Full Validation and Application to Clinical Research of a High-Performance Liquid Chromatography Method for the Assessment of Urinary 3-Indoxyl Sulfate in Pediatric Patients with Hematopoietic Stem Cell Transplant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Chromatographic Conditions
2.3. Stock and Working Solutions
2.4. Human Sample Preparation
2.5. Assay Validation
2.5.1. Selectivity and Sensitivity
2.5.2. Linearity
2.5.3. Intra- and Inter-Day Precision and Accuracy
2.5.4. Recovery
2.5.5. Stability
2.6. Clinical Research Samples
3. Results
3.1. Assay Development and Validation
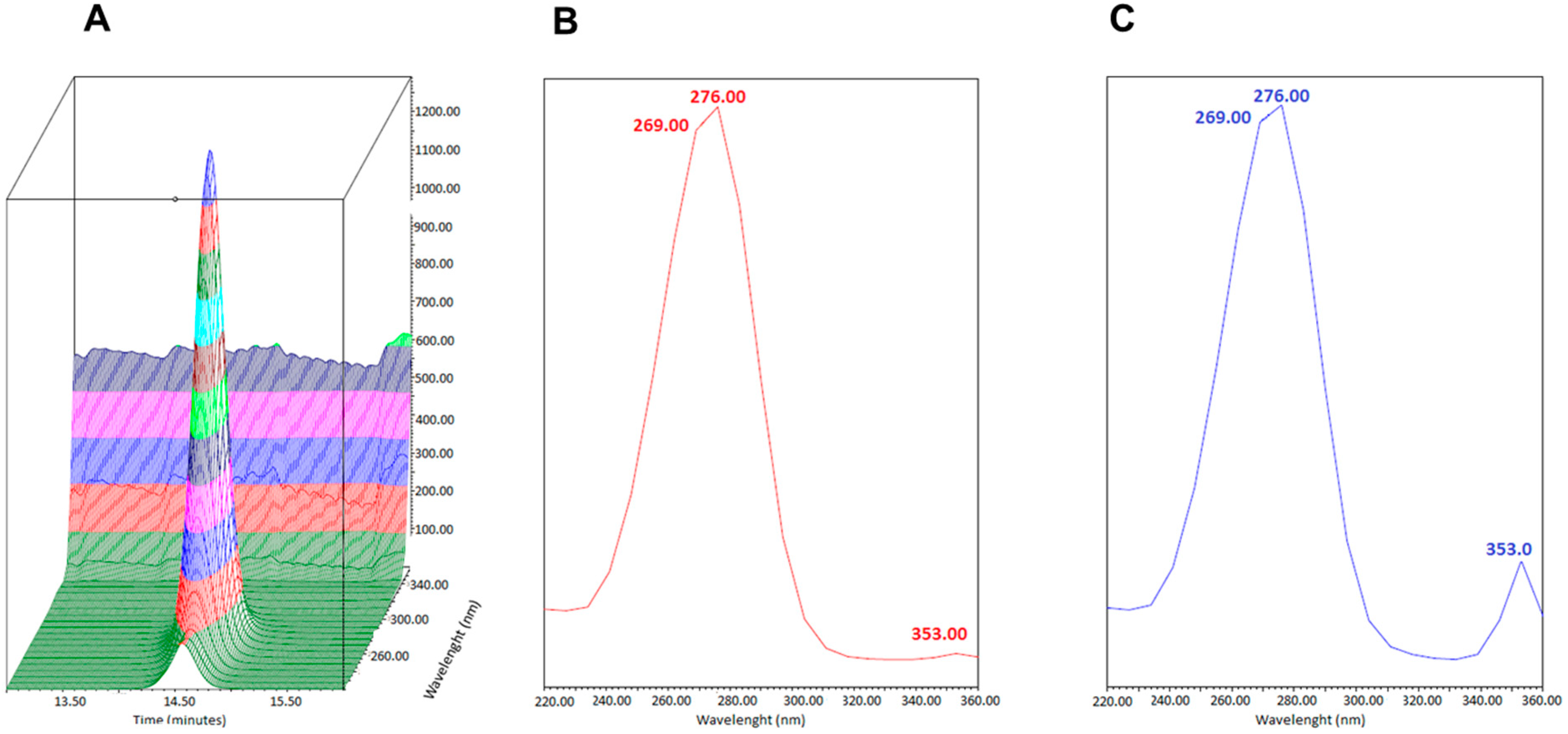
3.1.1. Chromatographic Conditions
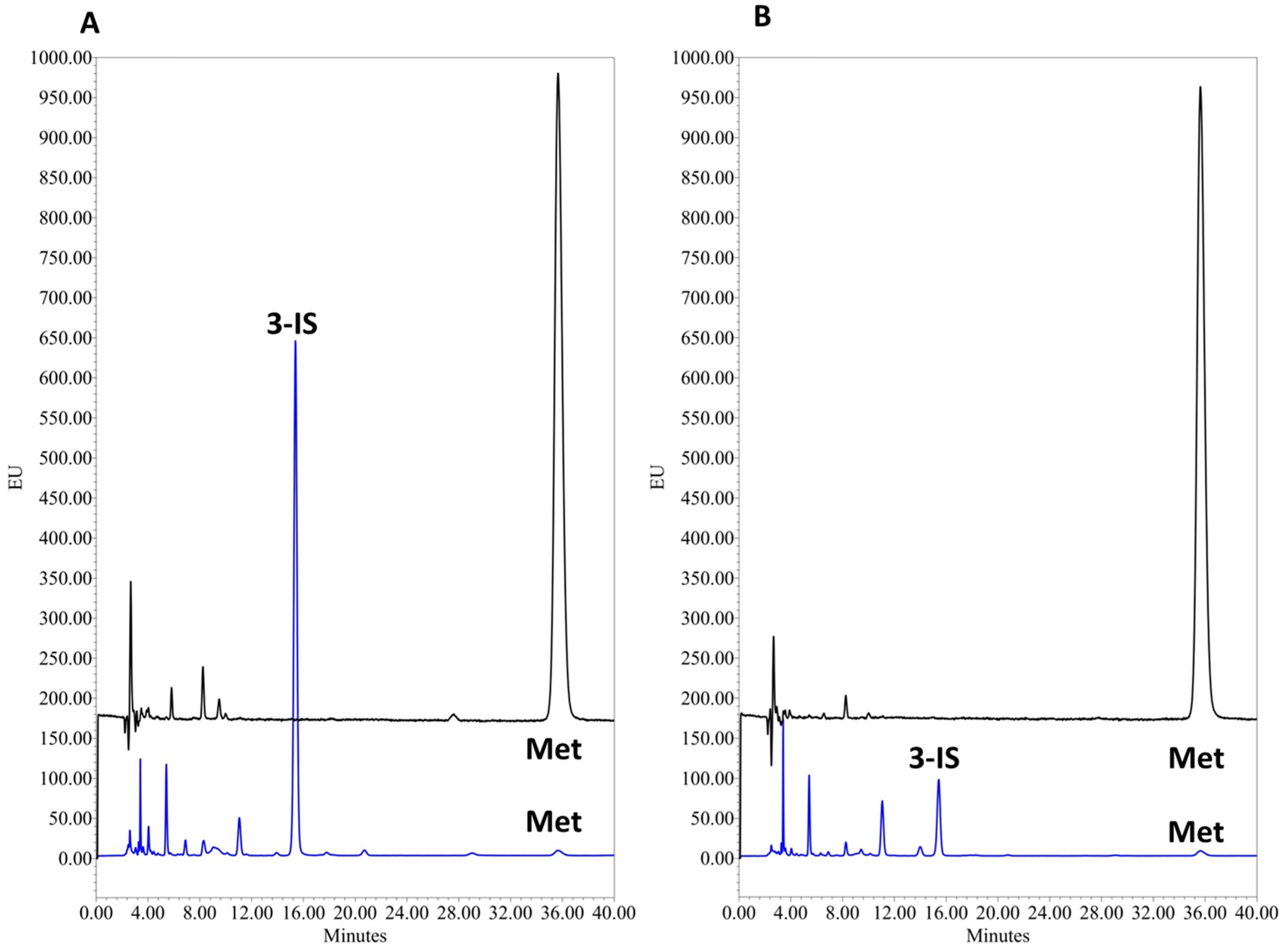
3.1.2. Selectivity and Sensitivity
3.1.3. Linearity
3.1.4. Accuracy and Precision
3.1.5. Recovery
3.1.6. Stability
3.2. Clinical Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Manos, J. The human microbiome in disease and pathology. APMIS 2022, 130, 690–705. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Vrankx, K.; Engberg, J.; Friis-Møller, A.; Brynskov, J.; Nordgaard-Lassen, I.; Petersen, A.M.; Krogfelt, K.A. Disease-Specific Enteric Microbiome Dysbiosis in Inflammatory Bowel Disease. Front. Med. 2018, 5, 304. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Gatza, E.; Reddy, P.; Choi, S.W. Prevention and Treatment of Acute Graft-versus-Host Disease in Children, Adolescents, and Young Adults. Biol. Blood Marrow Transplant. 2020, 26, e101–e112. [Google Scholar] [CrossRef]
- PDQ. Pediatric Treatment Editorial Board Complications, Graft-versus-Host Disease, and Late Effects after Pediatric Hematopoietic Stem Cell Transplant (PDQ®): Health Professional Version. 2002. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15994282 (accessed on 10 August 2024).
