Suitability of XRF for Routine Analysis of Multi-Elemental Composition: A Multi-Standard Verification
Abstract
:1. Introduction
2. Materials and Methods
2.1. XRF Analyzer and Standard Analysis
2.2. Recoveries and Statistical Analysis
3. Results
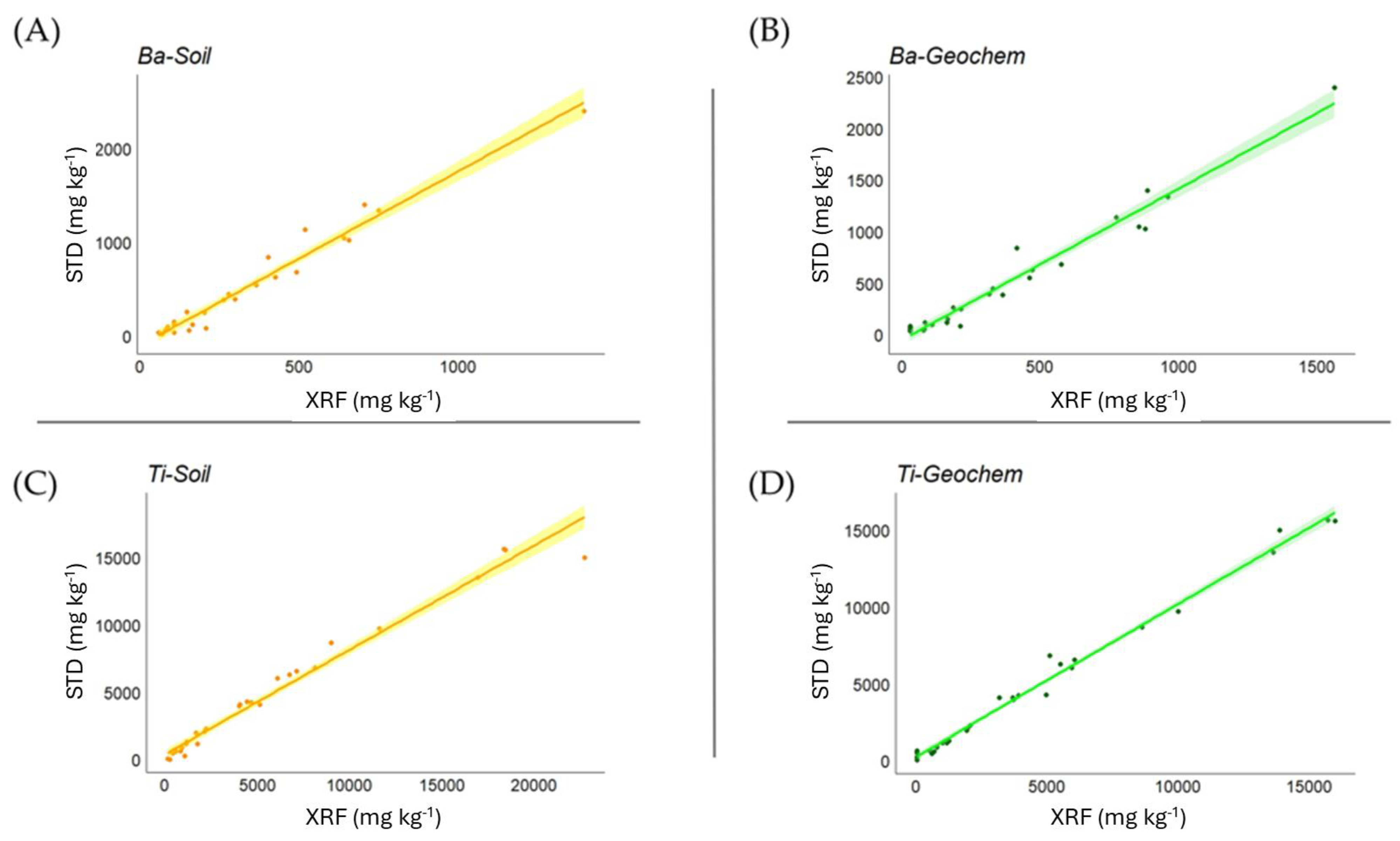
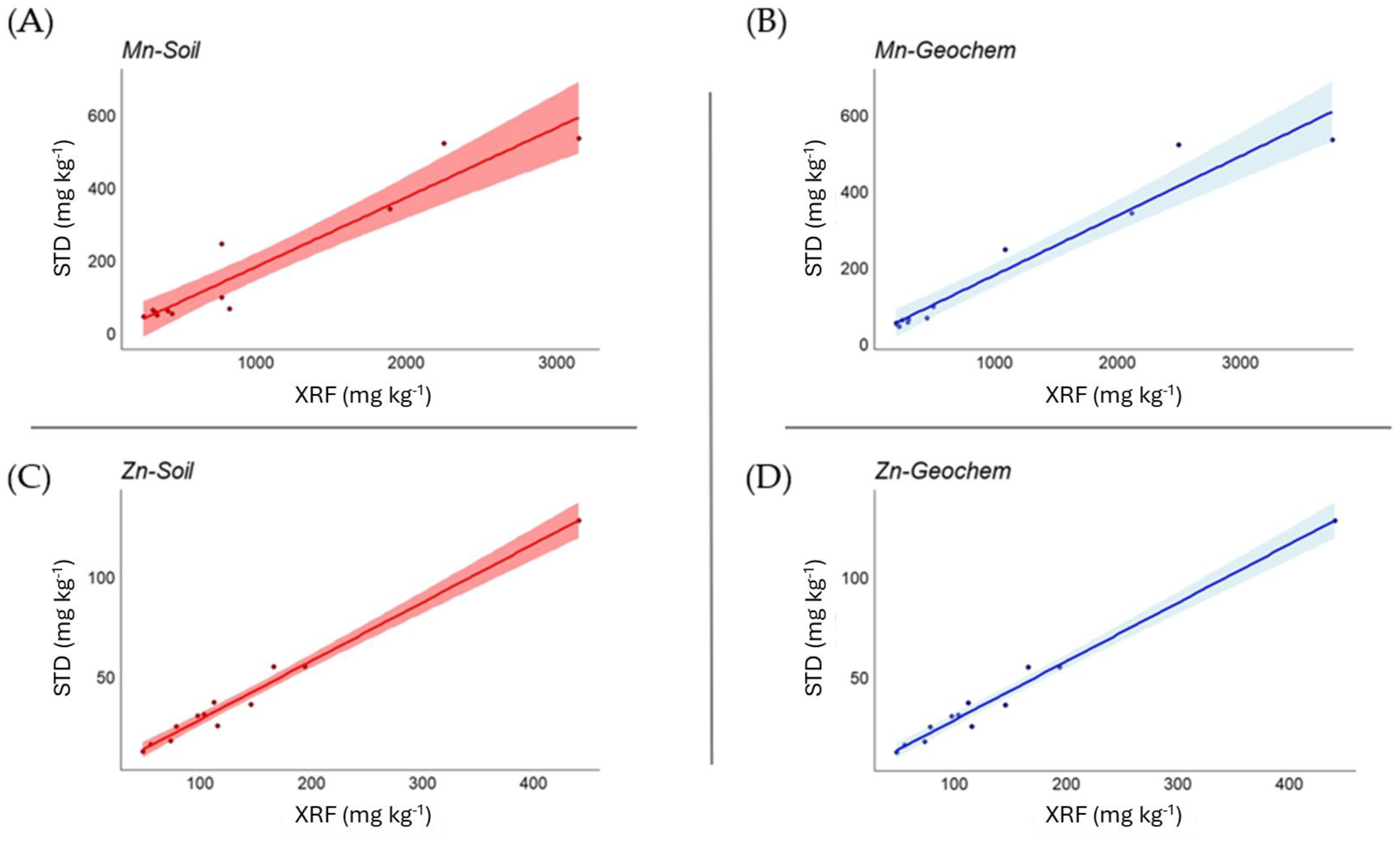
3.1. Soil Matrices
3.2. Plant Matrices
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albarede, F.; Telouk, P.; Blichert-Toft, J.; Boyet, M.; Agranier, A.; Nelson, B. Precise and accurate isotopic measurements using multiple-collector ICPMS. Geoch. Cosm. Acta 2004, 68, 2725–2744. [Google Scholar] [CrossRef]
- Butler, O.T.; Cairns, W.R.L.; Cook, J.M.; Davidson, C.M. Atomic spectrometry update. Environmental analysis. J. Anal. At. Spectrom. 2010, 25, 103–141. [Google Scholar] [CrossRef]
- Wheal, M.S.; Fowles, T.O.; Palmer, L.T. A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal. Methods 2011, 3, 2854–2863. [Google Scholar] [CrossRef]
- Steinnes, E. Neutron activation techniques in environmental studies. J. Radioanal. Nucl. Chem. 2000, 243, 235–239. [Google Scholar] [CrossRef]
- Pappalardo, L.; De Sanoit, J.; Marchetta, C.; Pappalardo, G.; Romano, F.P.; Rizzo, F. A portable spectrometer for simultaneous PIXE and XRF analysis. X-ray Spectrom. 2007, 36, 310–315. [Google Scholar] [CrossRef]
- Benyaïch, F.; Makhtari, A.; Torrisi, L.; Foti, G. PIXE and XRF comparison for applications to sediments analysis. Nucl. Instrum. 1997, 132, 481–488. [Google Scholar] [CrossRef]
- Malmqvist, K.G. Comparison between PIXE and XRF for applications in art and archaeology. Nucl. Instrum. 1986, 14, 86–92. [Google Scholar] [CrossRef]
- El Ouahabi, M.; Chene, G.; Strivay, D.; Vander Auwera, J.; Hubert-Ferrari, A. Inter-technique comparison of PIXE and XRF for lake sediments. J. Anal. At. Spectrom. 2018, 33, 883–892. [Google Scholar] [CrossRef]
