Mind-Body Therapies for Cancer Patients Living with Depression, Anxiety or Insomnia (MIRACLE): A Systematic Review with Individual Participant Data Network Meta-Analysis
Abstract
:1. Background
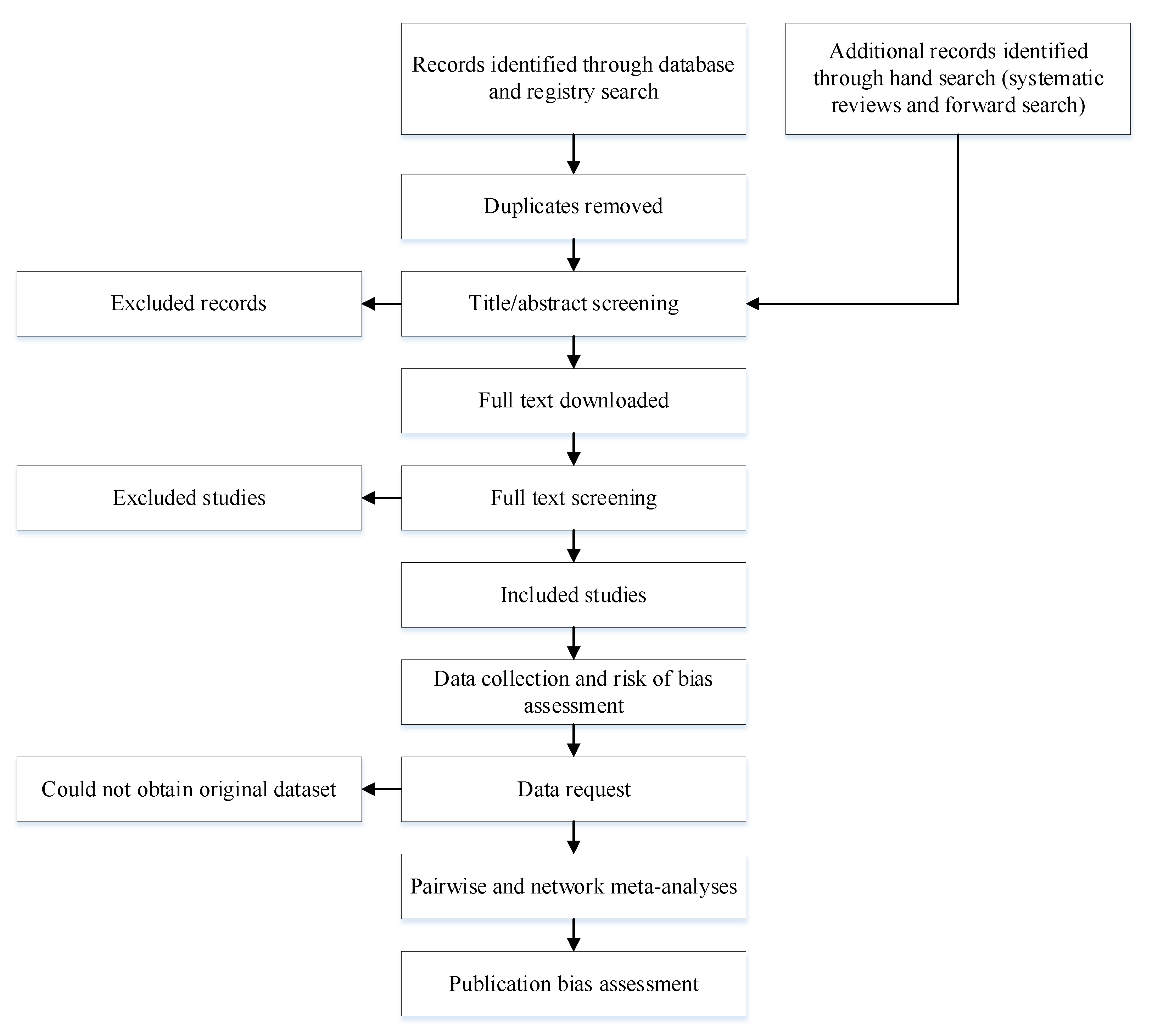
2. Methods
2.1. Study Selection
2.1.1. Study Design
2.1.2. Participants
2.1.3. Interventions
2.1.4. Controls
2.1.5. Outcomes
2.2. Search Strategy
2.3. Screening
2.4. Data Extraction
- List of authors and contact details
- Year of publication
- Treatment arms with sample size (intention-to-treat)
- Selection of participants according to insomnia, anxiety, or depression diagnosis and diagnostic tool
- Participant characteristics (gender, age, type and stage of cancer, type and stage of anticancer treatment)
- Intervention details (intervention name, treatment frequency and duration, session duration, mode of delivery, presence of home practice) for both experimental and control groups
- Outcome measures
- Outcome at baseline, during treatment, and at post-treatment
2.5. Data Request
- Participant covariates (gender, age, type and stage of cancer, type and stage of anti-cancer treatment, presence and type of psychotropic treatment)
- All relevant outcomes for any time point
- Adherence to treatment (number of sessions completed)
- Completion status, with the withdrawal time point and reason in the case of non-completion
2.6. Data Management and Preparation
2.7. Risk of Bias Assessment
2.8. Publication Bias Assessment
2.9. Heterogeneity and Consistency Assessment
2.10. Pairwise and Network Meta-Analyses
2.11. Subgroup and Sensitivity Analyses
- Cancer site
- Cancer stage, separating early stages (stages 0 and I) and late stages (stages II, III, and IV)
- Presence or absence of concomitant anti-cancer treatment
- Concomitant usage of psychotropic treatment
- Presence or absence of home practice
