Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. What is DECT and What Does it Add to Single-Energy CT?
3.2. What Physical Concepts Are Important to Understand?
3.3. Does “Dual-Energy” Mean Two Photon Energies?
3.4. Which Parameters Influence Imaging Quality in DECT?
3.5. How Is Postprocessing Performed in DECT Imaging and Why Is It Useful?
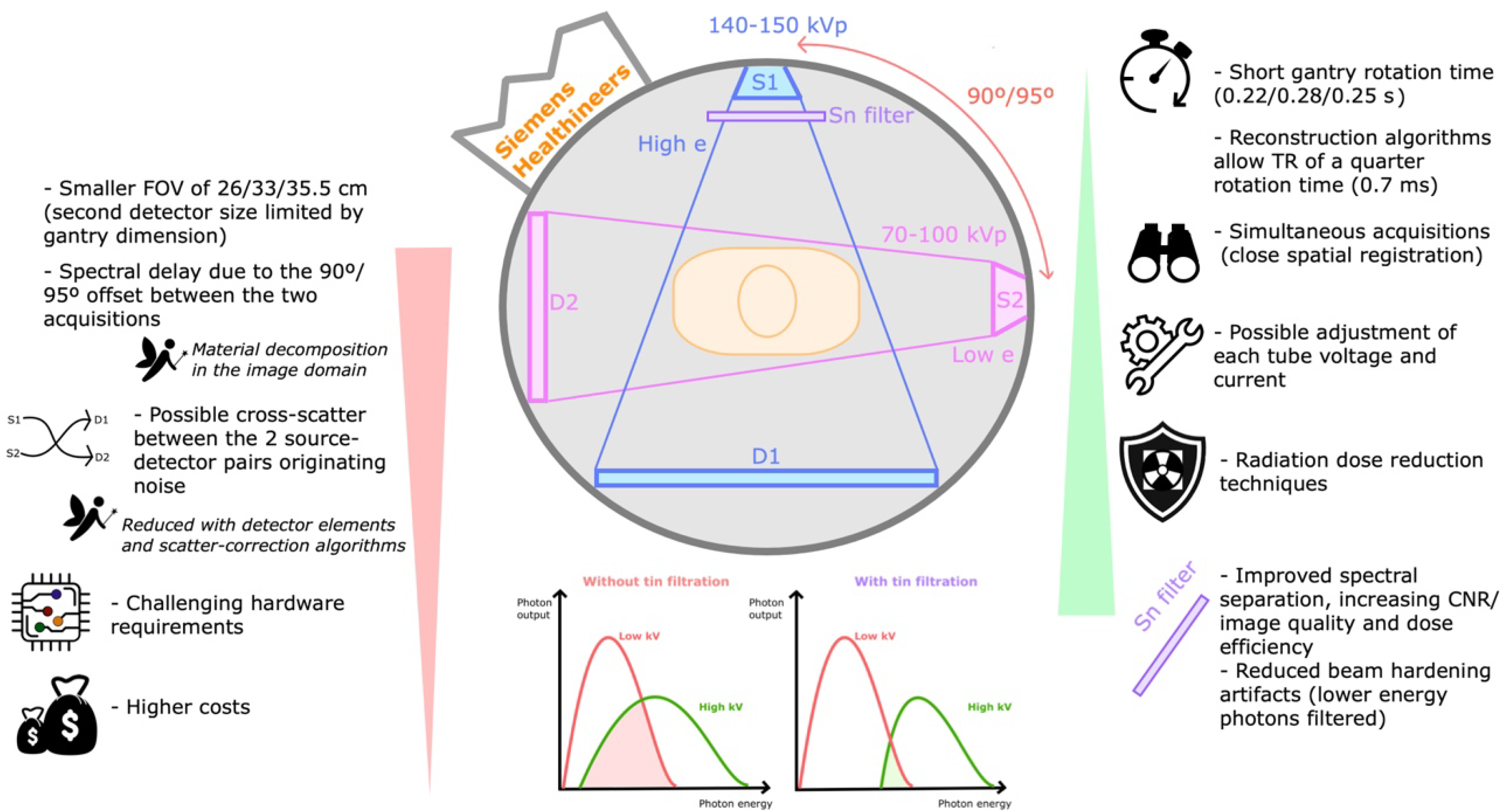
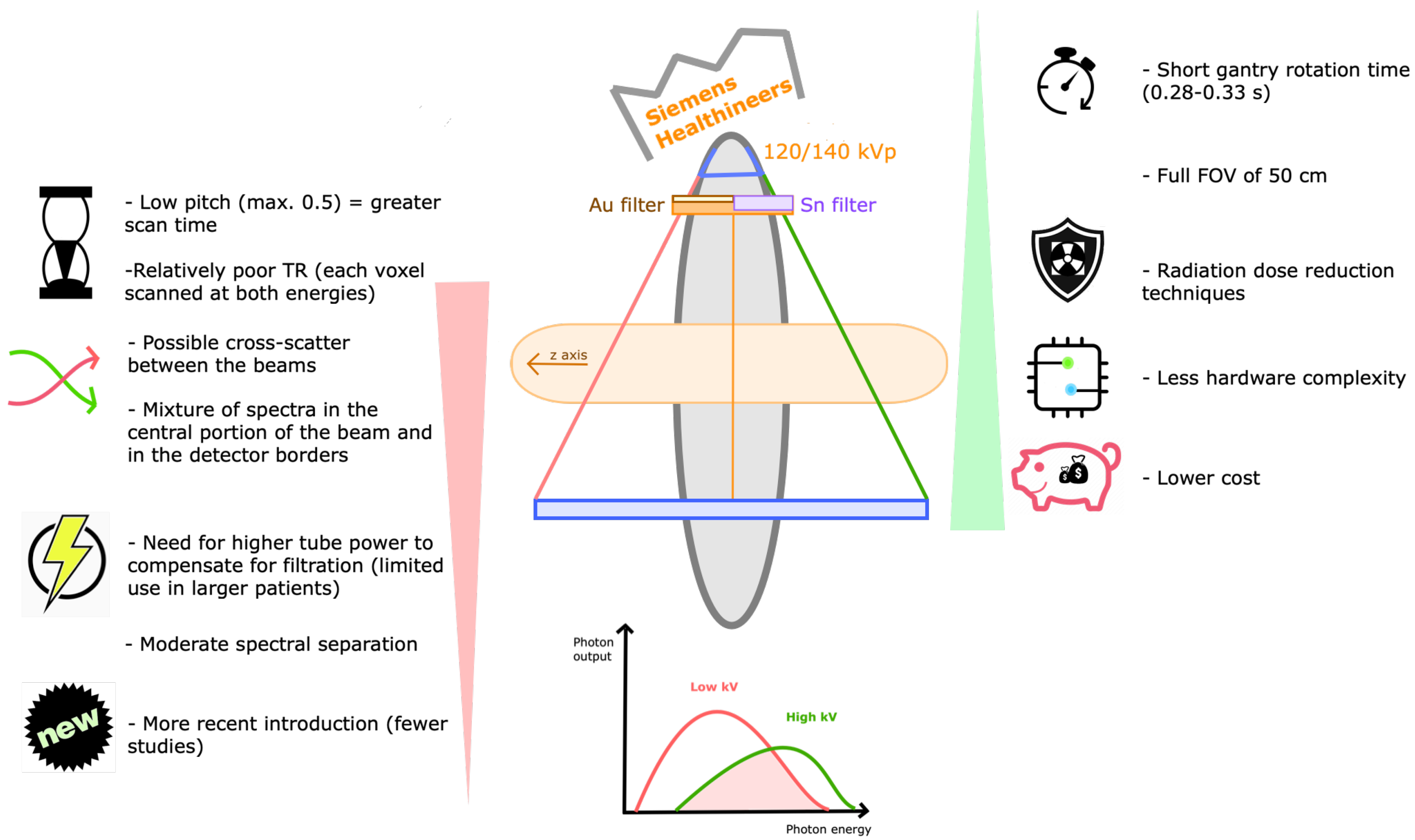
3.6. What Are the Technical Differences between Available DECT Systems?
3.7. Is DECT Imaging Applicable to Daily Routine Workflow?
3.8. What Are Beam Hardening Artifacts and How Are They Lessened?
3.9. Is DECT Imaging Also Prone to Artifacts?
3.10. Which DECT Scanners Are More Prone to Temporal Misregistration?
3.11. How Does the Patient Size Influence the DECT Image Quality?
3.12. How Is Contrast Enhancement Improved with DECT and Why Is It Advantageous?
3.13. Is DECT Imaging Associated with Greater Radiation Exposure?
3.14. How Do DECT Systems Differ in Terms of Imaging Quality?
3.15. Are DECT Images Reproducible among Different Scanners?
3.16. What Are the Main Clinical Applications of DECT?
3.17. What to Expect in the Future of Spectral Imaging?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNR | contrast-to-noise ratio |
| CT | computed tomography |
| DECT | dual-energy computed tomography |
| dsDECT | dual-source dual-energy computed tomography |
| FOV | field of view |
| keV | kiloelectronvolt |
| kVp | kilovolt peak |
| VMIs | virtual monochromatic images |
| VNC | virtual non-contrast |
| PACS | picture archiving and communication system |
| SECT | single-energy computed tomography |
References
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Mari, A.; Floridi, C.; Bruno, F.; Carotti, M.; Schicchi, N.; Barile, A.; Maggi, S.; Giovagnoni, A. Dual-energy CT: Theoretical principles and clinical applications. La Radiol. Med. 2019, 124, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Honda, Y.; Awai, K. Dual-energy CT: Minimal essentials for radiologists. Jpn. J. Radiol. 2022, 40, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Sodickson, A.D.; Keraliya, A.; Czakowski, B.; Primak, A.; Wortman, J.; Uyeda, J.W. Dual energy CT in clinical routine: How it works and how it adds value. Emerg. Radiol. 2020, 28, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Forghani, R.; De Man, B.; Gupta, R. Dual-Energy Computed Tomography: Physical Principles, Approaches to Scanning, Usage, and Implementation: Part 1. Neuroimaging Clin. N. Am. 2017, 27, 371–384. [Google Scholar] [CrossRef]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef] [Green Version]
- Garnett, R. A comprehensive review of dual-energy and multi-spectral computed tomography. Clin. Imaging 2020, 67, 160–169. [Google Scholar] [CrossRef]
