Quantification of Circulating Cell-Free DNA as a NETosis Marker in Trauma Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Neutrophil and NET Isolation
2.4. DNA Isolation from Neutrophils
2.5. Absolute Quantification of NETs by the Standard Curve Method
2.6. DNA Isolation from Serum Samples
2.7. Fluorometric Quantification of DNA in Serum Samples
2.8. ELISA
2.9. Statistical Analysis
3. Results
3.1. Study Cohort Characteristics
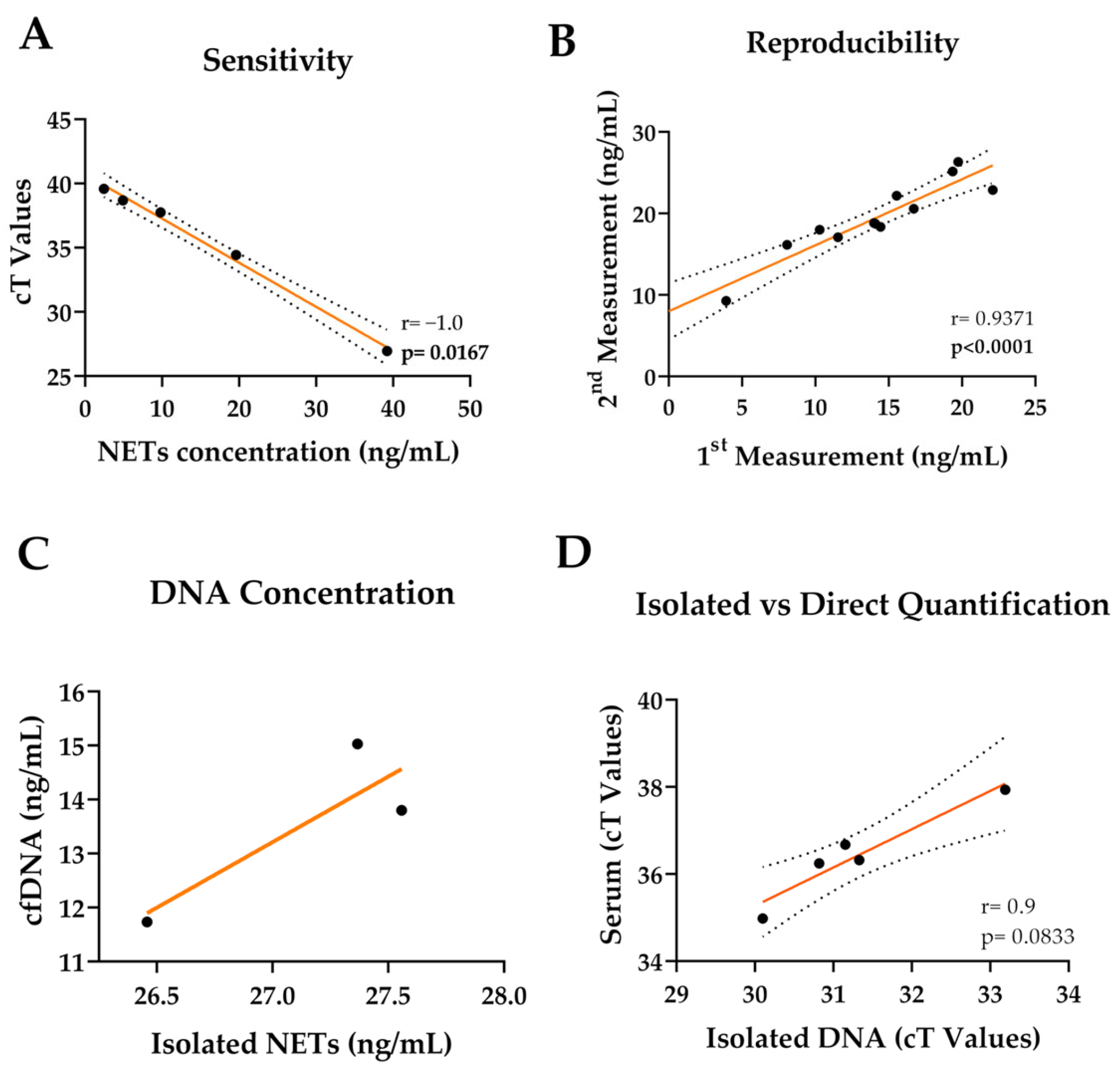
3.2. cfDNA Can Be Quantified from Serum Samples Directly
3.3. ELISA or Fluorometric Quantification Is Not Sensitive in Low Concentrations
| ELA2 (ELISA) | MPO (ELISA) | citH3 (ELISA) | cfDNA (Fluorometric) | cfDNA (qPCR) | ||
|---|---|---|---|---|---|---|
| LoD (ng/mL) | EDTA | 0.197 | 0.005 | N/A | 90.807 | N/A |
| Heparin | 0.378 | 0.002 | 52.356 | 26.529 | N/A | |
| Citrate | 0.334 | 0.017 | 8.777 | 69.217 | N/A | |
| Serum | 0.001 | 0.16 | 0.972 | 36.013 | 0.84 | |
| LoQ (ng/mL) | EDTA | 0.598 | 0.015 | N/A | 275.174 | N/A |
| Heparin | 1.145 | 0.006 | 158.655 | 80.393 | N/A | |
| Citrate | 1.012 | 0.053 | 26.599 | 209.751 | N/A | |
| Serum | 0.004 | 0.489 | 2.947 | 109.133 | 2.56 | |
| Sensitivity | EDTA | 14.3% | 46.2% | N/A | 60% | N/A |
| Heparin | 14.5% | 46.8% | 7.4% | 76% | N/A | |
| Citrate | 14.8% | 44.6% | 8.9% | 105% | N/A | |
| Serum | 15.9% | 47.9% | 16% | 107% | 98% | |
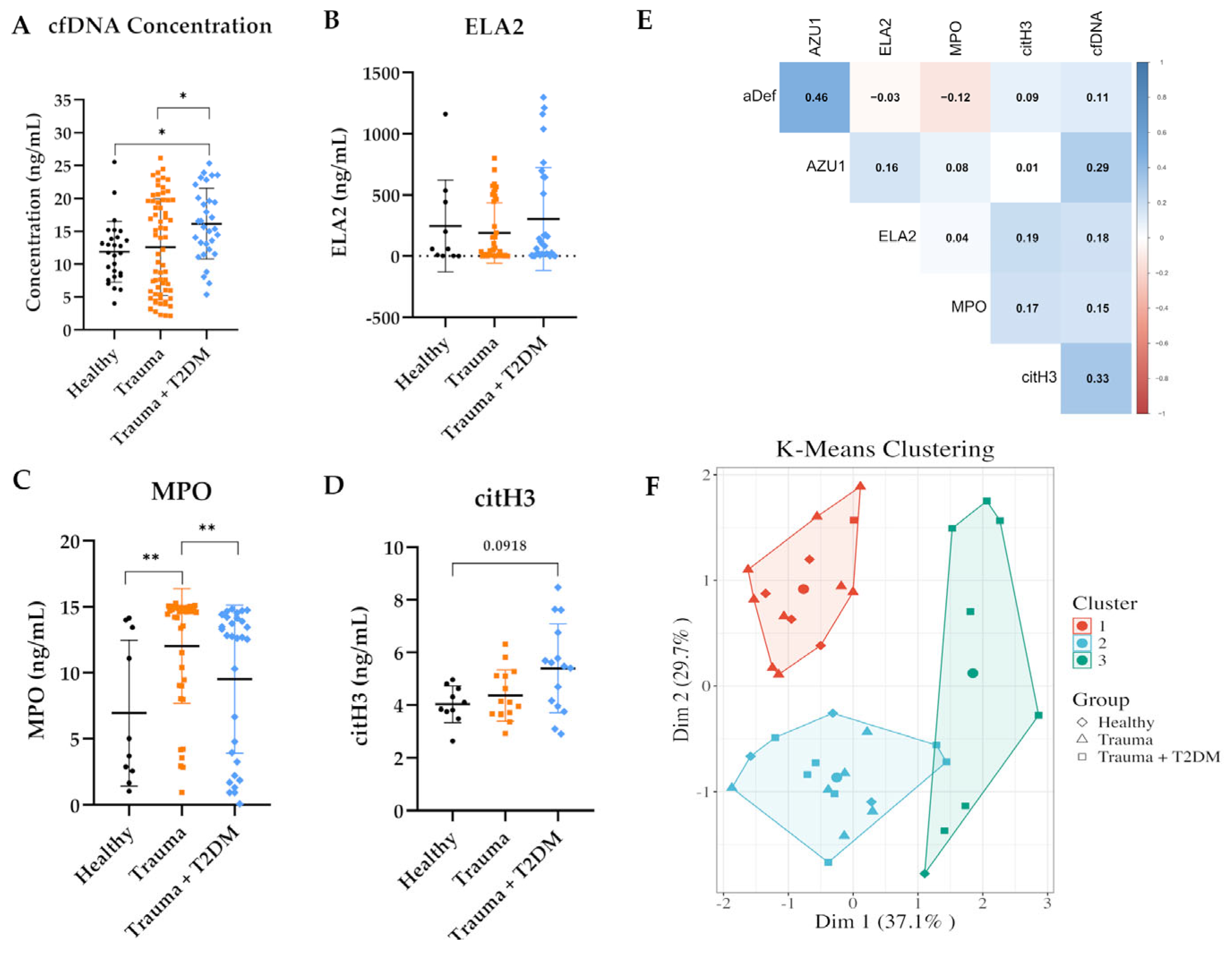
3.4. cfDNA and NET-Related Protein Concentrations in Trauma Patients
3.5. Correlation of qPCR and Fluorometric Quantification in Serum Samples
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoimenou, M.; Tzoros, G.; Skendros, P.; Chrysanthopoulou, A. Methods for the Assessment of NET Formation: From Neutrophil Biology to Translational Research. Int. J. Mol. Sci. 2022, 23, 15823. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Hamam, H.J.; Palaniyar, N. Post-Translational Modifications in NETosis and NETs-Mediated Diseases. Biomolecules 2019, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Yang, S.; Gu, Z.; Lu, C.; Zhang, T.; Guo, X.; Xue, G.; Zhang, L. Neutrophil Extracellular Traps Are Markers of Wound Healing Impairment in Patients with Diabetic Foot Ulcers Treated in a Multidisciplinary Setting. Adv. Wound Care 2019, 9, 16–27. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Disability Adjusted Life Years (DALYs). Disability Adjusted Life Years (DALYs). Disability adjusted life years (DALYs). In Encyclopedia of Public Health; Kirch, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 267–268. [Google Scholar]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Schreiner, A.J.; Stollhof, L.; Stöckle, U.; Nussler, A.; Fritsche, A.; Pscherer, S. Assessment of the Influence of Diabetes mellitus and Malnutrition on the Postoperative Complication Rate and Quality of Life of Patients in a Clinic Focused on Trauma Surgery. Z. Orthop. Unfall. 2019, 157, 173–182. [Google Scholar] [CrossRef]
- Pscherer, S.; Nussler, A.; Bahrs, C.; Reumann, M.; Ihle, C.; Stöckle, U.; Ehnert, S.; Freude, T.; Ochs, B.; Flesch, I.; et al. Retrospektive Analyse von Diabetikern im Hinblick auf Behandlungsdauer und Behandlungskosten in einem überregionalen Traumazentrum. Z. Orthop. Unfall. 2016, 12, 72–76. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Frechtel, G.; Cerrone, G.; Linari, M.; Gonzalez, C.; Casais, P.; Schattner, M. NETosis before and after hyperglycemic control in type 2 diabetes mellitus patients. PLoS ONE 2016, 11, e0168647. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, A.; Yamada, M.; Shida, H.; Nakazawa, D.; Kusunoki, Y.; Nakamura, A.; Miyoshi, H.; Tomaru, U.; Atsumi, T.; Ishizu, A. Circulating neutrophil extracellular trap levels in well-controlled Type 2 diabetes and pathway involved in their formation induced by high-dose glucose. Pathobiology 2016, 83, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.; Menegazzo, L.; Rigato, M.; Scattolini, V.; Poncina, N.; Bruttocao, A.; Ciciliot, S.; Mammano, F.; Ciubotaru, C.; Brocco, E. NETosis delays diabetic wound healing in mice and humans. Diabetes 2016, 65, 1061–1071. [Google Scholar] [CrossRef]
- de Buhr, N.; von Köckritz-Blickwede, M. Detection, Visualization, and Quantification of Neutrophil Extracellular Traps (NETs) and NET Markers. In Neutrophil: Methods and Protocols; Quinn, M.T., DeLeo, F.R., Eds.; Springer: New York, NY, USA, 2020; pp. 425–442. [Google Scholar]
- Schedel, F.; Mayer-Hain, S.; Pappelbaum, K.I.; Metze, D.; Stock, M.; Goerge, T.; Loser, K.; Sunderkötter, C.; Luger, T.A.; Weishaupt, C. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment Cell Melanoma Res. 2020, 33, 63–73. [Google Scholar] [CrossRef]
- Meddeb, R.; Dache, Z.A.A.; Thezenas, S.; Otandault, A.; Tanos, R.; Pastor, B.; Sanchez, C.; Azzi, J.; Tousch, G.; Azan, S.; et al. Quantifying circulating cell-free DNA in humans. Sci. Rep. 2019, 9, 5220. [Google Scholar] [CrossRef]