- Weindorf, D.C.; Bakr, N.; Zhu, Y. Advances in portable X-ray fluorescence (PXRF) for environmental, pedological, and agronomic applications. Adv. Agron. 2014, 128, 1–45. [Google Scholar]
- Potts, P.J.; Potts, P.J. X-ray fluorescence analysis: Principles and practice of wavelength dispersive spectrometry. A Handb. Silic. Rock Anal. 1987, 226–285. [Google Scholar]
- Rawat, K.; Sharma, N.; Singh, V.K. X-Ray Fluorescence and Comparison with Other Analytical Methods (AAS, ICP-AES, LA-ICP-MS, IC, LIBS, SEM-EDS, and XRD). In X-Ray Fluorescence in Biological Sciences: Principles, Instrumentation, and Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 1–20. [Google Scholar]
- Cannell, R.J. Prospecting the Physicochemical Past. Three Dimensional Geochemical Investigation into the Use of Space in Viking Age Sites in Southern Norway Using Portable XRF. Ph.D. Thesis, Bournemouth University, Poole, UK, 2022. [Google Scholar]
- Charlton, B.; Fisher, A.S.; Goodall, P.S.; Hinds, M.W.; Lancaster, S.; Salisbury, M. Atomic spectrometry update. Industrial analysis: Metals, chemicals and advanced materials. J. Anal. At. Spectrom. 2007, 22, 1517–1560. [Google Scholar] [CrossRef]
- Ingham, M.N.; Vrebos, B.A. High productivity geochemical XRF analysis. Adv. X-ray Anal. 1993, 37, 717–724. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 May 2024).
- Kilbride, C.; Poole, J.; Hutchings, T.R. A comparison of Cu, Pb, As, Cd, Zn, Fe, Ni and Mn determined by acid extraction/ICP–OES and ex situ field portable X-ray fluorescence analyses. Environ. Pollut. 2006, 143, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, N.; Guo, N.; Zhou, H.; Bu, J.; Ding, A. Comparative Study on the Determination of heavy metals in Soil by XRF and ICP-MS. J. Phys. Conf. Ser. 2021, 2009, 012075. [Google Scholar] [CrossRef]
- Rouillon, M.; Taylor, M.P. Can field portable X-ray fluorescence (pXRF) produce high quality data for application in environmental contamination research? Environ. Pollut. 2016, 214, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Huang, B.; Xing, Z.; Hu, W. In situ investigation of heavy metals at trace concentrations in greenhouse soils via portable X-ray fluorescence spectroscopy. Environ. Sci. Pollut. Res. 2018, 25, 11011–11022. [Google Scholar] [CrossRef] [PubMed]