- Gender
- Only studies in which the overall risk of bias was low
- Studies in which the IPD was available and studies in which the IPD was not available (the aggregated mean from the reports will be compared)
- Studies in which mental disorders were diagnosed according to a recognized diagnostic standard
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- #1.
- “mind-body therapies”[MeSH Terms] OR “mind body therap*”[Title/Abstract] OR “yoga”[MeSH Terms] OR “qigong”[MeSH Terms] OR “tai ji”[MeSH Terms] OR “mindfulness”[MeSH Terms] OR “meditation”[MeSH Terms] OR “yoga”[Title/Abstract] OR “qigong”[Title/Abstract] OR “qi gong”[Title/Abstract] OR “taiji”[Title/Abstract] OR “taichi”[Title/Abstract] OR “tai ji”[Title/Abstract] OR “tai chi”[Title/Abstract] OR “mindfulness”[Title/Abstract] OR “relaxation”[Title/Abstract] OR “meditation”[Title/Abstract].
- #2.
- “neoplasms”[MeSH Terms] OR “cancer survivors”[MeSH Terms] OR “neoplasms”[Title/Abstract] OR “tumor”[Title/Abstract] OR “cancer”[Title/Abstract] OR “leukemia”[Title/Abstract] OR “myeloma”[Title/Abstract] OR “lymphoma”[Title/Abstract].
- #3.
- “sleep initiation and maintenance disorders”[MeSH Terms] OR “insomnia”[Title/Abstract] OR “sleeplessness”[Title/Abstract] OR “sleep disturbances”[Title/Abstract] OR “sleep”[Title/Abstract] OR “depressive disorder”[MeSH Terms] OR “depression”[MeSH Terms] OR “depressive disorder”[MeSH Terms] OR “depressive disorder, major”[MeSH Terms] OR “dysthymic disorder”[MeSH Terms] OR “adjustment disorders”[MeSH Terms] OR “depression”[Title/Abstract] OR “mood”[Title/Abstract] OR “anxiety”[MeSH Terms] OR “anxiety”[Title/Abstract] OR “anxiety disorders”[Title/Abstract] OR “phobic disorders”[MeSH Terms] OR “mental health”[MeSH Terms] OR “mental disorders”[MeSH Terms] OR “psycholog*”[Title/Abstract].
- #4.
- #1 AND #2 AND #3.
