A Non-Invasive, Label-Free Method for Examining Tardigrade Anatomy Using Holotomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Tardigrade Culture
2.2. Quantification of Anesthetization and Recovery Durations in Tardigrades
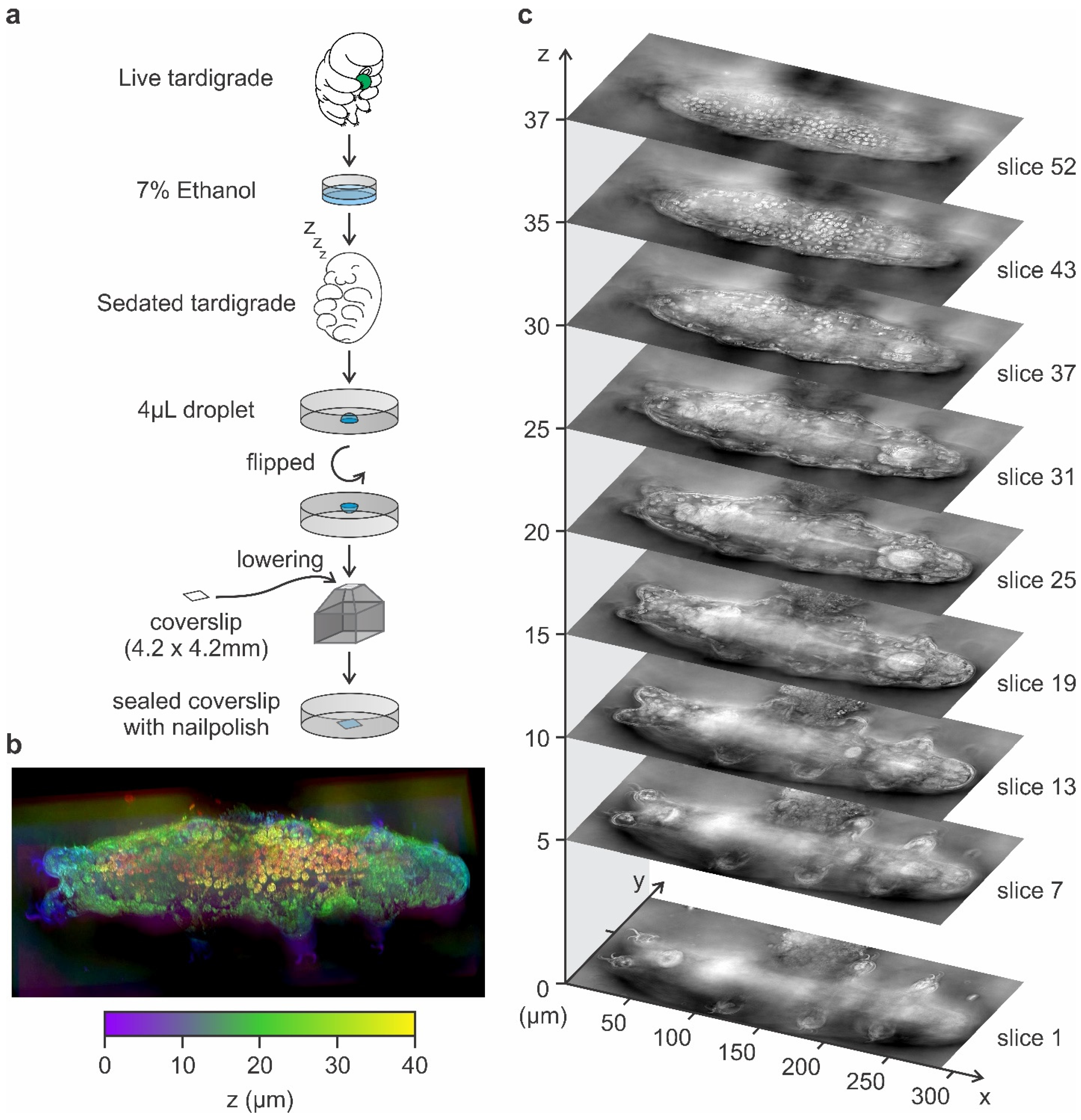
2.3. Tardigrade Sample Mounting for Holotomography Imaging
2.4. Brightfield Microscopy
2.5. Holotomography Using Tomocube HT-X1
2.6. Data Analysis and Visualization
3. Results
3.1. Tardigrade 3D Holotomography
3.2. Anatomy of Tardigrade Visualized Using Holotomography
3.2.1. External Morphology
3.2.2. Feeding Apparatus and Digestive Tract
3.2.3. Reproductive Organs
3.2.4. Salivary Glands and Musculature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popescu, G.; Park, Y. Quantitative phase imaging in biomedicine. J. Biomed. Opt. 2015, 20, 111201. [Google Scholar] [CrossRef]
- Lambert, A. Live Cell Imaging with Holotomography and Fluorescence. Microsc. Today 2020, 28, 18–23. [Google Scholar] [CrossRef]
- Park, J.; Bai, B.; Ryu, D.; Liu, T.; Lee, C.; Luo, Y.; Lee, M.J.; Huang, L.; Shin, J.; Zhang, Y.; et al. Artificial intelligence-enabled quantitative phase imaging methods for life sciences. Nat. Methods 2023, 20, 1645–1660. [Google Scholar] [CrossRef] [PubMed]
- Wełnicz, W.; Grohme, M.A.; Kaczmarek, L.; Schill, R.O.; Frohme, M. Anhydrobiosis in tardigrades—The last decade. J. Insect Physiol. 2011, 57, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Boothby, T.C.; Tapia, H.; Brozena, A.H.; Piszkiewicz, S.; Smith, A.E.; Giovannini, I.; Rebecchi, L.; Pielak, G.J.; Koshland, D.; Goldstein, B. Tardigrades Use Intrinsically Disordered Proteins to Survive Desiccation. Mol. Cell 2017, 65, 975–984.e5. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kunieda, T. DNA Protection Protein, a Novel Mechanism of Radiation Tolerance: Lessons from Tardigrades. Life 2017, 7, 26. [Google Scholar] [CrossRef]
- Hashimoto, T.; Horikawa, D.D.; Saito, Y.; Kuwahara, H.; Kozuka-Hata, H.; Shin, I.T.; Minakuchi, Y.; Ohishi, K.; Motoyama, A.; Aizu, T.; et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 2016, 7, 12808. [Google Scholar] [CrossRef]
- Clark-Hachtel, C.M.; Hibshman, J.D.; De Buysscher, T.; Stair, E.R.; Hicks, L.M.; Goldstein, B. The tardigrade Hypsibius exemplaris dramatically upregulates DNA repair pathway genes in response to ionizing radiation. Curr. Biol. 2024, 34, 1819–1830.e1816. [Google Scholar] [CrossRef]