- Eriguchi, Y.; Takashima, S.; Oka, H.; Shimoji, S.; Nakamura, K.; Uryu, H.; Shimoda, S.; Iwasaki, H.; Shimono, N.; Ayabe, T.; et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood 2012, 120, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Devlin, S.M.; Staffas, A.; Lumish, M.; Khanin, R.; Littmann, E.R.; Ling, L.; Kosuri, S.; Maloy, M.; Slingerland, J.B.; et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J. Clin. Oncol. 2017, 35, 1650–1659. [Google Scholar] [CrossRef]
- Weber, D.; Jenq, R.R.; Peled, J.U.; Taur, Y.; Hiergeist, A.; Koestler, J.; Dettmer, K.; Weber, M.; Wolff, D.; Hahn, J.; et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 845–852. [Google Scholar] [CrossRef]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2017, 23, 820–829. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Zhang, Y.; Li, Q.; Yu, L.; Li, Q.; Liu, C.; Xie, Y.; Chen, K.; Ye, F.; et al. Alterations of the Gut Microbiome Composition and Lipid Metabolic Profile in Radiation Enteritis. Front. Cell. Infect. Microbiol. 2020, 10, 541178. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.-A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Jenq, R.R. Intestinal microbiota-related effects on graft-versus-host disease. Int. J. Hematol. 2015, 101, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Golob, J.L.; Pergam, S.A.; Srinivasan, S.; Fiedler, T.L.; Liu, C.; Garcia, K.; Mielcarek, M.; Ko, D.; Aker, S.; Marquis, S.; et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin. Infect. Dis. 2017, 65, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Leardini, D.; Muratore, E.; Fabbrini, M.; D’Amico, F.; Zama, D.; Baccelli, F.; Gottardi, F.; Belotti, T.; Ussowicz, M.; et al. Gut microbiota diversity before allogeneic hematopoietic stem cell transplantation as a predictor of mortality in children. Blood 2023, 142, 1387–1398. [Google Scholar] [CrossRef]
- Elgarten, C.W.; Margolis, E.B.; Kelly, M.S. The Microbiome and Pediatric Transplantation. J. Pediatr. Infect. Dis. Soc. 2024, 13, S80–S89. [Google Scholar] [CrossRef] [PubMed]
- Ingham, A.C.; Kielsen, K.; Mordhorst, H.; Ifversen, M.; Aarestrup, F.M.; Müller, K.G.; Pamp, S.J. Microbiota long-term dynamics and prediction of acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Microbiome 2021, 9, 148. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic T reg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Stevens, A.P.; Dettmer, K.; Gottfried, E.; Hoves, S.; Kreutz, M.; Holler, E.; Canelas, A.B.; Kema, I.; Oefner, P.J. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Oefner, P.J.; Hiergeist, A.; Koestler, J.; Geßner, A.; Weber, M.; Hahn, J.; Wolff, D.; Stämmler, F.; Spang, R.; et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are aßociated with poor outcome. Blood 2015, 126, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Peled, J.U.; Li, S.; Mahabamunuge, J.; Dagher, Z.; Slingerland, A.E.; Del Rio, C.; Valles, B.; Kempner, M.E.; Smith, M.; et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018, 2, 745–753. [Google Scholar] [CrossRef]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Holler, B.; Wolff, D.; et al. Metagenomic Analysis of the Stool Microbiome in Patients Receiving Allogeneic Stem Cell Transplantation: Loss. Biol. Blood Marrow Transplant. 