- Megibow, A.J.; Kambadakone, A.; Ananthakrishnan, L. Dual-Energy Computed Tomography: Image Acquisition, Processing, and Workflow. Radiol. Clin. N. Am. 2018, 56, 507–520. [Google Scholar] [CrossRef]
- Tsang, D.S.; Merchant, T.E.; Merchant, S.E.; Smith, H.; Yagil, Y.; Hua, C.-H. Quantifying potential reduction in contrast dose with monoenergetic images synthesized from dual-layer detector spectral CT. Br. J. Radiol. 2017, 90, 20170290. [Google Scholar] [CrossRef]
- Siegel, M.J.; Kaza, R.K.; Bolus, D.N.; Boll, D.T.; Rofsky, N.M.; De Cecco, C.N.; Foley, W.D.; Morgan, D.E.; Schoepf, U.J.; Sahani, D.V.; et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 1: Technology and Terminology. J. Comput. Assist. Tomogr. 2016, 40, 841–845. [Google Scholar] [CrossRef]
- Catalano, C.; Geiger, D. Dual-, multi-, and mono-energy CT & iodine: Basic concepts and clinical applications. In CT of the Retroperitoneum; Springer: Milano, Italy, 2014; pp. 11–18. [Google Scholar] [CrossRef]
- Forghani, R.; De Man, B.; Gupta, R. Dual-Energy Computed Tomography: Physical Principles, Approaches to Scanning, Usage, and Implementation: Part 2. Neuroimaging Clin. N. Am. 2017, 27, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.C. Dual-Energy CT: General Principles. Am. J. Roentgenol. 2012, 199 (Suppl. S5), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Faby, S.; Merz, J.; Krauss, B.; Kuemmel, F. Spectral Imaging with Dual Energy CT—Comparison of Different Technologies and Workflows; Whitepaper; Siemens Healthcare GmbH: Erlangen, Germany, 2018. [Google Scholar]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and Minimizing Artifacts at Dual-Energy CT. Radiographics 2021, 41, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Toia, G.V.; Mileto, A.; Wang, C.L.; Sahani, D.V. Quantitative dual-energy CT techniques in the abdomen. Abdom. Imaging 2021, 47, 3003–3018. [Google Scholar] [CrossRef]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Mileto, A.; Mazziotti, S.; Gaeta, M.; Bottari, A.; Zimbaro, F.; Giardina, C.; Ascenti, G. Pancreatic dual-source dual-energy CT: Is it time to discard unenhanced imaging? Clin. Radiol. 2012, 67, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Michoux, N.; Coche, E.; Dragean, C. Virtual unenhanced phase with spectral dual-energy CT: Is it an alternative to conventional true unenhanced phase for abdominal tissues? Diagn. Interv. Imaging 2019, 100, 503–511. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Boll, D.T.; Bolus, D.N.; Foley, W.D.; Kaza, R.K.; Morgan, D.E.; Rofsky, N.M.; Sahani, D.V.; Schoepf, U.J.; Shuman, W.P.; et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 4: Abdominal and Pelvic Applications. J. Comput. Assist. Tomogr. 2017, 41, 8–14. [Google Scholar] [CrossRef]
