Real-Time Monitoring of Lysosomal Membrane Permeabilization Using Acridine Orange
Abstract
:1. Introduction
2. Experimental Design
2.1. Considerations When Designing the Experiment
2.1.1. Lysosomal pH, Volume and Numbers
2.1.2. Phototoxicity and Photobleaching
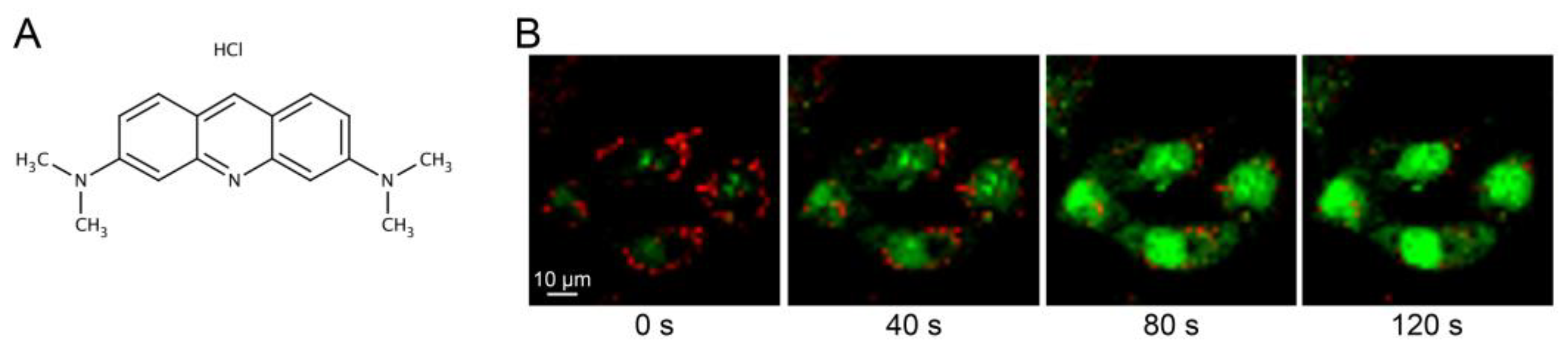
2.1.3. AO Staining of RNA and DNA
2.2. Materials
- Adherent primary cells or cell line of choice.
- Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 µg/mL streptomycin (all from Gibco, Paisley, UK), or other complete cell culture medium suitable for the cells of choice.
- Phenol-free DMEM supplemented with 10% FBS, 100 IU/mL penicillin and 100 µg/mL streptomycin, or other phenol-free complete cell culture medium.
- Acridine orange (Sigma-Aldrich, Cat. No.: 318337).
- Positive control 1: LLOMe (Sigma-Aldrich, Cat. No.: L7393).
- Positive control 2: GO (Sigma-Aldrich, Cat. No.: G2133).
- 96-Well Black Polystyrene Microplate with clear bottom (Corning, Cat. No.: 3603).
2.3. Equipment
- Fluorescence plate reader with heating, equipped with excitation/emission filter at 485/535 nm for green fluorescence and 465/650–710 nm (see Section 4 for choosing the correct emission wavelength) for red fluorescence. We kept the measurement time as short as possible. We have used a Spark 10 M reader (Tecan, Männedorf, Switzerland) with an integration time of 40 µs.
3. Procedure
3.1. Preparation of Stock Solutions
- 1 mg/mL AO in H2O. Store at 4 °C for up to 6 months in the dark;
- 0.1 M LLOMe in H2O. Store at −20 °C for up to 6 months;
- 1 mg/mL GO in 50 mM sodium acetate. Prepare fresh solution for each experiment.
3.2. Cell Seeding
- For experiment, use trypsinize cells and seed in a black 96-well plate for fluorescence reading. Use a cell density to allow 80–90% confluence at the day of experiment.
- Make triplicates of each sample and include at least one well without cells for measurement of background fluorescence.
- Allow cells to adhere for at least 24 h.
- Proper optimization of cell density vs. drug concentration must be performed before the start of the experiment. Also, low density of cells can generate uncertain intensity values, especially for red fluorescence.
3.3. Staining of Cells
- Prepare AO staining solution by diluting the AO stock in complete cell culture medium to reach a final concentration of 2–5 µg/mL;
- Remove culture medium from the cells and add 100 µL staining solution/well;
- Incubate for 15 min at 37 °C;
- Wash cells with 100 µL complete phenol-free medium for 2 × 5 min;
- Leave cells in 100 µL complete phenol-free medium. Remember to add medium to the well without cells.