2016, 20, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, H.; Zhang, G.; Sun, W.; Zhou, D.; Jiao, G.; Yu, Y. Development and validation of a ultra performance LC-ESI/MS method for analysis of metabolic phenotypes of healthy men in day and night urine samples. J. Sep. Sci. 2008, 31, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Olesova, D.; Galba, J.; Piestansky, J.; Celusakova, H.; Repiska, G.; Babinska, K.; Ostatnikova, D.; Katina, S.; Kovac, A. A novel uhplc-ms method targeting urinary metabolomic markers for autism spectrum disorder. Metabolites 2020, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.Z.; Dettmer, K.; Annavajhala, M.K.; Chong, D.H.; Uhlemann, A.-C.C.; Abrams, J.A.; Oefner, P.J.; Freedberg, D.E. Associations between urinary 3-indoxyl sulfate, a gut microbiome-derived biomarker, and patient outcomes after intensive care unit admission. J. Crit. Care 2021, 63, 15–21. [Google Scholar] [CrossRef]
- Kim, A.H.; Yoon, S.; Lee, Y.; Lee, J.; Bae, E.; Lee, H.; Kim, D.K.; Lee, S.; Yu, K.; Jang, I.-J.; et al. Assessment of Hepatic Cytochrome P450 3A Activity Using Metabolic Markers in Patients with Renal Impairment. J. Korean Med. Sci. 2018, 33, 1109811. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-S.; Yu, C.-P.; Hsieh, Y.-W.; Chao, P.-D.L.; Sweet, D.H.; Hou, Y.-C.; Lin, S.-P. Effects of antibiotics on the pharmacokinetics of indoxyl sulfate, a nephro-cardiovascular toxin. Xenobiotica 2020, 50, 588–592. [Google Scholar] [CrossRef]
- Valko-Rokytovská, M.; Hubková, B.; Birková, A.; Mašlanková, J.; Stupák, M.; Zábavníková, M.; Čižmárová, B.; Mareková, M. Specific urinary metabolites in malignant melanoma. Medicina 2019, 55, 145. [Google Scholar] [CrossRef] [PubMed]
- Banoglu, E.; King, R.S. Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2002, 27, 135–140. [Google Scholar] [CrossRef] [PubMed]
- NIH (US). Indoxyl Sulfate, National Center for Biotechnology Information. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Indoxyl-sulfate (accessed on 10 August 2024).
- Pretorius, C.J.; McWhinney, B.C.; Sipinkoski, B.; Johnson, L.A.; Rossi, M.; Campbell, K.L.; Ungerer, J.P.J. Reference ranges and biological variation of free and total serum indoxyl- and p-cresyl sulphate measured with a rapid UPLC fluorescence detection method. Clin. Chim. Acta 2013, 419, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.P.; Campagnolo, S.; Fernandes, S.R.; Marques, S.S.; Barreiros, L.; Sampaio-Maia, B.; Segundo, M.A. Rapid and sustainable HPLC method for the determination of uremic toxins in human plasma samples. Anal. Bioanal. Chem. 2023, 415, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Al Za’Abi, M.; Ali, B.; Al Toubi, M. HPLC-fluorescence method for measurement of the uremic toxin indoxyl sulfate in plasma. J. Chromatogr. Sci. 2013, 51, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Thongboonkerd, V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010, 402, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Perinchery, S.M.; Kuzhiumparambil, U.; Vemulpad, S.; Goldys, E.M. The