References
- Savard, J.; Ivers, H.; Villa, J.; Caplette-Gingras, A.; Morin, C.M. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J. Clin. Oncol. 2011, 29, 3580–3586. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Cao, X.-L.; Wang, S.-B.; Zhong, B.-L.; Zhang, L.; Ungvari, G.S.; Ng, C.H.; Li, L.; Chiu, H.F.; Lok, G.K.; Lu, J.-P. The prevalence of insomnia in the general population in China: A meta-analysis. PLoS ONE 2017, 12, e0170772. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; LeBlanc, M.; Bélanger, L.; Ivers, H.; Mérette, C.; Savard, J. Prevalence of insomnia and its treatment in Canada. Can. J. Psychiatry 2011, 56, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.J.; Scott, K.; Vos, T.; Whiteford, H. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897. [Google Scholar] [CrossRef]
- Schag, C.; Ganz, P.A.; Polinsky, M.L.; Fred, C.; Hirji, K.; Petersen, L. Characteristics of women at risk for psychosocial distress in the year after breast cancer. J. Clin. Oncol. 1993, 11, 783–793. [Google Scholar] [CrossRef]
- Fann, J.R.; Fan, M.-Y.; Unützer, J. Improving primary care for older adults with cancer and depression. J. Gen. Intern. Med. 2009, 24, 417–424. [Google Scholar] [CrossRef]
- Neckelmann, D.; Mykletun, A.; Dahl, A.A. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep 2007, 30, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.O.; Roth, T.; Breslau, N. The association of insomnia with anxiety disorders and depression: Exploration of the direction of risk. J. Psychiatr. Res. 2006, 40, 700–708. [Google Scholar] [CrossRef]
- Jansson-Fröjmark, M.; Lindblom, K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J. Psychosom. Res. 2008, 64, 443–449. [Google Scholar] [CrossRef]
- Redeker, N.S.; Lev, E.L.; Ruggiero, J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch. Inq. Nurs. Pract. 2000, 14, 275–290. [Google Scholar]
- Chen, M.-L.; Tseng, H.-C. Symptom clusters in cancer patients. Support. Care Cancer 2006, 14, 825–830. [Google Scholar] [CrossRef]
- Hoang, H.T.X.; Molassiotis, A.; Chan, C.W.; Nguyen, T.H. New-onset insomnia among cancer patients undergoing chemotherapy: Prevalence, risk factors, and its correlation with other symptoms. Sleep Breath. 2020, 24, 241–251. [Google Scholar] [CrossRef]
- Dong, S.T.; Costa, D.S.; Butow, P.N.; Lovell, M.R.; Agar, M.; Velikova, G.; Teckle, P.; Tong, A.; Tebbutt, N.C.; Clarke, S.J. Symptom clusters in advanced cancer patients: An empirical comparison of statistical methods and the impact on quality of life. J. Pain Symptom Manag. 2016, 51, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, Ó.; Angulo, L.P.; Núñez-Valencia, C.; Dorantes-Gallareta, Y.; Macedo, E.O.; Martínez-López, D.; Alvarado, S.; Corona-Cruz, J.-F.; Oñate-Ocaña, L.F. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann. Surg. Oncol. 2013, 20, 1941–1948. [Google Scholar] [CrossRef]
- Mausbach, B.T.; Schwab, R.B.; Irwin, S.A. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2015, 152, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiMatteo, M.R.; Haskard-Zolnierek, K.B. Impact of depression on treatment adherence and survival from cancer. Depress. Cancer 2011, 101–124. [Google Scholar]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Duberstein, P. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Li, J.-Q.; Shi, J.-F.; Que, J.-Y.; Liu, J.-J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.-Q.; Qiao, Y.-L. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Kissane, D.W.; Grabsch, B.; Clarke, D.M.; Christie, G.; Clifton, D.; Gold, S.; Hill, C.; Morgan, A.; McDermott, F.; Smith, G.C. Supportive-expressive group therapy: The transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psycho-Oncol. 2004, 13, 755–768. [Google Scholar] [CrossRef]
- Kissane, D. Beyond the psychotherapy and survival debate: The challenge of social disparity, depression and treatment adherence in psychosocial cancer care. Psycho-Oncol. 2009, 18, 1–5. [Google Scholar] [CrossRef]
- Zingone, A.; Brown, D.; Bowman, E.D.; Vidal, O.M.; Sage, J.; Neal, J.; Ryan, B.M. Relationship between anti-depressant use and lung cancer survival. Cancer Treat. Res. Commun. 2017, 10, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.G.; Boks, M.P.; Smeets, H.M.; Zainal, N.Z.; de Wit, N.J. Prescription patterns for psychotropic drugs in cancer patients; a large population study in the Netherlands. Psycho-Oncol. 2013, 22, 762–767. [Google Scholar] [CrossRef]