- Al Maliki, A.; Al-lami, A.K.; Hussain, H.M.; Al-Ansari, N. Comparison between inductively coupled plasma and X-ray fluorescence performance for Pb analysis in environmental soil samples. Environ. Earth Sci. 2017, 76, 433. [Google Scholar] [CrossRef]
- Barnett, M.C.; Forster, N.A.; Ray, G.A.; Li, L.; Guppy, C.N.; Hegarty, R.S. Using portable X-ray fluorescence (pXRF) to determine fecal concentrations of non-absorbable digesta kinetic and digestibility markers in sheep and cattle. Anim. Feed Sci. Technol. 2016, 212, 35–41. [Google Scholar] [CrossRef]
- Caporale, A.G.; Adamo, P.; Capozzi, F.; Langella, G.; Terribile, F.; Vingiani, S. Monitoring metal pollution in soils using portable-XRF and conventional laboratory-based techniques: Evaluation of the performance and limitations according to metal properties and sources. Sci. Total Environ. 2018, 643, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Shand, C.A.; Wendler, R. Portable X-ray fluorescence analysis of mineral and organic soils and the influence of organic matter. J. Geochem. Explor. 2014, 143, 31–42. [Google Scholar] [CrossRef]
| Soil | Geochem | |
|---|---|---|
| Ag | 4 | 2 |
| Al | - | 480 |
| As | 1 | 3 |
| Au | - | 8 |
| Bi | 5 | 4 |
| Ca | 30 | 40 |
| Cd | 5 | 3 |
| Cl | 40 | 30 |
| Co | 3 | 20 |
| Cr | 20 | 250 |
| Cu | 3 | 5 |
| Fe | 4 | 7 |
| Hg | 2 | 2 |
| K | 20 | 40 |
| Mg | - | 5030 |
| Mn | 10 | 15 |
| Mo | 2 | 3 |
| Nb | 1 | 1 |
| Ni | 4 | 5 |
| P | 250 | 30 |
| Pb | 2 | 4 |
| Rb | 1 | 2 |
| S | 40 | 30 |
| Sb | 8 | 4 |
| Se | 1 | 4 |
| Si | - | 320 |
| Sn | 7 | 3 |
| Sr | 2 | 2 |
| Th | 4 | 3 |
| Ti | 20 | 30 |
| U | 4 | 5 |
| V | 10 | 260 |
| W | 4 | 1 |
| Y | 2 | 2 |
| Zn | 2 | 20 |
| Zr | 2 | 1 |
| Standard | |
|---|---|
| Soil | Plant |