- Brankatschk, R.; Bodenhausen, N.; Zeyer, J.; Bürgmann, H. Simple absolute quantification method correcting for quantitative PCR efficiency variations for microbial community samples. Appl. Environ. Microbiol. 2012, 78, 4481–4489. [Google Scholar] [CrossRef]
- Masuda, S.; Shimizu, S.; Matsuo, J.; Nishibata, Y.; Kusunoki, Y.; Hattanda, F.; Shida, H.; Nakazawa, D.; Tomaru, U.; Atsumi, T.; et al. Measurement of NET formation in vitro and in vivo by flow cytometry. Cytometry Part A 2017, 91, 822–829. [Google Scholar] [CrossRef]
- Silva, L.M.; Moutsopoulos, N.; Bugge, T.H.; Doyle, A. Live Imaging and Quantification of Neutrophil Extracellular Trap Formation. Curr. Protoc. 2021, 1, e157. [Google Scholar] [CrossRef]
- Kraaij, T.; Tengström, F.C.; Kamerling, S.W.A.; Pusey, C.D.; Scherer, H.U.; Toes, R.E.M.; Rabelink, T.J.; van Kooten, C.; Teng, Y.K.O. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun. Rev. 2016, 15, 577–584. [Google Scholar] [CrossRef]
- Ruoß, M.; Kieber, V.; Rebholz, S.; Linnemann, C.; Rinderknecht, H.; Häussling, V.; Häcker, M.; Olde Damink, L.H.H.; Ehnert, S.; Nussler, A.K. Cell-Type-Specific Quantification of a Scaffold-Based 3D Liver Co-Culture. Methods Protoc. 2020, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Hamam, H.J.; Khan, M.A.; Palaniyar, N. Histone Acetylation Promotes Neutrophil Extracellular Trap Formation. Biomolecules 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; Deleo, F.R.; Quinn, M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2014, 1124, 3–10. [Google Scholar] [CrossRef]
- Sorvillo, N.; Cherpokova, D.; Martinod, K.; Wagner, D.D. Extracellular DNA NET-Works With Dire Consequences for Health. Circ. Res. 2019, 125, 470–488. [Google Scholar] [CrossRef]
- Stockley, R.; De Soyza, A.; Gunawardena, K.; Perrett, J.; Forsman-Semb, K.; Entwistle, N.; Snell, N. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir. Med. 2013, 107, 524–533. [Google Scholar] [CrossRef]
- Kraaij, T.; Kamerling, S.W.A.; de Rooij, E.N.M.; van Daele, P.L.A.; Bredewold, O.W.; Bakker, J.A.; Bajema, I.M.; Scherer, H.U.; Toes, R.E.M.; Huizinga, T.J.W.; et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J. Autoimmun. 2018, 91, 45–54. [Google Scholar] [CrossRef]
- Sperling, R.I.; Benincaso, A.I.; Anderson, R.J.; Coblyn, J.S.; Austen, K.F.; Weinblatt, M.E. Acute and chronic suppression of leukotriene B4 synthesis ex vivo in neutrophils from patients with rheumatoid arthritis beginning treatment with methotrexate. Arthritis Rheum. 1992, 35, 376–384. [Google Scholar] [CrossRef]
