In Vitro Methods for Measuring the Permeability of Cell Monolayers
Abstract
:1. Introduction
2. Overview of Permeability Assays
2.1. Macromolecular Tracer Assays
2.2. Resistance-Based Assays
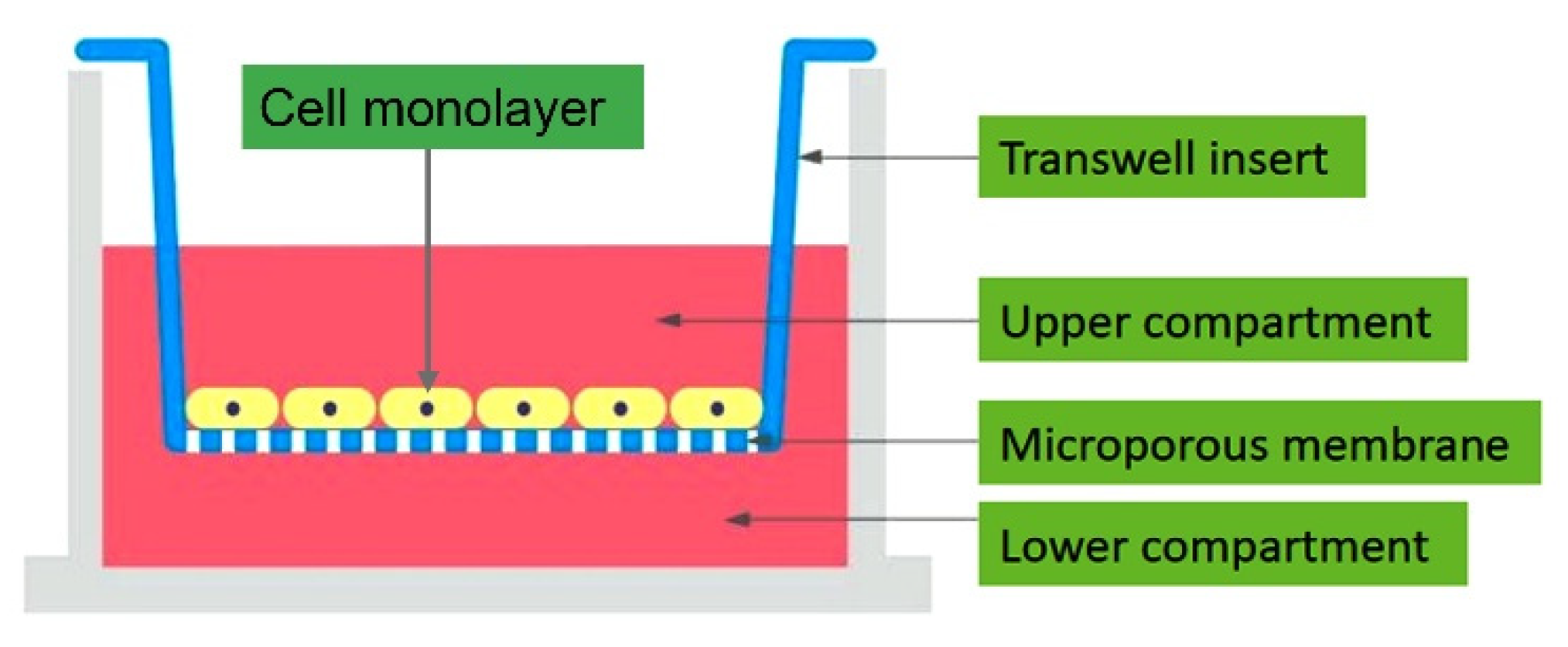
2.2.1. Transepithelial/Endothelial Electrical Resistance (TEER) Measurements
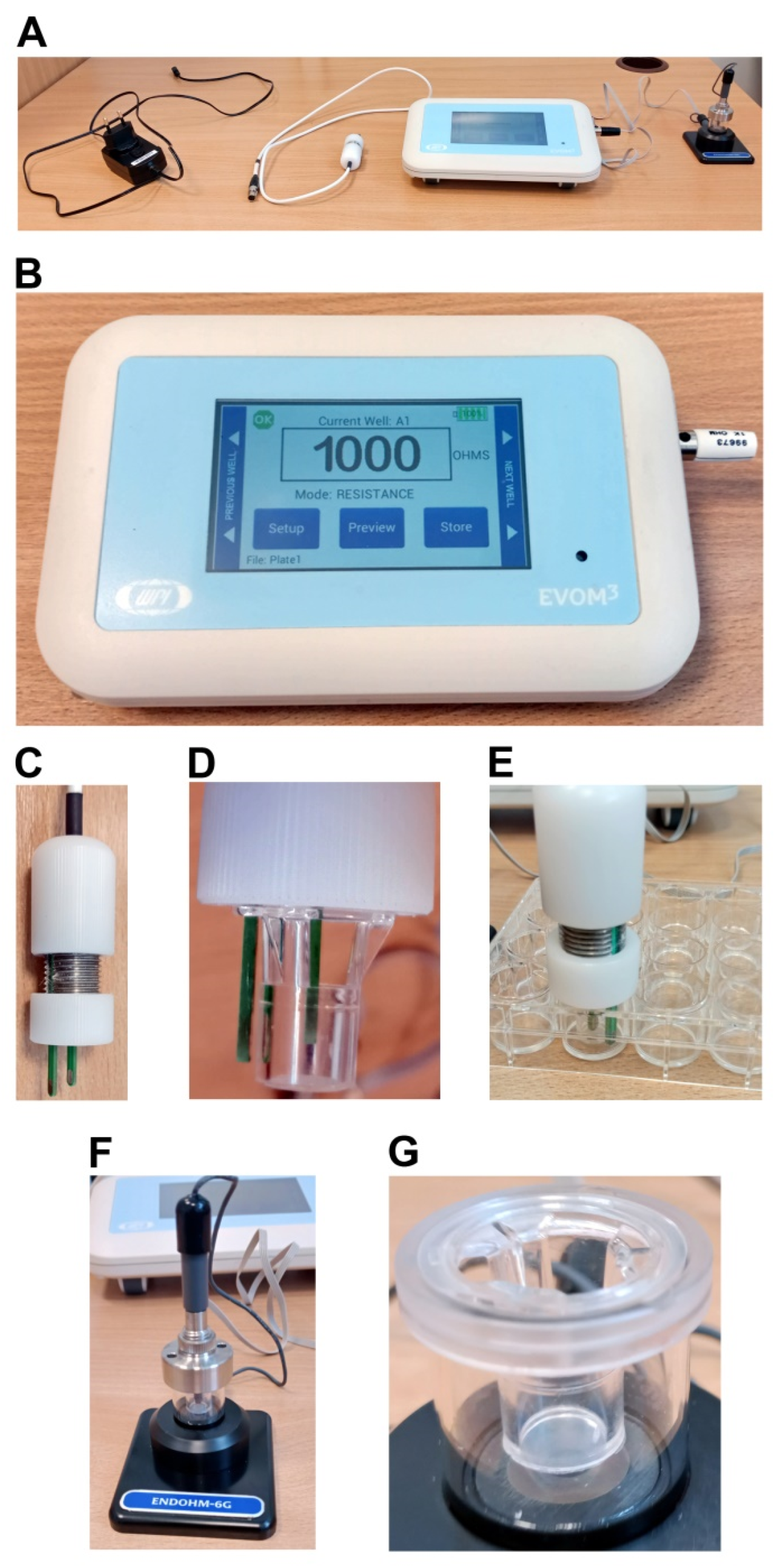
Epithelial Volt/Ohm Meter (EVOM)
2.2.2. Real-Time Cell Electrical Impedance Sensing
Ussing Chamber
ACEA xCELLigence® Real-Time Cell Analysis (RTCA)
ECIS
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mullin, J.M.; Agostino, N.; Rendon-Huerta, E.; Thornton, J.J. Keynote review: Epithelial and endothelial barriers in human disease. Drug Discov. Today 2005, 10, 395–408. [Google Scholar] [CrossRef]
- Bischoff, I.; Hornburger, M.C.; Mayer, B.A.; Beyerle, A.; Wegener, J.; Fürst, R. Pitfalls in assessing microvascular endothelial barrier function: Impedance-based devices versus the classic macromolecular tracer assay. Sci. Rep. 2016, 6, 23671. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Urbani, S.; Bradfield, P.F.; Imhof, B.A. Tight junction dynamics: The role of junctional adhesion molecules (JAMs). Cell Tissue Res. 2014, 355, 701–715. [Google Scholar] [CrossRef] [Green Version]
- Kornecki, E.; Walkowiak, B.; Naik, U.P.; Ehrlich, Y.H. Activation of human platelets by a stimulatory monoclonal antibody. J. Biol. Chem. 1990, 265, 10042–10048. [Google Scholar] [CrossRef]
- Martìn-Padura, I.; Lostaglio, S.; Schneemann, M.; Williams, L.; Romano, M.; Fruscella, P.; Panzeri, C.; Stoppacciaro, A.; Ruco, L.; Villa, A.; et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998, 142, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Bednarek, R.; Selmi, A.; Wojkowska, D.; Karolczak, K.; Popielarski, M.; Stasiak, M.; Salifu, M.O.; Babinska, A.; Swiatkowska, M. Functional inhibition of F11 receptor (F11R/junctional adhesion molecule-A/JAM-A) activity by a F11R-derived peptide in breast cancer and its microenvironment. Breast Cancer Res. Treat. 2020, 179, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Harhaj, N.S.; Antonetti, D.A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef]
- Frost, T.S.; Jiang, L.; Lynch, R.M.; Zohar, Y. Permeability of epithelial/endothelial barriers in transwells and microfluidic bilayer devices. Micromachines 2019, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Wegener, J.; Seebach, J. Experimental tools to monitor the dynamics of endothelial barrier function: A survey of in vitro approaches. Cell Tissue Res. 2014, 355, 485–514. [Google Scholar] [CrossRef]