- Cao, J.; Lennartz, S.; Pisuchpen, N.; Parakh, A.; Kambadakone, A. Attenuation values on virtual unenhanced images obtained with detector-based dual-energy computed tomography: Observations on single- and split-bolus contrast protocols. Abdom. Imaging 2021, 47, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Durieux, P.; Gevenois, P.A.; Van Muylem, A.; Howarth, N.; Keyzer, C. Abdominal Attenuation Values on Virtual and True Unenhanced Images Obtained with Third-Generation Dual-Source Dual-Energy CT. Am. J. Roentgenol. 2018, 210, 1042–1058. [Google Scholar] [CrossRef]
- Obmann, M.; Kelsch, V.; Cosentino, A.; Hofmann, V.; Boll, D.T.; Benz, M.R. Interscanner and Intrascanner Comparison of Virtual Unenhanced Attenuation Values Derived from Twin Beam Dual-Energy and Dual-Source, Dual-Energy Computed Tomography. Investig. Radiol. 2019, 54, 1–6. [Google Scholar] [CrossRef]
- Faby, S.; Kuchenbecker, S.; Sawall, S.; Simons, D.; Schlemmer, H.-P.; Lell, M.; Kachelrieß, M. Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: A simulation study. Med. Phys. 2015, 42, 4349–4366. [Google Scholar] [CrossRef]
- Kim, S.; Kang, B.S.M.; Kwon, W.-J.M.; Bang, M.; Lim, S.; Park, G.M.; Lee, T.Y. Abdominal Organs Attenuation Values and Abdominal Aortic Calcifications on Virtual and True Noncontrast Images Obtained with Third-Generation Dual-Source Dual-Energy Computed Tomography. J. Comput. Assist. Tomogr. 2020, 44, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: Comparison of attenuation values with unenhanced CT. Abdom. Imaging 2017, 42, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, S.; Parakh, A.; Cao, J.; Kambadakone, A. Longitudinal reproducibility of attenuation measurements on virtual unen-hanced images: Multivendor dual-energy CT evaluation. Eur. Radiol. 2021, 31, 9240–9249. [Google Scholar] [CrossRef] [PubMed]
- Wortman, J.R.; Sodickson, A.D. Pearls, Pitfalls, and Problems in Dual-Energy Computed Tomography Imaging of the Body. Radiol. Clin. N. Am. 2018, 56, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Nasir, M.U.; So, A.; Andrews, G.; Nicolaou, S.; Qamar, S.R. Clinical Applications of Dual-Energy CT. Korean J. Radiol. 2021, 22, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Sato, J.; Akahane, M.; Kunimatsu, A.; Abe, O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018, 38, 450–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godreau, J.-P.; Vulasala, S.S.R.; Gopireddy, D.; Rao, D.; Hernandez, M.; Lall, C.; Bhosale, P.; Virarkar, M.K. Introducing and Building a Dual-Energy CT Business. Semin. Ultrasound CT MRI 2022, 43, 355–363. [Google Scholar] [CrossRef]