- Stiefel, F.C.; Kornblith, A.B.; Holland, J.C. Changes in the prescription patterns of psychotropic drugs for cancer patients during a 10-year period. Cancer 1990, 65, 1048–1053. [Google Scholar] [CrossRef]
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D. Management of chronic insomnia disorder in adults: A clinical practice guideline from the american college of physicians. Ann. Intern. Med. 2016, 165, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.K.; Roth, T. Pharmacologic treatment of insomnia: Benzodiazepine receptor agonists. In Principles and Practices of Sleep Medicine, 5th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Saunders: Glendenning, Australia, 2011; pp. 905–915. [Google Scholar]
- Glass, J.; Lanctot, K.L.; Herrmann, N.; Sproule, B.A.; Busto, U.E. Sedative hypnotics in older people with insomnia: Meta-analysis of risks and benefits. BMJ 2005, 331, 1169. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J. Insomnia. JAMA 2013, 309, 706–716. [Google Scholar] [CrossRef] [Green Version]
- Licata, S.C.; Rowlett, J.K. Abuse and dependence liability of benzodiazepine-type drugs: GABA A receptor modulation and beyond. Pharmacol. Biochem. Behav. 2008, 90, 74–89. [Google Scholar] [CrossRef] [Green Version]
- Predictable, S. Side effects of antidepressants: An overview. Cleveland Clin. J. Med. 2006, 73, 351. [Google Scholar]
- Lovato, N.; Lack, L. Insomnia and mortality: A meta-analysis. Sleep Med. Rev. 2019, 43, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Wernli, K.J.; Hampton, J.M.; Trentham-Dietz, A.; Newcomb, P.A. Use of antidepressants and NSAIDs in relation to mortality in long-term breast cancer survivors. Pharmacoepidemiol. Drug Saf. 2011, 20, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Sundquist, J.; Ekedahl, A.; Johansson, S.-E. Sales of tranquillizers, hypnotics/sedatives and antidepressants and their relationship with underprivileged area score and mortality and suicide rates. Eur. J. Clin. Pharmacol. 1996, 51, 105–109. [Google Scholar] [CrossRef]
- Chen, P.-L.; Lee, W.-J.; Sun, W.-Z.; Oyang, Y.-J.; Fuh, J.-L. Risk of dementia in patients with insomnia and long-term use of hypnotics: A population-based retrospective cohort study. PLoS ONE 2012, 7, e49113. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jung, S.J.; Choi, J.-w.; Shin, A.; Lee, Y.J. Use of sedative-hypnotics and the risk of Alzheimer’s dementia: A retrospective cohort study. PLoS ONE 2018, 13, e0204413. [Google Scholar] [CrossRef]
- Andersohn, F.; Schade, R.; Suissa, S.; Garbe, E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am. J. Psychiatry 2009, 166, 591–598. [Google Scholar] [CrossRef]
- Kripke, D.F.; Langer, R.D.; Kline, L.E. Hypnotics’ association with mortality or cancer: A matched cohort study. BMJ Open 2012, 2, e000850. [Google Scholar] [CrossRef] [Green Version]
- Vincent, N.; Lionberg, C. Treatment preference and patient satisfaction in chronic insomnia. Sleep 2001, 24, 411. [Google Scholar] [CrossRef] [Green Version]
- Morin, C.M.; Gaulier, B.; Barry, T.; Kowatch, R.A. Patients’ acceptance of psychological and pharmacological therapies for insomnia. Sleep 1992, 15, 302. [Google Scholar] [CrossRef] [Green Version]
- Mehling, W.E.; Wrubel, J.; Daubenmier, J.J.; Price, C.J.; Kerr, C.E.; Silow, T.; Gopisetty, V.; Stewart, A.L. Body Awareness: A phenomenological inquiry into the common ground of mind-body therapies. Philos. Ethics Humanit. Med. 2011, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Morgan, N.; Irwin, M.R.; Chung, M.; Wang, C. The effects of mind-body therapies on the immune system: Meta-analysis. PLoS ONE 2014, 9, e100903. [Google Scholar] [CrossRef] [Green Version]
- Balasubramaniam, M.; Telles, S.; Doraiswamy, P.M. Yoga on our minds: A systematic review of yoga for neuropsychiatric disorders. Front. Psychiatry 2013, 3, 117. [Google Scholar] [CrossRef] [Green Version]
- Cramer, H.; Lauche, R.; Langhorst, J.; Dobos, G. Yoga for depression: A systematic review and meta-analysis. Depress. Anxiety 2013, 30, 1068–1083. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Sawyer, A.T.; Witt, A.A.; Oh, D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J. Consult. Clin. Psychol. 2010, 78, 169. [Google Scholar] [CrossRef]