- Robinson, B.D.; Shaji, C.A.; Lomas, A.; Tharakan, B. Measurement of Microvascular Endothelial Barrier Dysfunction and Hyperpermeability In Vitro. Methods Mol. Biol. 2018, 1717, 237–242. [Google Scholar] [CrossRef]
- Martins-Green, M.; Petreaca, M.; Yao, M. Chapter 8 An Assay System for In Vitro Detection of Permeability in Human “Endothelium”. Methods Enzymol. 2008, 443, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, W.; Bogner, E.; Wirth, M.; Trzeciak, J.; Lachmann, B.; Gabor, F.; Noe, C.R. A novel tool to characterize paracellular transport: The APTS-dextran ladder. Pharm. Res. 2006, 23, 1491–1501. [Google Scholar] [CrossRef]
- Turksen, K. Permeability barrier: Methods and protocols. Humana Press. Encycl. Ref. Genom. Proteom. Mol. Med. 2011, 763, 138–154. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Measuring Size-Dependent Permeability of the Tight Junction Using PEG Profiling. In Claudins; Humana Press: Totowa, NJ, USA, 2011; Volume 762, ISBN 9781617791840. [Google Scholar] [CrossRef]
- Bowman, P.D.; Ennis, S.R.; Rarey, K.E.; Lorris Betz, A.; Goldstein, G.W. Brain microvessel endothelial cells in tissue culture: A model for study of blood-brain barrier permeability. Ann. Neurol. 1983, 14, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.A.; Del Vecchio, P.J.; Minnear, F.L.; Burhop, K.E.; Selig, W.M.; Garcia, J.G.; Malik, A.B. Measurement of albumin permeability across endothelial monolayers in vitro. J. Appl. Physiol. 1987, 62, 1076–1083. [Google Scholar] [CrossRef]
- Kazakoff, P.W.; McGuire, T.R.; Hoie, E.B.; Cano, M.; Iversen, P.L. An in vitro model for endothelial permeability: Assessment of monolayer integrity. Vitr. Cell. Dev. Biol.-Anim. J. Soc. Vitr. Biol. 1995, 31, 846–852. [Google Scholar] [CrossRef]
- Deitch, E.A.; Adams, C.; Lu, Q.; Xu, D.Z. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery 2001, 129, 39–47. [Google Scholar] [CrossRef]
- Chandra, A.; Barillas, S.; Suliman, A.; Angle, N. A novel fluorescence-based cellular permeability assay. J. Biochem. Biophys. Methods 2007, 70, 329–333. [Google Scholar] [CrossRef]
- Konishi, Y. Modulations of food-derived substances on intestinal permeability in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2297–2299. [Google Scholar] [CrossRef]
- Duffy, S.L.; Murphy, J.T. Colorimetric Assay to Quantify Macromolecule Diffusion across Endothelial Monolayers. Biotechniques 2001, 31, 495–496. [Google Scholar] [CrossRef] [Green Version]