3.4. Measurement of Initial AO Fluorescence
- Preheat the instrument to 37 °C.
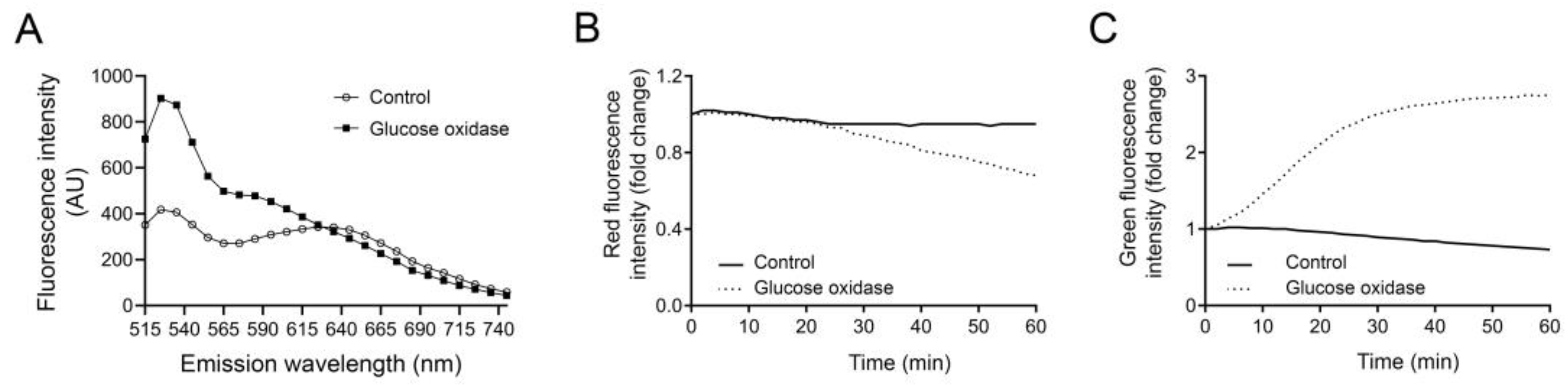
- Set to measure green fluorescence at excitation 485 nm and emission 535 nm. If red fluorescence is to be analyzed, use excitation 465 nm and emission 650–710 nm (see Section 4 for choosing the optimal emission wavelength).
- Be aware that green fluorescence is markedly increased upon LMP, thus, be cautious for signal saturation when setting the gain.
- Make a single-point measurement to record initial AO fluorescence before induction of LMP.
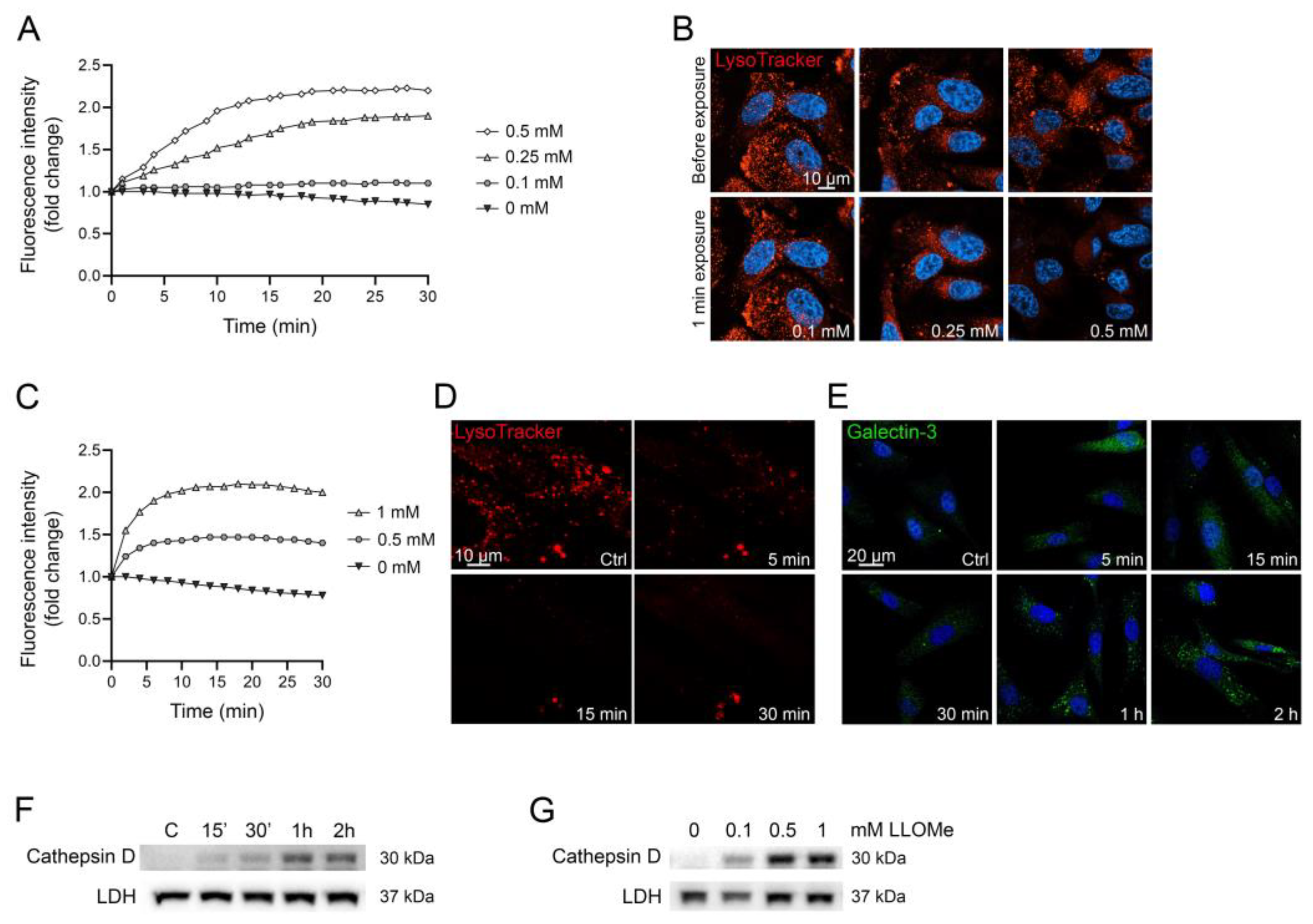
3.5. Induction of Lysosomal Leakage
- 5–50 µg/mL glucose oxidase;
- 0.1–5 mM LLOMe.
3.6. Measurement of AO Fluorescence
- Start the collection of data for fluorescence change over time at 37 °C immediately after the addition of LMP inducer. Use the same settings as for initial AO fluorescence measurement.
- The time between each measurement can vary depending on the LMP inducer. For lysosomal membrane-targeting drugs, such as LLOMe, maximal leakage usually occurs within 30 min and fluorescence intensity data are collected every minute. Indirect LMP inducers such as GO may need up to 2 h of data collection and data can be collected every 2nd–3rd min.
3.7. Data Analysis
- Subtract the background.
- Divide the fluorescence value from each time point with the initial fluorescence value to obtain a ratio of fluorescence change.
- Calculate the mean value of the triplicates for each time point.
