An Efficient, Simultaneous Electrochemical Assay of Rosuvastatin and Ezetimibe from Human Urine and Serum Samples
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
2.2. Equipment
3. Procedures
3.1. Standards and Sample Preparation
3.2. Biological Sample Preparation
4. Results and Discussion
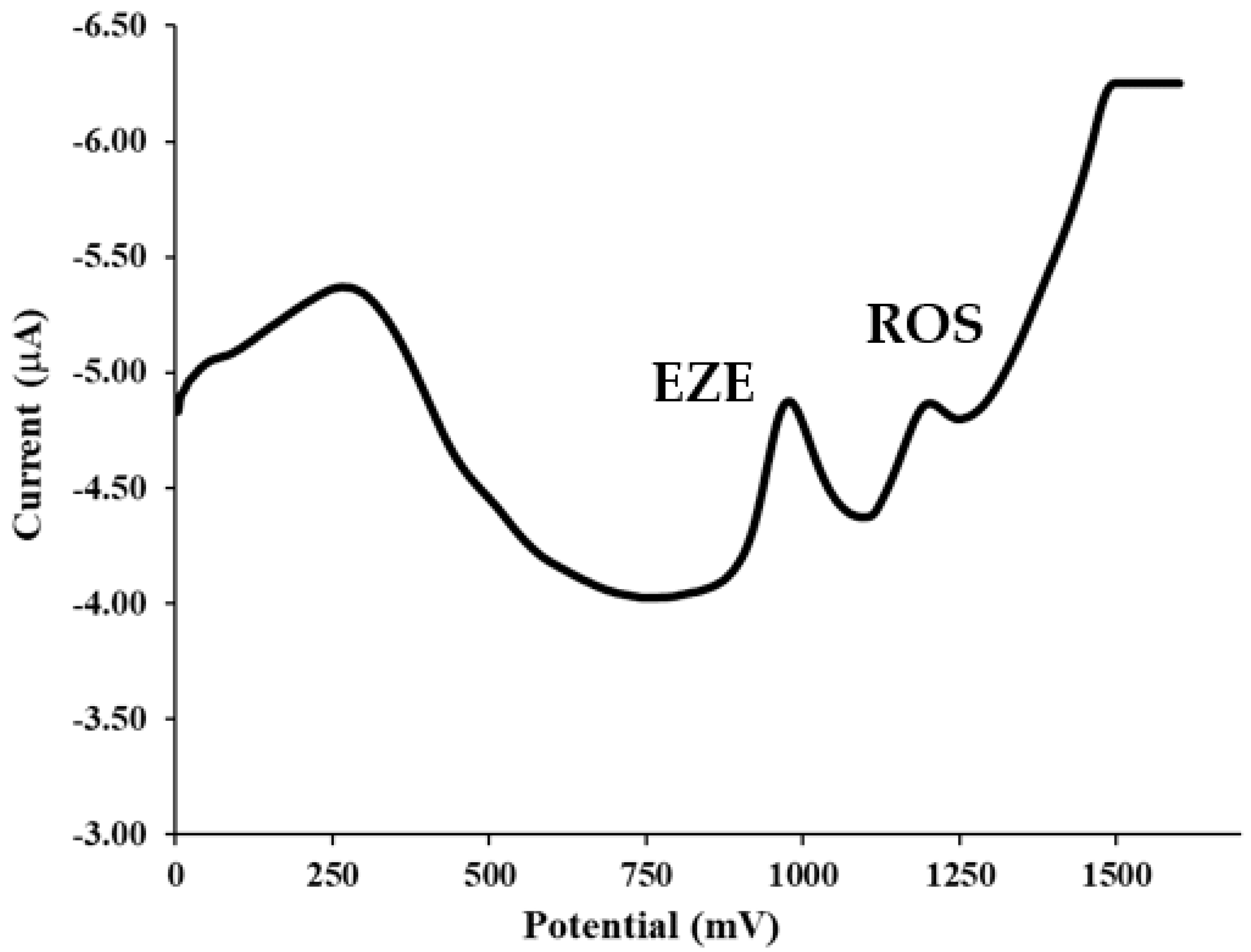
4.1. Voltammetric Behavior of ROS and EZE
4.2. Influence of the pH
4.3. Influence of the Scan Rate
4.4. Effect of Deposition Time and Potential
4.5. Analytical Characterization and Validation
4.6. Determination of Ezetimibe and Rosuvastatin in Biological Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, M.J.; McTaggart, F. Optimizing the pharmacology of statins: Characteristics of rosuvastatin. Atheroscler. Suppl. 2002, 2, 33–37. [Google Scholar] [CrossRef]
- Scott, L.J.; Curran, M.P.; Figgitt, D.P. Rosuvastatin: A review of its use in the management of dyslipidemia. Am. J. Cardiovasc. Drugs 2004, 4, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Jiang, X.; Wang, L. Pharmacokinetics and tolerability of multiple-dose rosuvastatin: An open-label, randomized-sequence, three-way crossover trial in healthy Chinese volunteers. Curr. Ther. Res.-Clin. Exp. 2009, 70, 392–404. [Google Scholar] [PubMed]
- Kosoglou, T.; Statkevich, P.; Johnson-Levonas, A.O.; Paolini, J.F.; Bergman, A.J.; Alton, K.B. Ezetimibe: A review of its metabolism, pharmacokinetics and drug interactions. Clin. Pharmacokinet. 2005, 44, 467–494. [Google Scholar] [CrossRef] [PubMed]
- Phan, B.A.P.; Dayspring, T.D.; Toth, P.P. Ezetimibe therapy: Mechanism of action and clinical update. Vasc. Health Risk Manag. 2012, 8, 415–427. [Google Scholar]
- Nakajima, N.; Miyauchi, K.; Yokoyama, T.; Ogita, M.; Miyazaki, T.; Tamura, H.; Nishino, A.; Yokoyama, K.; Okazaki, S.; Kurata, T.; et al. Effect of combination of ezetimibe and a statin on coronary plaque regression in patients with acute coronary syndrome: ZEUS trial (eZEtimibe Ultrasound Study). IJC Metab. Endocr. 2014, 3, 8–13. [Google Scholar] [CrossRef]
- Bays, H.E.; Davidson, M.; Massaad, R.; Flaim, D.; Lowe, R.; Tershakovec, A.; Jones-Burton, C. Efficacy and Safety of Ezetimibe Plus Rosuvastatin Versus Rosuvastatin Up-Titration in Hypercholesterolemic Patients at Risk for Atherosclerotic Coronary Heart Disease. J. Clin. Lipidol. 2011, 5, 217–218. [Google Scholar] [CrossRef]