influence of indoxyl sulfate and ammonium on the autofluorescence of human urine. Talanta 2010, 80, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Hiergeist, A.; Weber, M.; Dettmer, K.; Wolff, D.; Hahn, J.; Herr, W.; Gessner, A.; Holler, E. Detrimental Effect of Broad-spectrum Antibiotics on Intestinal Microbiome Diversity in Patients After Allogeneic Stem Cell Transplantation: Lack of Commensal Sparing Antibiotics. Clin. Infect. Dis. 2019, 68, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- CHMP. Guideline on Bioanalytical Method Validation; European Medicines Agency: Amsterdam, The Netherlands, 2011; Volume 4, pp. 1–26. [Google Scholar]
- FDA. Bioanalytical method validation. In New Drug Development: Regulatory Paradigms for Clinical Pharmacology and Biopharmaceutics; FDA: Silver Spring, MD, USA, 2018; pp. 138–159. ISBN 9780203026427. [Google Scholar] [CrossRef]
- Waikar, S.S.; Sabbisetti, V.S.; Bonventre, J.V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010, 78, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Tews, H.C.; Elger, T.; Gunawan, S.; Fererberger, T.; Sommersberger, S.; Loibl, J.; Huss, M.; Liebisch, G.; Müller, M.; Kandulski, A.; et al. Fecal short chain fatty acids and urinary 3-indoxyl sulfate do not discriminate between patients with Crohn’s disease and ulcerative colitis and are not of diagnostic utility for predicting disease severity. Lipids Health Dis. 2023, 22, 164. [Google Scholar] [CrossRef]
- van Lier, Y.F.; Vos, J.; Blom, B.; Hazenberg, M.D. Allogeneic hematopoietic cell transplantation, the microbiome, and graft-versus-host disease. Gut Microbes 2023, 15, 2178805. [Google Scholar] [CrossRef]
- Farowski, F.; Els, G.; Tsakmaklis, A.; Higgins, P.G.; Kahlert, C.R.; Stein-Thoeringer, C.K.; Bobardt, J.S.; Dettmer-Wilde, K.; Oefner, P.J.; Vehreschild, J.J.; et al. Assessment of urinary 3-indoxyl sulfate as a marker for gut microbiota diversity and abundance of Clostridiales. Gut Microbes 2019, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, B.; Księżarczyk, K.; Raćkowska, E.; Szlufik, S.; Koziorowski, D.; Giebułtowicz, J. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin. Chim. Acta 2020, 501, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Meibaum, J.; Krause, S.; Hillmer, H.; Marcelli, D.; Strohhöfer, C. Identification and characterisation of fluorescent substances in spent dialysis fluid. Int. J. Artif. Organs 2020, 43, 579–586. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; ALanazi, A.G.; Ali, I. A comparative and simultaneous analysis of indoxyl sulfate and sodium butyrate in human plasma by SPE and HPLC methods for kidney patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1159, 122356. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T.; Nakamura, M.; Tsutsumi, Y.; Suenaga, A.; Otagiri, M. Pharmacokinetics and tissue distribution of uraemic indoxyl sulphate in rats. Biopharm. Drug Dispos. 2003, 24, 345–355. [Google Scholar] [CrossRef]



| Artificial Urine | Artificial Urine + NH4 | % Bias | |||
|---|---|---|---|---|---|
| 3-IS (mg/L) | Ratio | Mean | Ratio | Mean | |
| 1 | 0.336 | 0.337 | 0.343 | 0.341 | 1.25% |