| ACE | B.C.R.-1135 |
| AGV-2 | GBW07603 |
| ALI | GBW07604 |
| ANG | IAEA-336 |
| BCR2 | IAEA-392 |
| BEN | M1 |
| BR | M2 |
| DRN | M3 |
| DTS-1 | NFA-36 |
| GA | NIST-1515 |
| GBW07108 | NIST-1543 |
| GBW07311 | NIST-1573a |
| GBW07411 | |
| GH | |
| GS-N | |
| GSP-2 | |
| JB-3 | |
| JGb-1 | |
| JR1 | |
| JR-2 | |
| JSO-2 | |
| MAN | |
| MICA-FE | |
| NIM-G | |
| NIM-N | |
| NIM-P | |
| NIM-S | |
| NIST-1944 | |
| SDC-1 | |
| SDO-1 | |
| SY-3 | |
| UB-N | |
| Soil | Geochem | |
|---|---|---|
| Al | - | 1.08 (5.5) |
| Ba | 0.71 (24.5) | 0.78 (11.1) |
| Ca | 1.06 (8.2) | 0.89 (6.0) |
| Ce | - | 0.82 (99.8) |
| Co | 0.08 (87.6) | 5.63 (75.2) |
| Cr | 0.65 (16.8) | 0.86 (32.5) |
| Cu | 0.98 (15.1) | 1.13 (19.7) |
| Fe | - | 0.90 (2.5) |
| K | 1.16 (10.5) | - |
| La | 0.55 (46.9) | 1.69 (177.1) |
| Mg | - | 1.16 (12.4) |
| Mn | 0.90 (7.8) | 0.89 (3.5) |
| Nb | 0.80 (15.3) | 0.86 (9.5) |
| Ni | 1.00 (11.3) | 0.71 (33.6) |
| P | 0.47 (42.8) | 1.37 (26.2) |
| Pb | 0.73 (13.6) | 0.72 (11.6) |
| Rb | 0.88 (2.9) | 0.89 (2.5) |
| S | 5.40 (40.6) | 3.93 (62.1) |
| Si | - | 1.02 (2.4) |
| Th | 0.38 (62.1) | 0.42 (56.2) |
| Ti | 1.05 (9.4) | 0.93 (6.1) |
| U | - | 0.57 (46.7) |
| V | 1.14 (68.3) | 0.77 (35.1) |
| Y | 0.66 (17.3) | 0.90 (6.7) |
| Zn | 0.97 (4.8) | 1.03 (6.8) |
| Zr | 0.72 (5.9) | 0.81 (7.9) |
| Soil | Geochem | |
|---|---|---|
| Al | - | 0.934 *** |
| Ba | 0.946 *** | 0.965 *** |
| Ca | 0.994 *** | 0.961 *** |
| Ce | - | 0.45 |
| Co | 0.333 | 0.700 *** |
| Cr | 0.664 *** | 0.656 *** |
| Cu | 0.916 *** | 0.855 *** |
| Fe | 0.994 *** | 0.994 *** |
| K | 0.975 *** | - |
| La | 0.448 | 0.363 |
| Mg | - | 0.923 *** |
| Mn | 0.976 *** | 0.984 *** |
| Nb | 0.640 *** | 0.646 *** |
| Ni | 0.933 *** | 0.875 *** |
| P | 0.800 *** | 0.900 *** |
| Pb | 0.951 *** | 0.949 *** |
| Rb | 0.940 *** | 0.932 *** |
| S | 0.560 ** | 0.298 |
| Si | - | 0.920 *** |
| Th | 0.836 *** | 0.676 ** |
| Ti | 0.983 *** | 0.981 *** |
| U | - | 0.744 *** |
| V | 0.673 *** | 0.729 *** |