- Nissen, S.E.; Nicholls, S.J.; Sipahi, I.; Libby, P.; Raichlen, J.S.; Ballantyne, C.M.; Davignon, J.; Erbel, R.; Fruchart, J.C.; Tardif, J.-C.; et al. Effect of Very High-Intensity Statin Therapy on Regression of Coronary Atherosclerosis. JAMA 2006, 295, 1556. [Google Scholar] [CrossRef]
- DeGorter, M.K.; Tirona, R.G.; Schwarz, U.I.; Choi, Y.H.; Dresser, G.K.; Suskin, N.; Myers, K.; Zou, G.Y.; Iwuchukwu, O.; Wei, W.Q.; et al. Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ. Cardiovasc. Genet. 2013, 6, 400–408. [Google Scholar] [CrossRef]
- Patel, J.; Sheehan, V.; Gurk-Turner, C. Ezetimibe (Zetia): A New Type of Lipid-Lowering Agent. Baylor Univ. Med. Cent. Proc. 2003, 16, 354–358. [Google Scholar] [CrossRef]
- Ozkan, S.A.; Uslu, B. From mercury to nanosensors: Past, present and the future perspective of electrochemistry in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2016, 130, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.A. Electroanalytical Methods in Pharmaceutical Analysis and Their Validation, 1st ed.; HNB Pub.: Palenville, NY, USA, 2012. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 677, ISBN 0471043729. [Google Scholar]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry; World Scientific Publishing Europe Ltd.: London, UK, 2010; ISBN 978-1-84816-585-4. [Google Scholar]
- Jain, R.; Yadav, R.K.; Dwivedi, A. Square-wave adsorptive stripping voltammetric behaviour of entacapone at HMDE and its determination in the presence of surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2010, 359, 25–30. [Google Scholar] [CrossRef]
- Beludari, M.I.; Prakash, K.V.; Mohan, G.K. RP-HPLC method for simultaneous estimation of Rosuvastatin and Ezetimibe from their combination tablet dosage form. Int. J. Chem. Anal. Sci. 2013, 4, 205–209. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Esim, O.; Ozkan, C.K.; Savaser, A.; Ozkan, Y.; Uslu, B.; Ozkan, S.A. Stability-indicating liquid chromatographic method for the simultaneous determination of rosuvastatin and ezetimibe from pharmaceuticals and biological samples. J. Turk. Chem. Soc. Sect. A Chem. 2020, 7, 865–874. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, M.C.; Kohli, D.V.; Chaturvedi, S.C. Micellar liquid chromatographic method development for determination of rosuvastatin calcium and ezetimibe in pharmaceutical combination dosage form. Der Pharma Chem. 2010, 2, 371–377. [Google Scholar]