| 1 | 0.336 | 0.339 | |||
| 1 | 0.338 | 0.340 | |||
| 10 | 3.402 | 3.400 | 3.344 | 3.339 | −1.79% |
| 10 | 3.395 | 3.339 | |||
| 10 | 3.403 | 3.334 | |||
| Curve Number | Slope | Intercept | r2 | Range (mg/L) |
|---|---|---|---|---|
| 1 | 0.4841 | −0.0014 | 0.997 | 0.10–10.00 |
| 2 | 0.4645 | 0.0030 | 0.997 | 0.10–10.00 |
| 3 | 0.4212 | 0.0036 | 0.999 | 0.10–10.00 |
| 4 | 0.4115 | −0.0012 | 0.997 | 0.10–10.00 |
| Intra-Day Precision and Accuracy | Inter-Day Precision and Accuracy | |||||||
|---|---|---|---|---|---|---|---|---|
| Level | Nominal 3-IS Conc. (mg/L) | Day | Mean Observed Conc. (mg/L) | Precision (%) | Accuracy (%) | Mean Observed Conc. (mg/L) | Precision (%) | Accuracy (%) |
| L-LOQ | 0.10 | 1 | 0.105 | 0.47 | 105.3 | 108.4 | ||
| 2 | 0.113 | 1.37 | 112.6 | 0.108 | 3.10 | |||
| 3 | 0.107 | 1.47 | 107.3 | |||||
| M-QC | 1.00 | 1 | 1.037 | 1.33 | 103.7 | |||
| 2 | 1.026 | 1.23 | 102.6 | 1.021 | 1.60 | 102.1 | ||
| 3 | 1.016 | 0.58 | 101.6 | |||||
| U-LOQ | 10.00 | 1 | 10.105 | 0.20 | 101.1 | |||
| 2 | 10.008 | 0.33 | 100.1 | 10.047 | 1.70 | 100.5 | ||
| 3 | 10.261 | 0.33 | 102.6 | |||||
| Sample | 3-IS Concentration before Spiking (mg/L) | %CV | Nominal Spiking Level (mg/L) | 3-IS Urine Concentration after Spiking (mg/L) | %CV | Recovery (%) |
|---|---|---|---|---|---|---|
| HV 1 | 3.65 | 0.02 | 0.1 | 3.74 | 0.15 | 92.7 |
| 0.5 | 4.11 | 0.18 | 96.9 | |||
| 1 | 4.61 | 0.12 | 95.9 | |||
| HV 2 | 3.13 | 0.18 | 0.1 | 3.22 | 0.08 | 92.1 |
| 0.5 | 3.59 | 0.17 | 96.1 | |||
| 1 | 4.03 | 0.06 | 90.0 |
| Condition | Time | Parameter | Sample | ||||
|---|---|---|---|---|---|---|---|
| L-LOQ | M-QC | U-LOQ | HV 1 | HV 2 | |||
| Benchtop | 0 h | 3-IS (mg/L) | 0.101 | 0.995 | 10.205 | 76.268 | 69.485 |
| Precision % | 0.35 | 0.04 | 0.29 | 5.15 | 1.48 | ||
| 1 h | 3-IS (mg/L) | 0.098 | 1.046 | 9.994 | 71.865 | 68.387 | |
| Precision % | 0.37 | 0.25 | 1.49 | 1.48 | 0.55 | ||
| % vs. t0 | 97.0 | 105.1 | 97.9 | 94.2 | 98.4 | ||
| 2 h | 3-IS (mg/L) | 0.086 | 0.989 | 9.485 | 70.671 | 67.600 | |
| Precision % | 0.62 | 0.21 | 0.29 | 0.16 | 0.14 | ||
| % vs. t0 | 85.1 | 99.4 | 92.9 | 92.7 | 97.3 | ||
| 4 h | 3-IS (mg/L) | 0.100 | 0.968 | 9.434 | 69.984 | 68.912 | |
| Precision % | 0.07 | 0.06 | 0.14 | 0.06 | 0.23 | ||
| % vs. t0 | 99.0 | 97.3 | 92.4 | 91.8 | 99.2 | ||
| 6 h | 3-IS (mg/L) | 0.102 | 0.986 | 9.975 | 70.903 | 67.794 | |
| Precision % | 0.25 | 1.86 | 0.23 | 0.21 | 0.43 | ||
| % vs. t0 | 100.1 | 99.1 | 97.7 | 93.0 | 97.6 | ||
| 7 days | 3-IS (mg/L) | 0.093 | 0.946 | 8.953 | 81.784 | 70.484 | |
| CV% | 0.24 | 0.02 | 0.21 | 0.17 | 0.07 | ||
| % vs. t0 | 92.1 | 95.1 | 87.7 | 107.2 | 101.4 | ||
| 14 days | 3-IS (mg/L) | 0.093 | 0.891 | 8.636 | 87.702 | 77.221 | |
| Precision % | 0.24 | 0.68 | 0.05 | 0.27 | 0.6 | ||
| % vs. t0 | 92.1 | 89.5 | 84.6 | 115.0 | 111.1 | ||
| 4 °C | 0 h | 3-IS (mg/L) | 0.101 | 0.995 | 10.205 | 76.268 | 69.486 |
| Precision % | 0.35 | 0.04 | 0.29 | 5.15 | 1.48 | ||
| 1 h | 3-IS (mg/L) | 0.112 | 1.086 | 9.892 | 79.635 | 71.544 | |
| Precision % | 0.29 | 0.23 | 0.45 | 0.38 | 0.24 | ||
| % vs. t0 | 110.9 | 109.1 | 96.9 | 104.4 | 102.03 | ||
| 2 h | 3-IS (mg/L) | 0.108 | 1.008 | 9.262 | 81.194 | 69.345 | |