- Ostafin, M.; Pruchniak, M.P.; Ciepiela, O.; Reznick, A.Z.; Demkow, U. Different procedures of diphenyleneiodonium chloride addition affect neutrophil extracellular trap formation. Anal. Biochem. 2016, 509, 60–66. [Google Scholar] [CrossRef]
- Kirchner, T.; Hermann, E.; Möller, S.; Klinger, M.; Solbach, W.; Laskay, T.; Behnen, M. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediat. Inflamm. 2013, 2013, 710239. [Google Scholar] [CrossRef]
- Handono, K.; Sidarta, Y.O.; Pradana, B.A.; Nugroho, R.A.; Hartono, I.A.; Kalim, H.; Endharti, A.T. Vitamin D prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Medica Indones. 2014, 46, 189–198. [Google Scholar]
- Trivedi, A.; Khan, M.A.; Bade, G.; Talwar, A. Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development. Biomedicines 2021, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ali, Z.S.; Sweezey, N.; Grasemann, H.; Palaniyar, N. Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation. Genes 2019, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Chamardani, T.M.; Amiritavassoli, S. Inhibition of NETosis for treatment purposes: Friend or foe? Mol. Cell. Biochem. 2022, 477, 673–688. [Google Scholar] [CrossRef]
- Timmermans, K.; Kox, M.; Scheffer, G.J.; Pickkers, P. Plasma Nuclear and Mitochondrial DNA Levels, and Markers of Inflammation, Shock, and Organ Damage in Patients with Septic Shock. Shock 2016, 45, 607–612. [Google Scholar] [CrossRef]
- Gautschi, O.; Bigosch, C.; Huegli, B.; Jermann, M.; Marx, A.; Chassé, E.; Ratschiller, D.; Weder, W.; Joerger, M.; Betticher, D.C.; et al. Circulating Deoxyribonucleic Acid As Prognostic Marker in Non–Small-Cell Lung Cancer Patients Undergoing Chemotherapy. J. Clin. Oncol. 2004, 22, 4157–4164. [Google Scholar] [CrossRef]
- Mahmoud, E.H.; Fawzy, A.; Ahmad, O.K.; Ali, A.M. Plasma Circulating Cell-free Nuclear and Mitochondrial DNA as Potential Biomarkers in the Peripheral Blood of Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2015, 16, 8299–8305. [Google Scholar] [CrossRef]
- Zachariah, R.R.; Schmid, S.; Buerki, N.; Radpour, R.; Holzgreve, W.; Zhong, X. Levels of Circulating Cell-Free Nuclear and Mitochondrial DNA in Benign and Malignant Ovarian Tumors. Obstet. Gynecol. 2008, 112, 843–850. [Google Scholar] [CrossRef]
- Aydın, Ş.; Özdemir, S.; Adıgüzel, A. The Potential of cfDNA as Biomarker: Opportunities and Challenges for Neurodegenerative Diseases. J. Mol. Neurosci. 2025, 75, 34. [Google Scholar] [CrossRef]