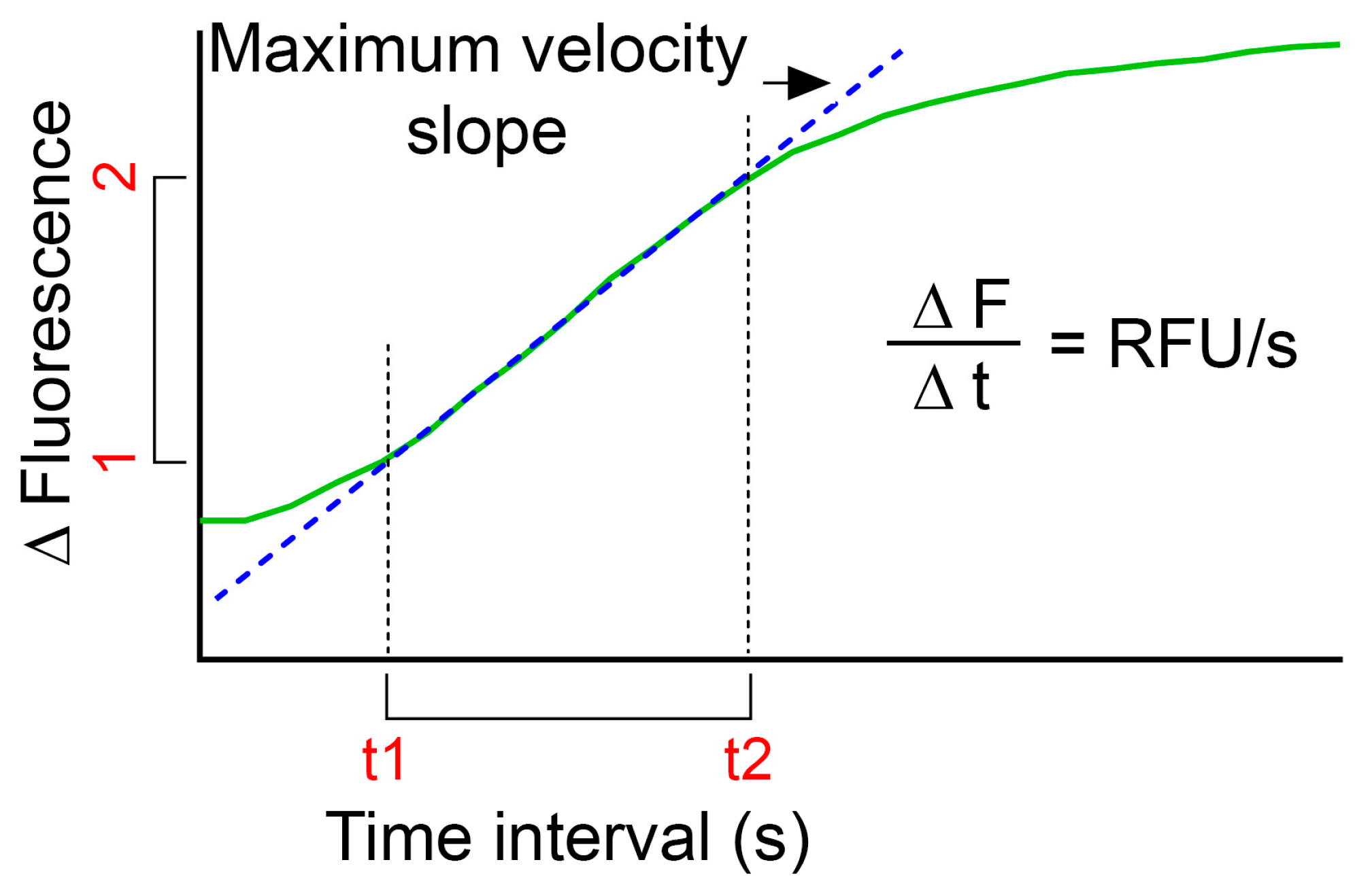
- Plot fluorescence intensity change over time (green curve in Figure 3).
- In order to compare LMP of several treatments, calculations of relative fluorescence unit per second (RFU/s) might be useful:
- o
- Use the plot of relative fluorescence over time as obtained above and define the highest slope of the curve (dotted blue line in Figure 3);
- o
- Calculate by subtracting the fluorescence value for the first time point (F1) to the fluorescence value for the end time point (F2) for the defined time interval. Divide the obtained value with the time interval (t2–t1) to obtain RFU/s;
- o
- Use the same time interval for all samples within one experiment.
We have observed a slight reduction in fluorescence over time in control cells not exposed to LMP inducers. Therefore, we recommend to always include unexposed controls for all conditions used in the experiment.