- Slavic, S.; Danielsson, M. Dual-energy: The GE approach. In Spectral Imaging: Dual-Energy, Multi-Energy and Photon-Counting CT: Medical Radiology; Alkadhi, H., Euler, A., Maintz, D., Sahani, D., Eds.; Springer International Publishing: New York, NY, USA, 2022; pp. 45–46. [Google Scholar]
- Boedeker, K.; Vaishnav, J.; Zhang, R.; Yu, Z.; Nakanishi, S. Dual-energy: The canon approach. In Spectral Imaging: Dual-Energy, Multi-Energy and Photon-Counting CT: Medical Radiology; Alkadhi, H., Euler, A., Maintz, D., Sahani, D., Eds.; Springer International Publishing: New York, NY, USA, 2022; pp. 63–64. [Google Scholar]
- Greffier, J.; Si-Mohamed, S.; Dabli, D.; de Forges, H.; Hamard, A.; Douek, P.; Beregi, J.P.; Frandon, J. Performance of four dual-energy CT platforms for abdominal imaging: A task-based image quality assessment based on phantom data. Eur. Radiol. 2021, 31, 5324–5334. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Flohr, T. Dual-energy: The siemens approach. In Spectral Imaging: Dual-Energy, Multi-Energy and Photon-Counting CT: Medical Radiology; Alkadhi, H., Euler, A., Maintz, D., Sahani, D., Eds.; Springer International Publishing: New York, NY, USA, 2022; pp. 15–17. [Google Scholar]
- Euler, A.; Parakh, A.; Falkowski, A.L.; Manneck, S.; Dashti, D.; Krauss, B.; Szucs-Farkas, Z.; Schindera, S.T. Initial Results of a Single-Source Dual-Energy Computed Tomography Technique Using a Split-Filter: Assessment of Image Quality, Radiation Dose, and Accuracy of Dual-Energy Applications in an In Vitro and In Vivo Study. Investig. Radiol. 2016, 51, 491–498. [Google Scholar] [CrossRef]
- Lambert, J.W.; Fitzgerald, P.F.; Edic, P.M.; Sun, Y.; Jr, P.J.B.; Colborn, R.E.; Yeh, B.M. The Effect of Patient Diameter on the Dual-Energy Ratio of Selected Contrast-Producing Elements. J. Comput. Assist. Tomogr. 2017, 41, 505–510. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.M.; Schoepf, U.J.; Steinbach, L.; Boll, D.T.; Foley, W.D.; Kaza, R.K.; Bolus, D.N.; Morgan, D.E.; Sahani, D.V.; Shuman, W.P.; et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 3: Vascular, Cardiac, Pulmonary, and Musculoskeletal Applications. J. Comput. Assist. Tomogr. 2017, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chatzaraki, V.; Born, C.; Kubik-Huch, R.A.; Froehlich, J.M.; Thali, M.J.; Niemann, T. Influence of Radiation Dose and Reconstruction Kernel on Fat Fraction Analysis in Dual-energy CT: A Phantom Study. Vivo 2021, 35, 3147–3155. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.; Reisinger, C.; Chou, H.; Munk, P.L.; Nicolaou, S.; Ouellette, H. Artifacts in Dual-Energy CT Gout Protocol: A Review of 50 Suspected Cases with an Artifact Identification Guide. Am. J. Roentgenol. 2014, 203, W103–W109. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamada, M.; Sugisawa, K.; Akita, H.; Shiomi, E.; Abe, T.; Okuda, S.; Jinzaki, M. Renal Cyst Pseudoenhancement: Intra-individual comparison between virtual monochromatic spectral images and conventional polychromatic 120-kVp images obtained during the same CT examination and comparisons among images reconstructed using filtered back projection, adaptive statistical iterative reconstruction, and model-based iterative reconstruction. Medicine 2015, 94, e754. [Google Scholar] [CrossRef] [PubMed]
