.; Reddy, R. Quantification of cartilage biomechanical and biochemical properties via T1ρ magnetic resonance imaging. Magn. Reson. Med. 2005, 54, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Regatte, R.R.; Akella, S.V.; Lonner, J.; Kneeland, J. T1ρ relaxation mapping in human osteoarthritis (OA) cartilage: Comparison of T1ρ with T2. J. Magn. Reson. Imaging 2006, 23, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, J.; Lin, K.; Saadat, E.; Bolbos, R.I.; Jobke, B.; Ries, M.D.; Horvai, A.; Link, T.M.; Majumdar, S. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: Correlation with biochemical measurements and histology. Magn. Reson. Imaging 2011, 29, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Stahl, R.; Luke, A.; Li, X.; Carballido-Gamio, J.; Ma, C.B.; Majumdar, S.; Link, T.M. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients—A 3.0-Tesla MRI study. Eur. Radiol. 2009, 19, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, D.U.; Souza, R.B.; Wu, S.; Luke, A.; Li, X.; Feeley, B.T. T1ρ Imaging Demonstrates Early Changes in the Lateral Patella in Patients with Patellofemoral Pain and Maltracking. Am. J. Sports Med. 2013, 41, 1813–1818. [Google Scholar] [CrossRef]
- Li, X.; Ma, B.C.; Bolbos, R.I.; Stahl, R.; Lozano, J.; Zuo, J.; Lin, K.; Link, T.M.; Safran, M.; Majumdar, S. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J. Magn. Reson. Imaging 2008, 28, 453–461. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Bolbos, R.I.; Link, T.M.; Majumdar, S. Longitudinal assessment of bone marrow edema-like lesions and cartilage degeneration in osteoarthritis using 3 T MR T1rho quantification. Skelet. Radiol. 2010, 39, 523–531. [Google Scholar] [CrossRef]
- Luke, A.C.; Stehling, C.; Stahl, R.; Li, X.; Kay, T.; Takamoto, S.; Ma, B.; Majumdar, S.; Link, T. High-Field Magnetic Resonance Imaging Assessment of Articular Cartilage before and after Marathon Running. Am. J. Sports Med. 2010, 38, 2273–2280. [Google Scholar] [CrossRef]
- Wang, L.; Vieira, R.L.R.; Rybak, L.D.; Babb, J.S.; Chang, G.; Krasnokutsky, S.; Abramson, S.; Regatte, R. Relationship between knee alignment and T1ρ values of articular cartilage and menisci in patients with knee osteoarthritis. Eur. J. Radiol. 2013, 82, 1946–1952. [Google Scholar] [CrossRef]
- A Yoon, M.; Hong, S.-J.; Im, A.L.; Kang, C.H.; Kim, B.H.; Kim, I.S. Comparison of T1rho and T2 Mapping of Knee Articular Cartilage in an Asymptomatic Population. Korean J. Radiol. 2016, 17, 912–918. [Google Scholar] [CrossRef]
- Bittersohl, B.; Hosalkar, H.S.; Werlen, S.; Trattnig, S.; Siebenrock, K.A.; Mamisch, T.C. dGEMRIC and subsequent T1 mapping of the hip at 1.5 Tesla: Normative data on zonal and radial distribution in asymptomatic volunteers. J. Magn. Reson. Imaging 2011, 34, 101–106. [Google Scholar] [CrossRef]
- Tiderius, C.J.; Svensson, J.; Leander, P.; Ola, T.; Dahlberg, L. dGEMRIC (delayed gadolinium-enhanced MRI of cartilage) indicates adaptive capacity of human knee cartilage. Magn. Reson. Med. 2004, 51, 286–290. [Google Scholar] [CrossRef]
- Watanabe, A.; Boesch, C.; Siebenrock, K.; Obata, T.; Anderson, S.E. T2mapping of hip articular cartilage in healthy volunteers at 3T: A study of topographic variation. J. Magn. Reson. Imaging 2007, 26, 165–171. [Google Scholar] [CrossRef]
- Bekkers, J.; Bartels, L.; Benink, R.; Tsuchida, A.; Vincken, K.; Dhert, W.; Creemers, L.; Saris, D. Delayed gadolinium enhanced MRI of cartilage (dGEMRIC) can be effectively applied for longitudinal cohort evaluation of articular cartilage regeneration. Osteoarthr. Cartil. 2013, 21, 943–949. [Google Scholar] [CrossRef]