- Ghim, M.; Alpresa, P.; Yang, S.W.; Braakman, S.T.; Gray, S.G.; Sherwin, S.J.; Van Reeuwijk, M.; Weinberg, P.D. Visualization of three pathways for macromolecule transport across cultured endothelium and their modification by flow. Am. J. Physiol.-Hear. Circ. Physiol. 2017, 313, H959–H973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simionescu, M.; Gafencu, A.; Antohe, F. Transcytosis of plasma macromolecules in endothelial cells: A cell biological survey. Microsc. Res. Tech. 2002, 57, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Rattner, D.W.; Ito, S.; Rutten, M.J.; Silen, W. A rapid method for culturing guinea pig gastric mucous cell monolayers. Vitr. Cell. Dev. Biol. 1985, 21, 453–462. [Google Scholar] [CrossRef]
- Audus, K.L.; Bartel, R.L.; Hidalgo, I.J.; Borchardt, R.T. The Use of Cultured Epithelial and Endothelial Cells for Drug Transport and Metabolism Studies. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1990, 7, 435–451. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Günzel, D.; Krug, S.M.; Rosenthal, R.; Fromm, M. Biophysical Methods to Study Tight Junction Permeability. Curr. Top. Membr. 2010, 65, 41–76. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The impact of food bioactives on health: In vitro and Ex Vivo models. Impact Food Bioact. Heal. Vitr. Ex Vivo Model. 2015, 1–327. [Google Scholar] [CrossRef]
- Li, H.; Sheppard, D.N.; Hug, M.J. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros. 2004, 3, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Stevens, L.J.; van Lipzig, M.M.H.; Erpelinck, S.L.A.; Pronk, A.; van Gorp, J.; Wortelboer, H.M.; van de Steeg, E. A higher throughput and physiologically relevant two-compartmental human ex vivo intestinal tissue system for studying gastrointestinal processes. Eur. J. Pharm. Sci. 2019, 137, 104989. [Google Scholar] [CrossRef]
- Lestari, F.; Hayes, A.J.; Green, A.R.; Chattopadhyay, G. In vitro cytotoxicity and morphological assessment of smoke from polymer combustion in human lung derived cells (A549). Int. J. Hyg. Environ. Health 2012, 215, 320–332. [Google Scholar] [CrossRef]
- Barrett, K.E. Epithelial transport in digestive diseases: Mice, monolayers, and mechanisms. Am. J. Physiol.-Cell Physiol. 2020, 318, C1136–C1143. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Ghiboub, M.; Donkers, J.M.; van de Steeg, E.; van Tol, E.A.F.; Hakvoort, T.B.M.; de Jonge, W.J. The progress of intestinal epithelial models from cell lines to gut-on-chip. Int. J. Mol. Sci. 2021, 22, 13472. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, C.A.; Yadav, S.; Bratcher, P.E.; Zeitlin, P.L. Receptor-mediated activation of CFTR via prostaglandin signaling pathways in the airway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Fu, H.; Cheng, H.; Cao, Q.; Zhao, Y.; Mou, X.; Zhang, X.; Liu, X.; Ke, Y. A dynamic real-time method for monitoring epithelial barrier function in vitro. Anal. Biochem. 2012, 425, 96–103. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Chen, R.; Xu, L.; Sun, X.; Yu, M.; Sun, G. Evaluation of drug myocardial toxicity and biological activity by real time xCELLigence analysis: A review. Sheng Wu Gong Cheng Xue Bao 2021, 37, 2425–2434. [Google Scholar] [CrossRef]
- Yah, C.S.; Simate, G.S. Engineered nanoparticle bio-conjugates toxicity screening: The xCELLigence cells viability impact. Bioimpacts 2020, 10, 195–203. [Google Scholar] [CrossRef]
- Morgan, K.; Gamal, W.; Samuel, K.; Morley, S.D.; Hayes, P.C.; Bagnaninchi, P.; Plevris, J.N. Application of Impedance-Based Techniques in Hepatology Research. J. Clin. Med. 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Du, Q.; Wei, X.; Miozzi, J.; Kang, C.; Wang, J.; Han, X.; Pan, J.; Xie, H.; Chen, J.; et al. Application of real-time cell electronic analysis system in modern pharmaceutical evaluation and analysis. Molecules 2018, 23, 3280. [Google Scholar] [CrossRef] [Green Version]
- Iwakura, T.; Marschner, J.A.; Zhao, Z.B.; Świderska, M.K.; Anders, H.-J. Electric cell-substrate impedance sensing in kidney research. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2021, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hedayatipour, A.; Aslanzadeh, S.; McFarlane, N. CMOS based whole cell impedance sensing: Challenges and future outlook. Biosens. Bioelectron. 2019, 143, 111600. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, I.; Toda, M.; Inomata, N.; Ono, T.; Li, F. Nano and microsensors for mammalian cell studies. Micromachines 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarek, R. In Vitro Methods for Measuring the Permeability of Cell Monolayers. Methods Protoc. 2022, 5, 17. https://doi.org/10.3390/mps5010017
Bednarek R. In Vitro Methods for Measuring the Permeability of Cell Monolayers. Methods and Protocols. 2022; 5(1):17. https://doi.org/10.3390/mps5010017
Chicago/Turabian StyleBednarek, Radoslaw. 2022. "In Vitro Methods for Measuring the Permeability of Cell Monolayers" Methods and Protocols 5, no. 1: 17. https://doi.org/10.3390/mps5010017
APA StyleBednarek, R. (2022). In Vitro Methods for Measuring the Permeability of Cell Monolayers. Methods and Protocols, 5(1), 17. https://doi.org/10.3390/mps5010017