- Petritsch, B.; Pannenbecker, P.; Weng, A.M.; Grunz, J.-P.; Veldhoen, S.; Bley, T.A.; Kosmala, A. Split-filter dual-energy CT pulmonary angiography for the diagnosis of acute pulmonary embolism: A study on image quality and radiation dose. Quant. Imaging Med. Surg. 2021, 11, 1817–1827. [Google Scholar] [CrossRef]
- Patel, B.N.; Alexander, L.; Allen, B.; Berland, L.; Borhani, A.; Mileto, A.; Moreno, C.; Morgan, D.; Sahani, D.; Shuman, W.; et al. Dual-energy CT workflow: Multi-institutional consensus on standardization of ab-dominopelvic MDCT protocols. Abdom. Radiol. 2017, 42, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Marsh, J.F.; Tao, A.; Michalak, G.J.; Rajendran, K.; McCollough, C.H.; Leng, S. Multi-energy CT imaging for large patients using dual-source photon-counting detector CT. Phys. Med. Biol. 2020, 65, 17NT01. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Higaki, T.; Kondo, S.; Kawashita, I.; Takahashi, I.; Awai, K. An introduction to photon-counting detector CT (PCD CT) for radiologists. Jpn. J. Radiol. 2022, 1–17. [Google Scholar] [CrossRef]
- Foley, W.D.; Shuman, W.P.; Siegel, M.J.; Sahani, D.V.; Boll, D.T.; Bolus, D.N.; De Cecco, C.N.M.; Kaza, R.K.; Morgan, D.E.; Schoepf, U.J.; et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 2: Radiation Dose and Iodine Sensitivity. J. Comput. Assist. Tomogr. 2016, 40, 846–850. [Google Scholar] [CrossRef]
- Schmidt, C.; Baessler, B.; Nakhostin, D.; Das, A.; Eberhard, M.; Alkadhi, H.; Euler, A. Dual-Energy CT-Based Iodine Quantification in Liver Tumors—Impact of Scan-, Patient-, and Position-Related Factors. Acad. Radiol. 2020, 28, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Euler, A.; Obmann, M.M.; Szucs-Farkas, Z.; Mileto, A.; Zaehringer, C.; Falkowski, A.L.; Winkel, D.J.; Marin, D.; Stieltjes, B.; Krauss, B.; et al. Comparison of image quality and radiation dose between split-filter dual-energy images and single-energy images in single-source abdominal CT. Eur. Radiol. 2018, 28, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Mager, P.; Yu, N.C.; Katz, D.P.; Brady, J.R.; Gupta, N. Iodine quantification and detectability thresholds among major dual-energy CT platforms. Br. J. Radiol. 2019, 92, 20190530. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.C.; Cressman, E.N.K.; Tamm, E.P.; Baluya, D.L.; Duan, X.; Cody, D.D.; Schellingerhout, D.; Layman, R.R. Dual-Energy CT: Lower Limits of Iodine Detection and Quantification. Radiology 2019, 292, 414–419. [Google Scholar] [CrossRef]