- Anandacoomarasamy, A.; Leibman, S.; Smith, G.; Caterson, I.; Giuffre, B.; Fransen, M.; Sambrook, P.N.; March, L. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann. Rheum. Dis. 2012, 71, 26–32. [Google Scholar] [CrossRef]
- Lazik, A.; Theysohn, J.M.; Geis, C.; Johst, S.; Ladd, M.E.; Quick, H.H.; Kraff, O. 7 Tesla quantitative hip MRI: T1, T2 and T2* mapping of hip cartilage in healthy volunteers. Eur. Radiol. 2016, 26, 1245–1253. [Google Scholar] [CrossRef]
- Savarino, E.; Chianca, V.; Bodini, G.; Albano, D.; Messina, C.; Tontini, G.E.; Sconfienza, L.M. Gadolinium accumulation after contrast-enhanced magnetic resonance imaging: Which implications in patients with Crohn’s disease? Dig. Liver Dis. 2017, 49, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Doniselli, F.M.; Albano, D.; Chianca, V.; Cimmino, M.A.; Sconfienza, L.M. Gadolinium accumulation after contrast-enhanced magnetic resonance imaging: What rheumatologists should know. Clin. Rheumatol. 2017, 36, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Kamp, B.; Frenken, M.; Henke, J.M.; Abrar, D.B.; Nagel, A.M.; Gast, L.V.; Oeltzschner, G.; Wilms, L.M.; Nebelung, S.; Antoch, G.; et al. Quantification of Sodium Relaxation Times and Concentrations as Surrogates of Proteoglycan Content of Patellar CARTILAGE at 3T MRI. Diagnostics 2021, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Brinkhof, S.; Froeling, M.; Janssen, R.P.A.; Ito, K.; Klomp, D.W.J. Can sodium MRI be used as a method for mapping of cartilage stiffness? Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zibetti, M.V.W.; Menon, R.G.; de Moura, H.L.; Zhang, X.; Kijowski, R.; Regatte, R.R. Updates on Compositional MRI Mapping of the Cartilage: Emerging Techniques and Applications. J. Magn. Reson. Imaging 2023, 58, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Newbould, R.D.; Miller, S.R.; Upadhyay, N.; Rao, A.W.; Swann, P.; Gold, G.E.; Strachan, R.K.; Matthews, P.M.; Taylor, P.C.; Brown, A.P. T1-Weighted Sodium MRI of the Articulator Cartilage in Osteoarthritis: A Cross Sectional and Longitudinal Study. PLoS ONE 2013, 8, e73067. [Google Scholar] [CrossRef] [PubMed]
- Zbýň, Š.; Mlynárik, V.; Juras, V.; Szomolanyi, P.; Trattnig, S. Evaluation of cartilage repair and osteoarthritis with sodium MRI. NMR Biomed. 2016, 29, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Albano, D.; Messina, C.; Cinnante, C.M.; Triulzi, F.M.; Sardanelli, F.; Sconfienza, L.M. Diffusion tensor imaging in the musculoskeletal and peripheral nerve systems: From experimental to clinical applications. Eur. Radiol. Exp. 2017, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Farley, M.; Nieminen, M.T.; Gray, M.; Burstein, D. Diffusion tensor imaging of native and degenerated human articular cartilage. Magn. Reson. Imaging 2007, 25, 168–171. [Google Scholar] [CrossRef]
- Raya, J.G.; Melkus, G.; Adam-Neumair, S.; Dietrich, O.; Mützel, E.; Reiser, M.F.; Putz, R.; Kirsch, T.; Jakob, P.M.; Glaser, C. Diffusion-Tensor Imaging of Human Articular Cartilage Specimens with Early Signs of Cartilage Damage. Radiology 2013, 266, 831–841. [Google Scholar] [CrossRef]
- Raya, J.G.; Horng, A.; Dietrich, O.; Krasnokutsky, S.; Beltran, L.S.; Storey, P.; Reiser, M.F.; Recht, M.P.; Sodickson, D.K.; Glaser, C. Articular Cartilage: In Vivo Diffusion-Tensor Imaging. Radiology 2012, 262, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; Peters, T.M.; Rutt, B.K. Quantitative diffusion imaging with steady-state free precession. Magn. Reson. Med. 2004, 51, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Bieri, O.; Ganter, C.; Scheffler, K. Quantitative in vivo diffusion imaging of cartilage using double echo steady-state free precession. Magn. Reson. Med. 2012, 68, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Ruiz, A.; Ferizi, U.; Bencardino, J.; Abramson, S.B.; Samuels, J.; Krasnokutsky-Samuels, S.; Raya, J.G. Diffusion tensor imaging of articular cartilage using a navigated radial imaging spin-echo diffusion (RAISED) sequence. Eur. Radiol. 