- Garland, E.L.; Brintz, C.E.; Hanley, A.W.; Roseen, E.J.; Atchley, R.M.; Gaylord, S.A.; Faurot, K.R.; Yaffe, J.; Fiander, M.; Keefe, F.J. Mind-body therapies for opioid-treated pain: A systematic review and meta-analysis. JAMA Intern. Med. 2020, 180, 91–105. [Google Scholar] [CrossRef]
- Cherkin, D.C.; Herman, P.M. Cognitive and mind-body therapies for chronic low back pain and neck pain: Effectiveness and value. JAMA Intern. Med. 2018, 178, 556–557. [Google Scholar] [CrossRef]
- Buffart, L.M.; van Uffelen, J.G.; Riphagen, I.I.; Brug, J.; van Mechelen, W.; Brown, W.J.; Chinapaw, M.J. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2012, 12, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, L.E. Distress management through mind-body therapies in oncology. JNCI Monogr. 2017, 52, 37–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, G.; Lockwood, S. Effect of yoga on sleep quality among adult cancer patients: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2014, 12, 382–419. [Google Scholar] [CrossRef] [Green Version]
- Kreutz, C.; Schmidt, M.E.; Steindorf, K. Effects of physical and mind–body exercise on sleep problems during and after breast cancer treatment: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 1–15. [Google Scholar] [CrossRef]
- So, W.K.; Law, B.M.; Chan, D.N.; Xing, W.; Chan, C.W.; McCarthy, A.L. The Effect of Nonpharmacological Interventions on Managing Symptom Clusters Among Cancer Patients: A Systematic Review. Cancer Nurs. 2019, 43, E304–E327. [Google Scholar] [CrossRef]
- Carlson, L.E.; Zelinski, E.; Toivonen, K.; Flynn, M.; Qureshi, M.; Piedalue, K.-A.; Grant, R. Mind-body therapies in cancer: What is the latest evidence? Curr. Oncol. Rep. 2017, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Piet, J.; Würtzen, H.; Zachariae, R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: A systematic review and meta-analysis. J. Consult. Clin. Psychol. 2012, 80, 1007. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Luo, T.; Xie, H.; Huang, M.; Cheng, A.S. Health benefits of qigong or tai chi for cancer patients: A systematic review and meta-analyses. Complement. Ther. Med. 2014, 22, 173–186. [Google Scholar] [CrossRef]
- Andersen, S.R.; Würtzen, H.; Steding-Jessen, M.; Christensen, J.; Andersen, K.K.; Flyger, H.; Mitchelmore, C.; Johansen, C.; Dalton, S.O. Effect of mindfulness-based stress reduction on sleep quality: Results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013, 52, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Tierney, J.F.; Stewart, L.A.; Clarke, M. Cochrane Individual Participant Data Meta-analysis Methods Group. Individual participant data. Cochrane Handb. Syst. Rev. Interv. 2019, 643–658. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Arroll, B.; Goodyear-Smith, F.; Crengle, S.; Gunn, J.; Kerse, N.; Fishman, T.; Falloon, K.; Hatcher, S. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann. Fam. Med. 2010, 8, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Vilagut, G.; Forero, C.G.; Barbaglia, G.; Alonso, J. Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): A systematic review with meta-analysis. PLoS ONE 2016, 11, e0155431. [Google Scholar] [CrossRef]
- Warmenhoven, F.; van Rijswijk, E.; Engels, Y.; Kan, C.; Prins, J.; Van Weel, C.; Vissers, K. The Beck Depression Inventory (BDI-II) and a single screening question as screening tools for depressive disorder in Dutch advanced cancer patients. Support. Care Cancer 2012, 20, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Aben, I.; Verhey, F.; Lousberg, R.; Lodder, J.; Honig, A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics 2002, 43, 386–393. [Google Scholar] [CrossRef]
- Wilkins, J.W.; Hamby, S.L.; Robertson, K.R.; Knorr, K.L.; Barry, N.S.; Hall, C.D. The Profile of Mood States as a screening test for major depression in HIV+ patients. Assessment 1995, 2, 181–188. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroenke, K.; Yu, Z.; Wu, J.; Kean, J.; Monahan, P.O. Operating characteristics of PROMIS four-item depression and anxiety scales in primary care patients with chronic pain. Pain Med. 2014, 15, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Dahm, J.; Wong, D.; Ponsford, J. Validity of the Depression Anxiety Stress Scales in assessing depression and anxiety following traumatic brain injury. J. Affect. Disord. 2013, 151, 392–396. [Google Scholar] [CrossRef]