- Swamy, C.; Boothby, T.C. Surviving extreme radiation. Elife 2024, 13, e100219. [Google Scholar] [CrossRef]
- Packebush, M.H.; Sanchez-Martinez, S.; Biswas, S.; Kc, S.; Nguyen, K.H.; Ramirez, J.F.; Nicholson, V.; Boothby, T.C. Natural and engineered mediators of desiccation tolerance stabilize Human Blood Clotting Factor VIII in a dry state. Sci. Rep. 2023, 13, 4542. [Google Scholar] [CrossRef]
- Traspas, A.; Burchell, M.J. Tardigrade Survival Limits in High-Speed Impacts-Implications for Panspermia and Collection of Samples from Plumes Emitted by Ice Worlds. Astrobiology 2021, 21, 845–852. [Google Scholar] [CrossRef]
- Smith, F.W.; Boothby, T.C.; Giovannini, I.; Rebecchi, L.; Jockusch, E.L.; Goldstein, B. The Compact Body Plan of Tardigrades Evolved by the Loss of a Large Body Region. Curr. Biol. 2016, 26, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nakano, T.; Watanabe, K.; Masuda, K.; Honda, G.; Kamata, S.; Yasui, R.; Kozuka-Hata, H.; Watanabe, C.; Chinen, T.; et al. Stress-dependent cell stiffening by tardigrade tolerance proteins that reversibly form a filamentous network and gel. PLoS Biol. 2022, 20, e3001780. [Google Scholar] [CrossRef]
- Gross, V.; Minich, I.; Mayer, G. External morphogenesis of the tardigrade Hypsibius dujardini as revealed by scanning electron microscopy. J. Morphol. 2017, 278, 563–573. [Google Scholar] [CrossRef]
- Massa, E.; Rebecchi, L.; Guidetti, R. Effects of synthetic acid rain and organic and inorganic acids on survival and CaCO3 piercing stylets in tardigrades. J. Exp. Zool. A Ecol. Integr. Physiol. 2023, 339, 578–589. [Google Scholar] [CrossRef]
- Richaud, M.; Le Goff, E.; Cazevielle, C.; Ono, F.; Mori, Y.; Saini, N.L.; Cuq, P.; Baghdiguian, S.; Godefroy, N.; Galas, S. Ultrastructural analysis of the dehydrated tardigrade Hypsibius exemplaris unveils an anhydrobiotic-specific architecture. Sci. Rep. 2020, 10, 4324. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.W.; Bartels, P.J.; Goldstein, B. A Hypothesis for the Composition of the Tardigrade Brain and its Implications for Panarthropod Brain Evolution. Integr. Comp. Biol. 2017, 57, 546–559. [Google Scholar] [CrossRef]