2019, 29, 2598–2607. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Albano, D.; Rizzo, S.; Maas, M.; Sconfienza, L.M.; Del Grande, F. Inter-vendor and inter-observer reliability of diffusion tensor imaging in the musculoskeletal system: A multiscanner MR study. Insights Imaging 2023, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jang, H.; Jerban, S.; Chang, E.Y.; Chung, C.B.; Bydder, G.M.; Du, J. Making the invisible visible-ultrashort echo time magnetic resonance imaging: Technical developments and applications. Appl. Phys. Rev. 2022, 9, 041303. [Google Scholar] [CrossRef]
- Jerban, S.; Oei, E.H.G.; Ding, J. Editorial: Cartilage assessment using quantitative MRI. Front. Endocrinol. 2022, 13, 1092354. [Google Scholar] [CrossRef] [PubMed]
- Afsahi, A.M.; Ma, Y.; Jang, H.; Jerban, S.; Chung, C.B.; Chang, E.Y.; Du, J. Ultrashort Echo Time Magnetic Resonance Imaging Techniques: Met and Unmet Needs in Musculoskeletal Imaging. J. Magn. Reson. Imaging 2022, 55, 1597–1612. [Google Scholar] [CrossRef]
- Robson, M.D.; Gatehouse, P.D.; Bydder, M.; Bydder, G.M. Magnetic Resonance: An Introduction to Ultrashort TE (UTE) Imaging. J. Comput. Assist. Tomogr. 2003, 27, 825–846. [Google Scholar] [CrossRef]
- Grodzki, D.M.; Jakob, P.M.; Heismann, B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn. Reson. Med. 2012, 67, 510–518. [Google Scholar] [CrossRef]
- Ji, Z.; Li, Y.; Dou, W.; Zhu, Y.; Shi, Y.; Zou, Y. Ultra-short echo time MR imaging in assessing cartilage endplate damage and relationship between its lesion and disc degeneration for chronic low back pain patients. BMC Med Imaging 2023, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Aletras, A.; Balaban, R. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Regatte, R.R.; Navon, G.; Jerschow, A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc. Natl. Acad. Sci. USA 2008, 105, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Matzat, S.J.; van Tiel, J.; Gold, G.E.; Oei, E.H.G. Quantitative MRI techniques of cartilage composition. 2013, 3, 162–174–174. [CrossRef]
- Soellner, S.; Welsch, G.; Gelse, K.; Goldmann, A.; Kleyer, A.; Schett, G.; Pachowsky, M. gagCEST imaging at 3 T MRI in patients with articular cartilage lesions of the knee and intraoperative validation. Osteoarthr. Cartil. 2021, 29, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Rehnitz, C.; Kupfer, J.; Streich, N.; Burkholder, I.; Schmitt, B.; Lauer, L.; Kauczor, H.-U.; Weber, M.-A. Comparison of biochemical cartilage imaging techniques at 3 T MRI. Osteoarthr. Cartil. 2014, 22, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Brinkhof, S.; Nizak, R.; Khlebnikov, V.; Prompers, J.J.; Klomp, D.W.; Saris, D.B. Detection of early cartilage damage: Feasibility and potential of gagCEST imaging at 7T. Eur. Radiol. 2018, 28, 2874–2881. [Google Scholar] [CrossRef]

| Techinque | Sequences Used/Component Assessed | Pros | Cons |
|---|---|---|---|
| T2-mapping | T2*, UTE-T2*, T2w SE, FSE, Multi Echo SE, Turbo Gradient SE, DESS Cartilage hydratation, Cartilage, Water | High reproducibility. T2* and UTE sequences allow a better visualization of deep cartilage and osteochondral junction. Predictive for OA in areas with much compression loading. No contrast administration. | Susceptible of magic angle artifact and magnetic field inhomogeneity. |