- Sagen, U.; Vik, T.G.; Moum, T.; Mørland, T.; Finset, A.; Dammen, T. Screening for anxiety and depression after stroke: Comparison of the Hospital Anxiety and Depression Scale and the Montgomery and Åsberg Depression Rating Scale. J. Psychosom. Res. 2009, 67, 325–332. [Google Scholar] [CrossRef]
- Beck, A.; Steer, R.; Brown, G. BDI-II, Beck Depression Inventory: Manual, 2nd ed.; Harcourt, Brace, and Company: Boston, MA, USA, 1996. [Google Scholar]
- Plummer, F.; Manea, L.; Trepel, D.; McMillan, D. Screening for anxiety disorders with the GAD-7 and GAD-2: A systematic review and diagnostic metaanalysis. Gen. Hosp. Psychiatry 2016, 39, 24–31. [Google Scholar] [CrossRef]
- Kummer, A.; Cardoso, F.; Teixeira, A.L. Generalized anxiety disorder and the Hamilton Anxiety Rating Scale in Parkinson’s disease. Arq. Neuropsiquiatr. 2010, 68, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.; Carter, O.; Adams, C.; Waterer, G.; Chung, L.P.; Hawkins, M.; Rudd, C.; Ziman, M.; Strobel, N. Discriminant validity of the Hospital Anxiety and Depression Scale, Beck Depression Inventory (II) and Beck Anxiety Inventory to confirmed clinical diagnosis of depression and anxiety in patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 2016, 13, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the Stait-Trait Anxiety Inventory; Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Higginson, C.I.; Fields, J.A.; Koller, W.C.; Tröster, A.I. Questionnaire assessment potentially overestimates anxiety in Parkinson’s disease. J. Clin. Psychol. Med. Settings 2001, 8, 95–99. [Google Scholar] [CrossRef]
- Behar, E.; Alcaine, O.; Zuellig, A.R.; Borkovec, T. Screening for generalized anxiety disorder using the Penn State Worry Questionnaire: A receiver operating characteristic analysis. J. Behav. Ther. Exp. Psychiatry 2003, 34, 25–43. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. The diagnostic validity of the Athens Insomnia Scale. J. Psychosom. Res. 2003, 55, 263–267. [Google Scholar] [CrossRef]
- Spencer, J.W.; Jacobs, J.J.M.D. Complementary and Alternative Medicine: An Evidence-Based Approach, 2nd ed.; Mosby: St. Louis, MO, USA, 2003. [Google Scholar]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Chaimani, A.; Higgins, J.P.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Stuck, A.E.; Rubenstein, L.Z.; Wieland, D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998, 316, 469. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, D.M. An overview of conducting systematic reviews with network meta-analysis. Syst. Rev. 2014, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birling, Y.; Nevitt, S.; Bhuyan, D.J.; Jia, M.; Feng, F.; Carlson, L.E.; Pham, T.; Liu, J.; Ayati, Z.; Nyiam, L.; et al. Mind-Body Therapies for Cancer Patients Living with Depression, Anxiety or Insomnia (MIRACLE): A Systematic Review with Individual Participant Data Network Meta-Analysis. Methods Protoc. 2021, 4, 76. https://doi.org/10.3390/mps4040076
Birling Y, Nevitt S, Bhuyan DJ, Jia M, Feng F, Carlson LE, Pham T, Liu J, Ayati Z, Nyiam L, et al. Mind-Body Therapies for Cancer Patients Living with Depression, Anxiety or Insomnia (MIRACLE): A Systematic Review with Individual Participant Data Network Meta-Analysis. Methods and Protocols. 2021; 4(4):76. https://doi.org/10.3390/mps4040076
Chicago/Turabian StyleBirling, Yoann, Sarah Nevitt, Deep Jyoti Bhuyan, Mingxian Jia, Fan Feng, Linda Ellen Carlson, Tiffany Pham, Jing Liu, Zahra Ayati, Liyi Nyiam, and et al. 2021. "Mind-Body Therapies for Cancer Patients Living with Depression, Anxiety or Insomnia (MIRACLE): A Systematic Review with Individual Participant Data Network Meta-Analysis" Methods and Protocols 4, no. 4: 76. https://doi.org/10.3390/mps4040076
APA StyleBirling, Y., Nevitt, S., Bhuyan, D. J., Jia, M., Feng, F., Carlson, L. E., Pham, T., Liu, J., Ayati, Z., Nyiam, L., Yu, Z., & Fahey, P. (2021). Mind-Body Therapies for Cancer Patients Living with Depression, Anxiety or Insomnia (MIRACLE): A Systematic Review with Individual Participant Data Network Meta-Analysis. Methods and Protocols, 4(4), 76. https://doi.org/10.3390/mps4040076