4. Expected Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- De Duve, C. Lysosomes revisited. Eur. J. Biochem. 1983, 137, 391–397. [Google Scholar] [CrossRef]
- Schroder, B.A.; Wrocklage, C.; Hasilik, A.; Saftig, P. The proteome of lysosomes. Proteomics 2010, 10, 4053–4076. [Google Scholar] [CrossRef]
- Appelqvist, H.; Waster, P.; Kagedal, K.; Ollinger, K. The lysosome: From waste bag to potential therapeutic target. J. Mol. Cell Biol. 2013, 5, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Aits, S.; Jaattela, M. Lysosomal cell death at a glance. J. Cell Sci. 2013, 126, 1905–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boya, P.; Andreau, K.; Poncet, D.; Zamzami, N.; Perfettini, J.L.; Metivier, D.; Ojcius, D.M.; Jaattela, M.; Kroemer, G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J. Exp. Med. 2003, 197, 1323–1334. [Google Scholar] [CrossRef]
- Repnik, U.; Stoka, V.; Turk, V.; Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 2012, 1824, 22–33. [Google Scholar] [CrossRef]
- Roberg, K.; Ollinger, K. Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am. J. Pathol. 1998, 152, 1151–1156. [Google Scholar]
- Reinheckel, T.; Tholen, M. Low-level lysosomal membrane permeabilization for limited release and sublethal functions of cathepsin proteases in the cytosol and nucleus. FEBS Open Bio 2022, 12, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, M.; Schink, K.O.; Wenzel, E.M.; Nahse, V.; Bongiovanni, A.; Lafont, F.; Stenmark, H. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 2018, 37, e99753. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, M.L.; Schlesinger, P.H.; Naismith, T.V.; Hanson, P.I. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 2018, 360, eaar5078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl-Meyer, J.; Stahl-Meyer, K.; Jaattela, M. Control of mitosis, inflammation, and cell motility by limited leakage of lysosomes. Curr. Opin. Cell Biol. 2021, 71, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, T.J.; Lloyd-Lewis, B.; Resemann, H.K.; Ramos-Montoya, A.; Skepper, J.; Watson, C.J. Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat. Cell Biol. 2014, 16, 1057–1068. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, X.; Boini, K.M.; Pitzer, A.L.; Gulbins, E.; Zhang, Y.; Li, P.L. Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim. Biophys. Acta 2015, 1853, 396–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, H.L.; Vainikka, L.K. Increased Lysosomal Membrane Permeabilization in Oxidant-exposed Macrophages of Human Fibrotic Lungs. J. Cell Death 2013, 6, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehay, B.; Bove, J.; Rodriguez-Muela, N.; Perier, C.; Recasens, A.; Boya, P.; Vila, M. Pathogenic lysosomal depletion in Parkinson’s disease. J. Neurosci. 2010, 30, 12535–12544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, I.; Vainikka, L.; Waster, P.; Ollinger, K. Lysosomal function and intracellular position determine the malignant phenotype in malignant melanoma. J. Investig. Dermatol. 2023. [Google Scholar] [CrossRef]
- Hsu, S.P.C.; Kuo, J.S.; Chiang, H.C.; Wang, H.E.; Wang, Y.S.; Huang, C.C.; Huang, Y.C.; Chi, M.S.; Mehta, M.P.; Chi, K.H. Temozolomide, sirolimus and chloroquine is a new therapeutic combination that synergizes to disrupt lysosomal function and cholesterol homeostasis in GBM cells. Oncotarget 2018, 9, 6883–6896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Puebla, A.; Boya, P. Lysosomal membrane permeabilization as a cell death mechanism in cancer cells. Biochem. Soc. Trans. 2018, 46, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, N.; Gyrd-Hansen, M.; Poulsen, B.; Felbor, U.; Kallunki, T.; Boes, M.; Weber, E.; Leist, M.; Jaattela, M. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res. 2004, 64, 5301–5310. [Google Scholar] [CrossRef] [Green Version]
- De Duve, C.; de Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Toprak, M.; Noory, M.A.; Robertson, G.P. Effect of lysosomotropic molecules on cellular homeostasis. Pharmacol. Res. 2017, 117, 177–184. [Google Scholar] [CrossRef]