| Precision % | 0.03 | 0.15 | 0.25 | 0.10 | 0.02 | ||
| % vs. t0 | 106.9 | 101.3 | 90.7 | 106.5 | 99.8 | ||
| 4 h | 3-IS (mg/L) | 0.110 | 0.995 | 9.473 | 80.498 | 70.346 | |
| Precision % | 0.04 | 0.08 | 0.98 | 0.39 | 0.47 | ||
| % vs. t0 | 108.9 | 100.0 | 92.8 | 105.5 | 101.2 | ||
| 6 h | 3-IS (mg/L) | 0.112 | 1.039 | 10.099 | 83.783 | 73.008 | |
| Precision % | 0.47 | 0.31 | 0.42 | 1.06 | 0.39 | ||
| % vs. t0 | 110.9 | 104.4 | 99.0 | 109.9 | 105.1 | ||
| 7 days | 3-IS (mg/L) | 0.100 | 1.014 | 9.227 | 81.169 | 71.142 | |
| Precision % | 0.59 | 1.23 | 0.27 | 0.65 | 0.46 | ||
| % vs. t0 | 99.0 | 101.9 | 90.4 | 106.4 | 102.4 | ||
| 14 days | 3-IS (mg/L) | 0.108 | 0.940 | 9.347 | 80.680 | 70.085 | |
| Precision % | 0.75 | 0.45 | 0.77 | 0.49 | 0.63 | ||
| % vs. t0 | 106.9 | 94.5 | 91.6 | 105.8 | 100.9 |
| Condition | Time | Parameter | Sample | ||||
|---|---|---|---|---|---|---|---|
| L-LOQ | M-QC | U-LOQ | HV 1 | HV 2 | |||
| −20 °C | 1 month | ISu (mg/L) | 0.091 | 0.973 | 10.14 | 85.83 | 70.531 |
| Precision% | 1.06 | 0.41 | 0.12 | 0.12 | 0.06 | ||
| % vs. t0 | 90.1 | 97.8 | 99.4 | 112.5 | 101.5 | ||
| 2 months | ISu (mg/L) | 0.101 | 0.962 | 9.939 | 79.157 | 68.649 | |
| CV% | 0.9 | 0.7 | 0.26 | 0.12 | 0.2 | ||
| % vs. t0 | 100.0 | 96.7 | 97.4 | 103.8 | 98.8 |
| Patient ID | Age (Years) | Sex | Time * (Days) | Concomitant Drugs | Underlying Disease | [3-IS] (mg/L) [3-IS] (μmol3-IS/mmolcreat) | |
|---|---|---|---|---|---|---|---|
| 1 | 3 | M | −7 | ACY, CAP, CID, ITRA, PRO, TRI/SULFA, VALGA | Chronic granulomatous disease | 13.6 | 67.9 |
| 2 | 8 | F | +25 | ACY, AMPT, BU, LOR, MPN, OMP, OND, TRI/SULFA, UDC, VANC | Myelodysplasia | 2.0 | 8.3 |
| 3 | 6 | M | −14 | DIP, FAM, LVM, TRM/SMX | Acute myeloid leukemia | 31.9 | 22.5 |
| 4 | 10 | F | −4 | FAM, HYD, TAC, VALGA, VCZ | Medullar aplasia | 8.1 | 3.4 |
| 5 | 13 | F | −8 | FAM, FLU, LVM, OND, PIP/TAZO, TRI/SULFA | Acute myeloid leukemia | 2.8 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivetti, C.E.; Fernández, M.F.; Stojanova, J.; Ruvinsky, S.; Mangano, A.; Schaiquevich, P. Full Validation and Application to Clinical Research of a High-Performance Liquid Chromatography Method for the Assessment of Urinary 3-Indoxyl Sulfate in Pediatric Patients with Hematopoietic Stem Cell Transplant. Methods Protoc. 2024, 7, 64. https://doi.org/10.3390/mps7040064
Olivetti CE, Fernández MF, Stojanova J, Ruvinsky S, Mangano A, Schaiquevich P. Full Validation and Application to Clinical Research of a High-Performance Liquid Chromatography Method for the Assessment of Urinary 3-Indoxyl Sulfate in Pediatric Patients with Hematopoietic Stem Cell Transplant. Methods and Protocols. 2024; 7(4):64. https://doi.org/10.3390/mps7040064
Chicago/Turabian StyleOlivetti, Christian Ezequiel, María Florencia Fernández, Jana Stojanova, Silvina Ruvinsky, Andrea Mangano, and Paula Schaiquevich. 2024. "Full Validation and Application to Clinical Research of a High-Performance Liquid Chromatography Method for the Assessment of Urinary 3-Indoxyl Sulfate in Pediatric Patients with Hematopoietic Stem Cell Transplant" Methods and Protocols 7, no. 4: 64. https://doi.org/10.3390/mps7040064