- Varghese, S.J.; Ravi, T.K. Determination of rosuvastatin and ezetimibe in a combined tablet dosage form using high-performance column liquid chromatography and high-performance thin-layer chromatography. J. AOAC Int. 2010, 93, 1222–1227. [Google Scholar] [CrossRef]
- Ashfaq, M.; Ahmad, H.; Khan, I.U.; Mustafa, G. Lc determination of rosuvastatin and ezetimibe in human Plasma. J. Chil. Chem. Soc. 2013, 58, 2177–2181. [Google Scholar] [CrossRef]
- Pandya, C.B.; Channabasavaraj, K.P.; Shridhara, H.S. Simultaneous estimation of Rosuvastatin calcium and ezetimibe in bulk and tablet dosage form by simultaneous equation method. Int. J. ChemTech Res. 2010, 2140–2144. [Google Scholar]
- Varghese, S.J.; Ravi, T.K. Development and validation of a liquid chromatography/ mass spectrometry method for the simultaneous quantitation of rosuvastatin and ezetimibe in human plasma. J. AOAC Int. 2013, 96, 307–312. [Google Scholar] [CrossRef]
- Bhadoriya, A.; Sanyal, M.; Shah, P.A.; Shrivastav, P.S. Simultaneous quantitation of rosuvastatin and ezetimibe in human plasma by LC–MS/MS: Pharmacokinetic study of fixed-dose formulation and separate tablets. Biomed. Chromatogr. 2018, 32, e4291. [Google Scholar] [CrossRef]
- Laviron, E. Surface linear potential sweep voltammetry: Equation of the peaks for a reversible reaction when interactions between the adsorbed molecules are taken into account. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 395–402. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kurbanoglu, S.; Uslu, B.; Ozkan, S.A. Differential Pulse Voltammetric Determination of Rosuvastatin Via Glassy Carbon Electrode. Rev. Roum. Chim. 2017, 62, 581–588. [Google Scholar]
- Karadas-Bakirhan, N.; Gumustas, M.; Uslu, B.; Ozkan, S.A. Simultaneous determination of amlodipine besylate and rosuvastatin calcium in binary mixtures by voltammetric and chromatographic techniques. Ionics (Kiel). 2016, 22, 277–288. [Google Scholar] [CrossRef]
- Silva, T.A.; Zanin, H.; Vicentini, F.C.; Corat, E.J.; Fatibello-Filho, O. Electrochemical determination of rosuvastatin calcium in pharmaceutical and human body fluid samples using a composite of vertically aligned carbon nanotubes and graphene oxide as the electrode material. Sens. Actuators B Chem. 2015, 218, 51–59. [Google Scholar] [CrossRef]
- Kul, D.; Uslu, B.; Ozkan, S.A. Electrochemical Determination of Anti-Hyperlipidemic Drug Ezetimibe Based on its Oxidation on Solid Electrodes. Anal. Lett. 2011, 44, 1341–1357. [Google Scholar]