- Gross, V.; Mayer, G. Neural development in the tardigrade Hypsibius dujardini based on anti-acetylated α-tubulin immunolabeling. Evodevo 2015, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, M.; Miernik, A.; Sojka, J.; Student, S.; Śliwińska, M.A.; Gross, V.; Poprawa, I. Oogenesis in the tardigrade Hypsibius exemplaris Gąsiorek, Stec, Morek & Michalczyk, 2018 (Eutardigrada, Hypsibiidae). Micron 2021, 150, 103126. [Google Scholar] [CrossRef]
- Gabriel, W.N.; Goldstein, B. Segmental expression of Pax3/7 and engrailed homologs in tardigrade development. Dev. Genes. Evol. 2007, 217, 421–433. [Google Scholar] [CrossRef]
- Laissue, P.P.; Alghamdi, R.A.; Tomancak, P.; Reynaud, E.G.; Shroff, H. Assessing phototoxicity in live fluorescence imaging. Nat. Methods 2017, 14, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, J.; Swartz, M.E.; Eberhart, J.K.; Chowdhury, S. DMD and microlens array as a switchable module for illumination angle scanning in optical diffraction tomography. Biomed. Opt. Express 2024, 15, 5932–5946. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, J.; Kalkman, J. Large-scale high-sensitivity optical diffraction tomography of zebrafish. Biomed. Opt. Express 2019, 10, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, M.; Kwak, H.; Kim, Y.S.; Shim, J.; Jung, J.H.; Park, W.S.; Park, J.H.; Lee, S.; Park, Y. High-fidelity optical diffraction tomography of live organisms using iodixanol refractive index matching. Biomed. Opt. Express 2022, 13, 6404–6415. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chen, M.; Eckert, R.; Ren, D.; Wu, F.; Repina, N.; Waller, L. High-resolution 3D refractive index microscopy of multiple-scattering samples from intensity images. Optica 2019, 6, 1211–1219. [Google Scholar] [CrossRef]
- Coquoz, S.; Marchand, P.J.; Bouwens, A.; Mouchiroud, L.; Sorrentino, V.; Szlag, D.; Auwerx, J.; Lasser, T. Label-free three-dimensional imaging of Caenorhabditis elegans with visible optical coherence microscopy. PLoS ONE 2017, 12, e0181676. [Google Scholar] [CrossRef]
- Roszkowska, M.; Wojciechowska, D.; Kmita, H.; Cerbin, S.; Dziuba, M.K.; Fiałkowska, E.; Sobkowiak, R.; Szydło, W.; Kaczmarek, Ł. Tips and tricks how to culture water bears: Simple protocols for culturing eutardigrades (Tardigrada) under laboratory conditions. Eur. Zool. J. 2021, 88, 449–465. [Google Scholar] [CrossRef]
- Ramløv, H.; Westh, P. Cryptobiosis in the Eutardigrade adorybiotus (Richtersius) coronifer: Tolerance to Alcohols, Temperature and de novo Protein Synthesis. Zool. Anz. A J. Comp. Zool. 2001, 240, 517–523. [Google Scholar] [CrossRef]