| T1ρ | FSE, TSE, balanced GRE Tissue proteins and composition, GAGs | Quantitative and sequence-independent voxel values. Excellent reproducibility and sensitivity in detecting GAGs depletion. No contrast administration. No special hardware needed. | High SAR, long acquisition time, pulse sequence must be adapted. |
| dGEMRIC (delayed gadolinium-enhanced) | T1-mapping after gadolinium administration GAG content | Evaluation of cartilage repair following regenerative treatments. Suitable for visualization of osteo-chondral interface and zonal variation of GAG content. | Intravascular or intrarticular administration of contrast. No standard time for intracartilage diffusion (usually after 90 min). Values not directly correlated with GAG concentration. |
| Sodium MRI | UTE T1w Na+ concentration of cartilage ECM | Promising technique for non-invasive evaluation of articular cartilage and repair tissue. Direct correlation with Na+ cartilage content. No contrast administration. | Low Na SNR, need of 3T or higher. Partial volume effect for intra- and extra-cellular sodium signals. Need of specialized RF coils and customized UTE sequences. |
| DTI (Diffusion Tensor Imaging) | Pulse sequences with steady precession, double echo SSFO, RAISED Collagen, PG | High sensitivity and specificity detecting early cartilage degeneration and collagen structure alteration. No contrast administration. | Limited SNR, motion sensitive, long acquisition time. High-field MRI (3T). |
| UTE (Ultrashort time echo) MRI | T1, T2, T2*, T1ρ, magnetization transfer (MT), DESS, IR, quantitative susceptibility mapping Cartilage deep layers, osteochondral junction | Assessment of thin articular structures with short-T2 other than long-T2 structure. Lower susceptibility artifacts and magic angle effect than conventional quantitative MRI. Excellent interobserver agreement to cartilage endplate damage and intervertebral disc degeneration. | Not well validated. Inhomogeneous data on compositional quantification. |
| GAG-CEST (glycosaminoglycan chemical exchange saturation transfer imaging) | Magnetization transfer (MT) | Direct quantification of GAG content. Promising for identification of initial knee-joint cartilage damage. Comparable to dGEMRIC and T2 mapping. Good to excellent reproducibility at 7T MRI. | High-field MRI (3T or more). Not yet well validated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, D.; Viglino, U.; Esposito, F.; Rizzo, A.; Messina, C.; Gitto, S.; Fusco, S.; Serpi, F.; Kamp, B.; Müller-Lutz, A.; et al. Quantitative and Compositional MRI of the Articular Cartilage: A Narrative Review. Tomography 2024, 10, 949-969. https://doi.org/10.3390/tomography10070072
Albano D, Viglino U, Esposito F, Rizzo A, Messina C, Gitto S, Fusco S, Serpi F, Kamp B, Müller-Lutz A, et al. Quantitative and Compositional MRI of the Articular Cartilage: A Narrative Review. Tomography. 2024; 10(7):949-969. https://doi.org/10.3390/tomography10070072
Chicago/Turabian StyleAlbano, Domenico, Umberto Viglino, Francesco Esposito, Aldo Rizzo, Carmelo Messina, Salvatore Gitto, Stefano Fusco, Francesca Serpi, Benedikt Kamp, Anja Müller-Lutz, and et al. 2024. "Quantitative and Compositional MRI of the Articular Cartilage: A Narrative Review" Tomography 10, no. 7: 949-969. https://doi.org/10.3390/tomography10070072
APA StyleAlbano, D., Viglino, U., Esposito, F., Rizzo, A., Messina, C., Gitto, S., Fusco, S., Serpi, F., Kamp, B., Müller-Lutz, A., D’Ambrosi, R., Sconfienza, L. M., & Sewerin, P. (2024). Quantitative and Compositional MRI of the Articular Cartilage: A Narrative Review. Tomography, 10(7), 949-969. https://doi.org/10.3390/tomography10070072