- Schmidt, D.; Söderberg, M.; Nilsson, M.; Lindvall, H.; Christoffersen, C.; Leander, P. Evaluation of image quality and radiation dose of abdominal dual-energy CT. Acta Radiol. 2017, 59, 845–852. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Hardie, A.D.; Schoepf, U.J.; Felmly, L.M.; Perry, J.D.; Varga-Szemes, A.; Mangold, S.; Caruso, D.; Canstein, C.; Vogl, T.J.; et al. Single- and dual-energy CT of the abdomen: Comparison of radiation dose and image quality of 2nd and 3rd gen-eration dual-source CT. Eur. Radiol. 2017, 27, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Grajo, J.R.; Sahani, D.V. Dual-Energy CT of the Abdomen and Pelvis: Radiation Dose Considerations. J. Am. Coll. Radiol. 2018, 15, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Krauss, B.; Grant, K.L.; Schmidt, B.T.; Flohr, T.G. The Importance of Spectral Separation: An assessment of dual-energy spectral separation for quantitative ability and dose efficiency. Investig. Radiol. 2015, 50, 114–118. [Google Scholar] [CrossRef]
- Almeida, I.P.; Schyns, L.E.J.R.; Öllers, M.C.; Van Elmpt, W.; Parodi, K.; Landry, G.; Verhaegen, F. Dual-energy CT quantitative imaging: A comparison study between twin-beam and dual-source CT scanners. Med. Phys. 2016, 44, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Chen, J.; Ren, H.; Wang, Y.; Tao, X.; Zhan, K. Comparison of image quality between split-filter twin beam dual energy and single energy images in abdominal CT. Eur. J. Radiol. 2019, 121, 108702. [Google Scholar] [CrossRef]
- Euler, A.; Laqua, F.C.; Cester, D.; Lohaus, N.; Sartoretti, T.; dos Santos, D.P.; Alkadhi, H.; Baessler, B. Virtual Monoenergetic Images of Dual-Energy CT—Impact on Repeatability, Reproducibility, and Classification in Radiomics. Cancers 2021, 13, 4710. [Google Scholar] [CrossRef]
- Itani, M.; Bresnahan, B.W.; Rice, K.; Gunn, M.L.; Wang, S.S.; Revels, J.; Mileto, A. Clinical and Payer-Based Analysis of Value of Dual-Energy Computed Tomography for Workup of Incidental Abdominal Findings. J. Comput. Assist. Tomogr. 2019, 43, 605–611. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Tirumani, S.H.; Lennartz, S.; Hokamp, N.G.; Gupta, A.; Pennig, L.; Persigehl, T.; Gilkeson, R.; Ramaiya, N. Evaluation of equivocal small cystic pancreatic lesions with spectral-detector computed tomography. Acta Radiol. 2020, 62, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kruis, M.F. Improving radiation physics, tumor visualisation, and treatment quantification in radiotherapy with spectral or dual-energy CT. J. Appl. Clin. Med. Phys. 2022, 23, e13468. [Google Scholar] [CrossRef] [PubMed]