- Ellegaard, A.M.; Dehlendorff, C.; Vind, A.C.; Anand, A.; Cederkvist, L.; Petersen, N.H.T.; Nylandsted, J.; Stenvang, J.; Mellemgaard, A.; Osterlind, K.; et al. Repurposing Cationic Amphiphilic Antihistamines for Cancer Treatment. EBioMedicine 2016, 9, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toriyama, K.; Takano, N.; Kokuba, H.; Kazama, H.; Moriya, S.; Hiramoto, M.; Abe, S.; Miyazawa, K. Azithromycin enhances the cytotoxicity of DNA-damaging drugs via lysosomal membrane permeabilization in lung cancer cells. Cancer Sci. 2021, 112, 3324–3337. [Google Scholar] [CrossRef]
- Pisonero-Vaquero, S.; Medina, D.L. Lysosomotropic Drugs: Pharmacological Tools to Study Lysosomal Function. Curr. Drug Metab. 2017, 18, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Homolak, J.; Kodvanj, I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106044. [Google Scholar] [CrossRef]
- Roos, N.J.; Mancuso, R.V.; Sanvee, G.M.; Bouitbir, J.; Krahenbuhl, S. Imatinib disturbs lysosomal function and morphology and impairs the activity of mTORC1 in human hepatocyte cell lines. Food Chem. Toxicol. 2022, 162, 112869. [Google Scholar] [CrossRef]
- Tao, L.; Qing, Y.; Cui, Y.; Shi, D.; Liu, W.; Chen, L.; Cao, Y.; Dai, Z.; Ge, X.; Zhang, L. Lysosomal membrane permeabilization mediated apoptosis involve in perphenazine-induced hepatotoxicity in vitro and in vivo. Toxicol. Lett. 2022, 367, 76–87. [Google Scholar] [CrossRef]
- Aits, S.; Jaattela, M.; Nylandsted, J. Methods for the quantification of lysosomal membrane permeabilization: A hallmark of lysosomal cell death. Methods Cell Biol. 2015, 126, 261–285. [Google Scholar] [CrossRef]
- Wang, F.; Gomez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Thome, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattia, C.A.; Mazzarella, L.; Vitagliano, V.; Puliti, R. Stacking interactions in the acridine dyes: Spectrophotometric data and crystal structure of acridine orange hydroiodide and acridine orange hydrochloride monohydrate. J. Crystallogr. Spectrosc. Res. 1984, 14, 71–87. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Roth, A.G.; Petersen, N.H.; Mahalka, A.K.; Olsen, O.D.; Moilanen, I.; Zylicz, A.; Knudsen, J.; Sandhoff, K.; Arenz, C.; et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010, 463, 549–553. [Google Scholar] [CrossRef]
- Olsson, G.M.; Rungby, J.; Rundquist, I.; Brunk, U.T. Evaluation of lysosomal stability in living cultured macrophages by cytofluorometry. Effect of silver lactate and hypotonic conditions. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989, 56, 263–269. [Google Scholar] [CrossRef]
- Olsson, M.; Rundquist, I.; Brunk, U. Flow cytofluorometry of lysosomal acridine orange uptake by living cultured cells. Effect of trypsinization and starvation. Acta Pathol. Microbiol. Immunol. Scand. A 1987, 95, 159–165. [Google Scholar] [CrossRef]
- Persson, H.L.; Vainikka, L.K. Lysosomal iron in pulmonary alveolar proteinosis: A case report. Eur. Respir. J. 2009, 33, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Persson, H.L.; Sioutas, A.; Jacobson, P.; Vainikka, L.K. Human Lung Macrophages Challenged to Oxidants ex vivo: Lysosomal Membrane Sensitization is Associated with Inflammation and Chronic Airflow Limitation. J. Inflamm. Res. 2020, 13, 925–932. [Google Scholar] [CrossRef]
- Thiele, D.L.; Lipsky, P.E. The action of leucyl-leucine methyl ester on cytotoxic lymphocytes requires uptake by a novel dipeptide-specific facilitated transport system and dipeptidyl peptidase I-mediated conversion to membranolytic products. J. Exp. Med. 1990, 172, 183–194. [Google Scholar] [CrossRef]
- Repnik, U.; Borg Distefano, M.; Speth, M.T.; Ng, M.Y.W.; Progida, C.; Hoflack, B.; Gruenberg, J.; Griffiths, G. L-leucyl-L-leucine methyl ester does not release cysteine cathepsins to the cytosol but inactivates them in transiently permeabilized lysosomes. J. Cell Sci. 2017, 130, 3124–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, D.L.; Lipsky, P.E. Mechanism of L-leucyl-L-leucine methyl ester-mediated killing of cytotoxic lymphocytes: Dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proc. Natl. Acad. Sci. USA 1990, 87, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Cadenas, E.; Brunk, U.T. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem. J. 2001, 356, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.L.; Yu, Z.; Tirosh, O.; Eaton, J.W.; Brunk, U.T. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radic. Biol. Med. 2003, 34, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Persson, H.L.; Eaton, J.W.; Brunk, U.T. Intralysosomal iron: A major determinant of oxidant-induced cell death. Free Radic. Biol. Med. 2003, 34, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Brunk, U.T.; Dalen, H.; Roberg, K.; Hellquist, H.B. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 1997, 23, 616–626. [Google Scholar] [CrossRef]