- Özden, D.Ş.; Durmuş, Z.; Dinç, E. Electrochemical oxidation behavior of ezetimibe and its adsorptive stripping determination in pharmaceutical dosage forms and biological fluids. Res. Chem. Intermed. 2015, 41, 1803–1818. [Google Scholar] [CrossRef]





| Buffer | Serum | Urine | ||||
|---|---|---|---|---|---|---|
| Compounds | EZE | ROS | EZE | ROS | EZE | ROS |
| Linearity range (M) | 1 × 10−6–2.5 × 10−5 | 5 × 10−6–1.25 × 10−5 | 3.0 × 10−6–1.0 × 10−5 | 2.0 ×10−5–6.0 × 10−5 | 3.0 × 10−6–1.0 × 10−5 | 2.0 × 10−5–6.0 × 10−5 |
| Slope (μA/mM) | 76.93 | 24.20 | 30.54 | 11.31 | 49.05 | 14.19 |
| Intercept (mM) | −0.03 | −0.08 | 0.03 | −0.12 | 0.09 | −0.05 |
| Determination coefficient | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| LOD (M) | 3.0 × 10−7 | 2.0 × 10−6 | 1.0 × 10−6 | 1.0 × 10−6 | 3.0 × 10−7 | 1.0 × 10−6 |
| LOQ (M) | 1.0 × 10−6 | 6.6 × 10−6 | 2.0 × 10−6 | 4.0 × 10−6 | 1.0 × 10−6 | 4.0 × 10−6 |
| Within-day Repeatability (RSD %) * | 1.20 | 1.74 | 1.45 | 1.42 | 1.68 | 1.67 |
| Between-day Repeatability (RSD %) * | 1.48 | 1.72 | 1.83 | 1.51 | 1.87 | 1.98 |
| ROS | EZE | |||
|---|---|---|---|---|
| Human Urine | Human Serum | Human Urine | Human Serum | |
| Added (mg) | 5.00 | 5.00 | 5.00 | 5.00 |
| Found (mg) * | 4.87 | 4.92 | 4.82 | 4.89 |
| Recovery (%) * | 97.4 | 98.4 | 96.4 | 97.8 |
| RSD (%) | 1.10 | 0.80 | 1.30 | 0.90 |
| Bias (%) | 2.60 | 1.60 | 3.60 | 2.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadurmus, L.; Kurbanoglu, S.; Uslu, B.; Ozkan, S.A. An Efficient, Simultaneous Electrochemical Assay of Rosuvastatin and Ezetimibe from Human Urine and Serum Samples. Methods Protoc. 2022, 5, 90. https://doi.org/10.3390/mps5060090
Karadurmus L, Kurbanoglu S, Uslu B, Ozkan SA. An Efficient, Simultaneous Electrochemical Assay of Rosuvastatin and Ezetimibe from Human Urine and Serum Samples. Methods and Protocols. 2022; 5(6):90. https://doi.org/10.3390/mps5060090
Chicago/Turabian StyleKaradurmus, Leyla, Sevinc Kurbanoglu, Bengi Uslu, and Sibel A. Ozkan. 2022. "An Efficient, Simultaneous Electrochemical Assay of Rosuvastatin and Ezetimibe from Human Urine and Serum Samples" Methods and Protocols 5, no. 6: 90. https://doi.org/10.3390/mps5060090
APA StyleKaradurmus, L., Kurbanoglu, S., Uslu, B., & Ozkan, S. A. (2022). An Efficient, Simultaneous Electrochemical Assay of Rosuvastatin and Ezetimibe from Human Urine and Serum Samples. Methods and Protocols, 5(6), 90. https://doi.org/10.3390/mps5060090