| Y | 0.754 *** | 0.966 *** |
| Zn | 0.995 *** | 0.993 *** |
| Zr | 0.988 *** | 0.989 *** |
| Soil | Geochem | |
|---|---|---|
| Al | - | 8.24 (131.8) |
| Ba | 3.79 (95.6) | - |
| Ca | 2.52 (64.8) | 4.87 (85.8) |
| Cl | 3.28 (79.7) | 7.80 (147.4) |
| Cu | 5.15 (34.8) | 4.23 (24.2) |
| Fe | 3.63 (23.5) | 4.29 (18.9) |
| K | 3.26 (35.5) | - |
| Mn | 5.86 (20.7) | 4.89 (13.3) |
| P | 0.99 (26.5) | 2.96 (37.6) |
| Pb | 0.90 (66.9) | 2.85 (63.6) |
| Rb | 1.44 (86.5) | 1.15 (22.6) |
| S | 2.44 (51.3) | 4.45 (81.5) |
| Sr | 2.95 (109.8) | 1.23 (63.9) |
| Zn | 3.43 (9.7) | 3.88 (8.8) |
| Soil | Geochem | |
|---|---|---|
| Al | - | −0.429 |
| Ba | 0.512 | - |
| Ca | 0.794 *** | 0.733 * |
| Cl | 0.952 *** | 0.988 *** |
| Cu | 0.800 ** | 0.929 *** |
| Fe | 0.916 *** | 0.916 *** |
| K | 0.879 ** | - |
| Mn | 0.867 *** | 0.965 *** |
| P | 0.915 *** | 0.818 ** |
| Pb | 0.900 * | 0.900 * |
| Rb | 0.690 *** | 0.857 *** |
| S | 0.750 *** | 0.750 *** |
| Sr | 0.975 ** | 0.975 ** |
| Zn | 0.942 *** | 0.979 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedeli, R.; Di Lella, L.A.; Loppi, S. Suitability of XRF for Routine Analysis of Multi-Elemental Composition: A Multi-Standard Verification. Methods Protoc. 2024, 7, 53. https://doi.org/10.3390/mps7040053
Fedeli R, Di Lella LA, Loppi S. Suitability of XRF for Routine Analysis of Multi-Elemental Composition: A Multi-Standard Verification. Methods and Protocols. 2024; 7(4):53. https://doi.org/10.3390/mps7040053
Chicago/Turabian StyleFedeli, Riccardo, Luigi Antonello Di Lella, and Stefano Loppi. 2024. "Suitability of XRF for Routine Analysis of Multi-Elemental Composition: A Multi-Standard Verification" Methods and Protocols 7, no. 4: 53. https://doi.org/10.3390/mps7040053
APA StyleFedeli, R., Di Lella, L. A., & Loppi, S. (2024). Suitability of XRF for Routine Analysis of Multi-Elemental Composition: A Multi-Standard Verification. Methods and Protocols, 7(4), 53. https://doi.org/10.3390/mps7040053