- Petersen, N.H.; Kirkegaard, T.; Olsen, O.D.; Jaattela, M. Connecting Hsp70, sphingolipid metabolism and lysosomal stability. Cell Cycle 2010, 9, 2305–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierzynska-Mach, A.; Janowski, P.A.; Dobrucki, J.W. Evaluation of acridine orange, LysoTracker Red, and quinacrine as fluorescent probes for long-term tracking of acidic vesicles. Cytom. Part A 2014, 85, 729–737. [Google Scholar] [CrossRef]
- Delic, J.; Coppey, J.; Magdelenat, H.; Coppey-Moisan, M. Impossibility of acridine orange intercalation in nuclear DNA of the living cell. Exp. Cell Res. 1991, 194, 147–153. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Murata, H.; Takeshita, H.; Hashiguchi, S.; Nozaki, T.; Emoto, K.; Ashihara, T.; Hirasawa, Y. Intracellular binding sites of acridine orange in living osteosarcoma cells. Anticancer Res. 2000, 20, 971–975. [Google Scholar]
- Kusuzaki, K.; Matsubara, T.; Satonaka, H.; Matsumine, A.; Nakamura, T.; Sudo, A.; Murata, H.; Hosogi, S.; Baldini, N. Intraoperative Photodynamic Surgery (iPDS) with Acridine Orange for Musculoskeletal Sarcomas. Cureus 2014, 6, e204. [Google Scholar] [CrossRef] [Green Version]
- Repnik, U.; Cesen, M.H.; Turk, B. The Use of Lysosomotropic Dyes to Exclude Lysosomal Membrane Permeabilization. Cold Spring Harb. Protoc. 2016, 2016, 452–476. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, I.; Waster, P.; Ollinger, K. Restoration of lysosomal function after damage is accompanied by recycling of lysosomal membrane proteins. Cell Death Dis. 2020, 11, 370. [Google Scholar] [CrossRef]
- Aits, S.; Kricker, J.; Liu, B.; Ellegaard, A.M.; Hamalisto, S.; Tvingsholm, S.; Corcelle-Termeau, E.; Hogh, S.; Farkas, T.; Holm Jonassen, A.; et al. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 2015, 11, 1408–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaattela, M.; Nylandsted, J. Quantification of Lysosomal Membrane Permeabilization by Cytosolic Cathepsin and beta-N-Acetyl-Glucosaminidase Activity Measurements. Cold Spring Harb. Protoc. 2015, 2015, 1017–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repnik, U.; Hafner Cesen, M.; Turk, B. Strategies for Assaying Lysosomal Membrane Permeabilization. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Eriksson, I.; Joosten, M.; Roberg, K.; Ollinger, K. The histone deacetylase inhibitor trichostatin A reduces lysosomal pH and enhances cisplatin-induced apoptosis. Exp. Cell Res. 2013, 319, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Maejima, I.; Takahashi, A.; Omori, H.; Kimura, T.; Takabatake, Y.; Saitoh, T.; Yamamoto, A.; Hamasaki, M.; Noda, T.; Isaka, Y.; et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 2013, 32, 2336–2347. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksson, I.; Vainikka, L.; Persson, H.L.; Öllinger, K. Real-Time Monitoring of Lysosomal Membrane Permeabilization Using Acridine Orange. Methods Protoc. 2023, 6, 72. https://doi.org/10.3390/mps6040072
Eriksson I, Vainikka L, Persson HL, Öllinger K. Real-Time Monitoring of Lysosomal Membrane Permeabilization Using Acridine Orange. Methods and Protocols. 2023; 6(4):72. https://doi.org/10.3390/mps6040072
Chicago/Turabian StyleEriksson, Ida, Linda Vainikka, Hans Lennart Persson, and Karin Öllinger. 2023. "Real-Time Monitoring of Lysosomal Membrane Permeabilization Using Acridine Orange" Methods and Protocols 6, no. 4: 72. https://doi.org/10.3390/mps6040072
APA StyleEriksson, I., Vainikka, L., Persson, H. L., & Öllinger, K. (2023). Real-Time Monitoring of Lysosomal Membrane Permeabilization Using Acridine Orange. Methods and Protocols, 6(4), 72. https://doi.org/10.3390/mps6040072