- Poprawa, I.; Bartylak, T.; Kulpla, A.; Erdmann, W.; Roszkowska, M.; Chajec, Ł.; Kaczmarek, Ł.; Karachitos, A.; Kmita, H. Verification of Hypsibius exemplaris Gąsiorek et al., 2018 (Eutardigrada; Hypsibiidae) application in anhydrobiosis research. PLoS ONE 2022, 17, e0261485. [Google Scholar] [CrossRef]
- Guidetti, R.; Bonifacio, A.; Altiero, T.; Bertolani, R.; Rebecchi, L. Distribution of Calcium and Chitin in the Tardigrade Feeding Apparatus in Relation to its Function and Morphology. Integr. Comp. Biol. 2015, 55, 241–252. [Google Scholar] [CrossRef]
- Gross, V.; Müller, M.; Hehn, L.; Ferstl, S.; Allner, S.; Dierolf, M.; Achterhold, K.; Mayer, G.; Pfeiffer, F. X-ray imaging of a water bear offers a new look at tardigrade internal anatomy. Zoological Lett. 2019, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Camarda, D.; Massa, E.; Guidetti, R.; Lisi, O. A new, simplified, drying protocol to prepare tardigrades for scanning electron microscopy. Microsc. Res. Tech. 2024, 87, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Osorio, S.; Velasquez, Z.D.; Conejeros, I.; Taubert, A.; Hermosilla, C. Morphometric analysis of aerobic Eimeria bovis sporogony using live cell 3D holotomographic microscopy imaging. Parasitol. Res. 2022, 121, 1179–1189. [Google Scholar] [CrossRef]
- Bergaglio, T.; Bhattacharya, S.; Thompson, D.; Nirmalraj, P.N. Label-Free Digital Holotomography Reveals Ibuprofen-Induced Morphological Changes to Red Blood Cells. ACS Nanosci. Au 2023, 3, 241–255. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.-T.; Lee, G.; Chang, Y.-T. A Non-Invasive, Label-Free Method for Examining Tardigrade Anatomy Using Holotomography. Tomography 2025, 11, 34. https://doi.org/10.3390/tomography11030034
Hong M-T, Lee G, Chang Y-T. A Non-Invasive, Label-Free Method for Examining Tardigrade Anatomy Using Holotomography. Tomography. 2025; 11(3):34. https://doi.org/10.3390/tomography11030034
Chicago/Turabian StyleHong, Minh-Triet, Giyoung Lee, and Young-Tae Chang. 2025. "A Non-Invasive, Label-Free Method for Examining Tardigrade Anatomy Using Holotomography" Tomography 11, no. 3: 34. https://doi.org/10.3390/tomography11030034
APA StyleHong, M.-T., Lee, G., & Chang, Y.-T. (2025). A Non-Invasive, Label-Free Method for Examining Tardigrade Anatomy Using Holotomography. Tomography, 11(3), 34. https://doi.org/10.3390/tomography11030034