- Le Calvez-Kelm, F.; Foll, M.; Wozniak, M.B.; Delhomme, T.M.; Durand, G.; Chopard, P.; Pertesi, M.; Fabianova, E.; Adamcakova, Z.; Holcatova, I.; et al. KRAS mutations in blood circulating cell-free DNA: A pancreatic cancer case-control. Oncotarget 2016, 7, 78827–78840. [Google Scholar] [CrossRef]
- Nakano, Y.; Kitago, M.; Matsuda, S.; Nakamura, Y.; Fujita, Y.; Imai, S.; Shinoda, M.; Yagi, H.; Abe, Y.; Hibi, T.; et al. KRAS mutations in cell-free DNA from preoperative and postoperative sera as a pancreatic cancer marker: A retrospective study. Br. J. Cancer 2018, 118, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef]
- Matta, B.; Battaglia, J.; Barnes, B.J. Detection of neutrophil extracellular traps in patient plasma: Method development and validation in systemic lupus erythematosus and healthy donors that carry IRF5 genetic risk. Front. Immunol. 2022, 13, 951254. [Google Scholar] [CrossRef]
- Altrichter, J.; Zedler, S.; Kraft, R.; Faist, E.; Mitzner, S.R.; Sauer, M.; Windolf, J.; Scholz, M.; Lögters, T. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur. J. Trauma Emerg. Surg. 2010, 36, 551–557. [Google Scholar] [CrossRef]
- Margraf, S.; Lögters, T.; Reipen, J.; Altrichter, J.; Scholz, M.; Windolf, J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): A potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock 2008, 30, 352–358. [Google Scholar] [CrossRef]
- de Buhr, N.; von Köckritz-Blickwede, M. How Neutrophil Extracellular Traps Become Visible. J. Immunol. Res. 2016, 2016, 4604713. [Google Scholar] [CrossRef]
- Gavillet, M.; Martinod, K.; Renella, R.; Harris, C.; Shapiro, N.I.; Wagner, D.D.; Williams, D.A. Flow cytometric assay for direct quantification of neutrophil extracellular traps in blood samples. Am. J. Hematol. 2015, 90, 1155–1158. [Google Scholar] [CrossRef]
- Kasprzycka, W.; Homa-Mlak, I.; Mlak, R.; Małecka-Massalska, T. Direct and indirect methods of evaluating the NETosis process. J. Pre-Clin. Clin. Res. 2019, 13, 50–56. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Autar, A.S.A.; Sultan, A.R.; Abraham, T.E.; van Cappellen, W.A.; Houtsmuller, A.B.; van Wamel, W.J.B.; van Beusekom, H.M.M.; van Neck, J.W.; de Maat, M.P.M. In vitro induction of NETosis: Comprehensive live imaging comparison and systematic review. PLoS ONE 2017, 12, e0176472. [Google Scholar] [CrossRef]




| Control | Trauma | Trauma + T2DM | |||
|---|---|---|---|---|---|
| Age (Years) | 45 ± 15.6 | 50.7 ± 14.4 | 62.06 ± 9.2 | ||