- van Elmpt, W.; Landry, G.; Das, M.; Verhaegen, F. Dual energy CT in radiotherapy: Current applications and future outlook. Radiother. Oncol. 2016, 119, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, I.P.; Schyns, L.E.J.R.; Vaniqui, A.; van der Heyden, B.; Dedes, G.; Resch, A.F.; Kamp, F.; Zindler, J.D.; Parodi, K.; Landry, G.; et al. Monte Carlo proton dose calculations using a radiotherapy specific dual-energy CT scanner for tissue segmentation and range assessment. Phys. Med. Biol. 2018, 63, 115008. [Google Scholar] [CrossRef]
- Han, X.; An, W.; Cao, Q.; Liu, C.; Shang, S.; Zhao, L. Noninvasive evaluation of esophageal varices in cirrhotic patients based on spleen hemodynamics: A dual-energy CT study. Eur. Radiol. 2020, 30, 3210–3216. [Google Scholar] [CrossRef]
- Vlahos, I.; Jacobsen, M.C.; Godoy, M.C.; Stefanidis, K.; Layman, R.R. Dual-energy CT in pulmonary vascular disease. Br. J. Radiol. 2022, 95, 20210699. [Google Scholar] [CrossRef]
- Tsurusaki, M.; Sofue, K.; Hori, M.; Sasaki, K.; Ishii, K.; Murakami, T.; Kudo, M. Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications. Diagnostics 2021, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Lestra, T.; Mulé, S.; Millet, I.; Carsin-Vu, A.; Taourel, P.; Hoeffel, C. Applications of dual energy computed tomography in abdominal imaging. Diagn. Interv. Imaging 2016, 97, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Kaza, R.K.; Platt, J.F.; Cohan, R.H.; Caoili, E.M.; Al-Hawary, M.M.; Wasnik, A.P. Dual-Energy CT with Single- and Dual-Source Scanners: Current Applications in Evaluating the Genitourinary Tract. Radiographics 2012, 32, 353–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Jiang, B.; Wu, X.; Zhang, Z.; Chen, H.; Cai, H.; Fu, C. Diagnostic accuracy of dual-energy computed tomography angiography in the differentiation of benign and malignant pelvic masses. Eur. J. Radiol. 2022, 150, 110240. [Google Scholar] [CrossRef]
- Kim, C.; Kim, W.; Park, S.-J.; Lee, Y.H.; Hwang, S.H.; Yong, H.S.; Oh, Y.-W.; Kang, E.-Y.; Lee, K.Y. Application of Dual-Energy Spectral Computed Tomography to Thoracic Oncology Imaging. Korean J. Radiol. 2020, 21, 838–850. [Google Scholar] [CrossRef]
- De Santis, D.; Eid, M.; De Cecco, C.N.; Jacobs, B.E.; Albrecht, M.H.; Varga-Szemes, A.; Tesche, C.; Caruso, D.; Laghi, A.; Schoepf, U.J. Dual-Energy Computed Tomography in Cardiothoracic Vascular Imaging. Radiol. Clin. N. Am. 2018, 56, 521–534. [Google Scholar] [CrossRef]
- Wong, W.D.; Shah, S.; Murray, N.; Walstra, F.; Khosa, F.; Nicolaou, S. Advanced Musculoskeletal Applications of Dual-Energy Computed Tomography. Radiol. Clin. N. Am. 2018, 56, 587–600. [Google Scholar] [CrossRef]