| Gender | 14 Female, 13 Male | 30 Female, 37 Male | 12 Female, 19 Male | ||
| Random Blood Glucose (mg/dL) | N/A | 100.2 ± 15.4 | 191.6 ± 63.4 | ||
| HbA1c (%) | N/A | N/A | 7.9 ± 1.1 | ||
| CRP (mg/L) | N/A | 23.7 ± 28.3 | 49.5 ± 75.6 | ||
| Trauma Types | Bone Complications | Pseudoarthrosis | - | 11 | 11 |
| Osteomyelitis | - | 8 | 3 | ||
| Joint Empyema | - | 7 | 1 | ||
| Arthrodesis | - | 2 | - | ||
| Fracture Infection | - | 7 | 3 | ||
| Wound Complications | Wound Infection | - | 24 | 20 | |
| Outer Malleolus Formation | - | 1 | - | ||
| Foot Necrosis | - | 5 | 3 | ||
| Fistula | - | 1 | - | ||
| Limp Ulceration | - | 1 | - | ||
| Primer | GenBank Accession | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Amplicon Size (bp) | Ta (°C) |
|---|---|---|---|---|---|
| KRAS | NC_000012.12 | CCTTGGGTTT–CAAGTTATATG | CCCTGACATA–CTCCCAAGGA | 67 | 60 |
| Target Protein | Order No. | Company | Dilution Factor | Chromogenic Substrate |
|---|---|---|---|---|
| α-Defensin | DY8198-05 | R&D Systems | 20 | Tetramethylbenzidine |
| AZU1 | ELH-AZU1 | RayBio | 5 | Tetramethylbenzidine |
| citH3 | Cay501620-96 | Cayman Chemicals | 25 | Tetramethylbenzidine |
| ELA2 | DY9167-05 | R&D Systems | 25 | Tetramethylbenzidine |
| MPO | DY3174 | R&D Systems | 25 | Tetramethylbenzidine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin, F.; Breinbauer, R.; Linnemann, C.; Tombaz, M.; Nussler, A.K.; Ehnert, S. Quantification of Circulating Cell-Free DNA as a NETosis Marker in Trauma Patients with Type 2 Diabetes Mellitus. Methods Protoc. 2025, 8, 42. https://doi.org/10.3390/mps8020042
Sahin F, Breinbauer R, Linnemann C, Tombaz M, Nussler AK, Ehnert S. Quantification of Circulating Cell-Free DNA as a NETosis Marker in Trauma Patients with Type 2 Diabetes Mellitus. Methods and Protocols. 2025; 8(2):42. https://doi.org/10.3390/mps8020042
Chicago/Turabian StyleSahin, Filiz, Regina Breinbauer, Caren Linnemann, Melike Tombaz, Andreas K. Nussler, and Sabrina Ehnert. 2025. "Quantification of Circulating Cell-Free DNA as a NETosis Marker in Trauma Patients with Type 2 Diabetes Mellitus" Methods and Protocols 8, no. 2: 42. https://doi.org/10.3390/mps8020042
APA StyleSahin, F., Breinbauer, R., Linnemann, C., Tombaz, M., Nussler, A. K., & Ehnert, S. (2025). Quantification of Circulating Cell-Free DNA as a NETosis Marker in Trauma Patients with Type 2 Diabetes Mellitus. Methods and Protocols, 8(2), 42. https://doi.org/10.3390/mps8020042