- Liu, L.P.; Shapira, N.; Chen, A.A.; Shinohara, R.T.; Sahbaee, P.; Schnall, M.; Litt, H.I.; Noël, P.B. First-generation clinical dual-source photon-counting CT: Ultra-low-dose quantitative spectral imaging. Eur. Radiol. 2022, 32, 8579–8587. [Google Scholar] [CrossRef]
- Hagen, F.; Hofmann, J.; Wrazidlo, R.; Gutjahr, R.; Schmidt, B.; Faby, S.; Nikolaou, K.; Horger, M. Image quality and dose exposure of contrast-enhanced abdominal CT on a 1st generation clinical dual-source photon-counting detector CT in obese patients vs. a 2nd generation dual-source dual energy integrating detector CT. Eur. J. Radiol. 2022, 151, 110325. [Google Scholar] [CrossRef]
- Gutjahr, R.; Halaweish, A.F.; Yu, Z.; Leng, S.; Yu, L.; Li, Z.; Jorgensen, S.M.; Ritman, E.L.; Kappler, S.; McCollough, C.H. Human Imaging with Photon Counting–Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Investig. Radiol. 2016, 51, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Younis, M.H.; Wang, H.; Zhang, J.; Cai, W.; Ni, D. Spectral computed tomography with inorganic nanomaterials: State-of-the-art. Adv. Drug Deliv. Rev. 2022, 189, 114524. [Google Scholar] [CrossRef]












| Topic of Analysis Article | DECT Technique * | Scanner Specifications ** | Clinical Applications | Future Perspectives |
|---|---|---|---|---|
| [1] | X | X | X | |
| [2] | X | X | ||
| [3] | X | |||
| [4] | X | X | ||
| [5,6] | X | X | X | |
| [7] | X | X | X | |
| [8,9] | X | |||
| [10] | X | X | ||
| [11] | X | |||
| [12,13] | X | X | ||
| [14] | X | X | X | |
| [15] | X | X | ||
| [16] | X | X | X | |
| [17] | X | X | ||
| [18,19] | X | |||
| [20] | X | X | ||
| [21,22,23,24,25,26,27,28] | X | |||
| [29] | X | X | ||
| [30] | X | |||
| [31] | X | |||
| [32] | X | X | ||
| [33] | X | |||
| [34] | X | X | ||
| [35,36] | X | |||
| [37] | X | |||
| [38] | X | X | ||
| [39,40,41,42,43] | X | |||
| [44,45] | X | X | ||
| [46] | X | X | ||
| [47,48,49,50] | X | X | ||
| [51,52,53,54] | X | |||
| [55,56,57] | X | X | ||
| [58,59,60,61,62,63,64,65,66,67,68,69,70,71] | X | |||
| [72,73,74] | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, A.P.; Antunes, C.; Curvo-Semedo, L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography 2023, 9, 195-216. https://doi.org/10.3390/tomography9010017
Borges AP, Antunes C, Curvo-Semedo L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography. 2023; 9(1):195-216. https://doi.org/10.3390/tomography9010017
Chicago/Turabian StyleBorges, Ana P., Célia Antunes, and Luís Curvo-Semedo. 2023. "Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”" Tomography 9, no. 1: 195-216. https://doi.org/10.3390/tomography9010017
APA StyleBorges, A. P., Antunes, C., & Curvo-Semedo, L. (2023). Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography, 9(1), 195-216. https://doi.org/10.3390/tomography9010017






