Abstract
A pharyngeal jaw and loose pharyngeal teeth from Prairie Creek, Indiana, and loose pharyngeal teeth and two basioccipital pharyngeal processes from Bell Cave, Alabama, appear to be those of Moxostoma hubbsi (Copper Redhorse). Among suckers, only M. carinatum, M. hubbsi, M. robustum, and M. ugidatli have molariform teeth on their pharyngeal jaws, with M. hubbsi having the teeth of the largest relative size. Measurements of most of the teeth from Prairie Creek overlap with those of M. hubbsi, and the largest tooth from Bell Cave is the largest pharyngeal tooth measured. The more complete pharyngeal process of the basioccipital possesses a small condyle and stout processes along it that are indicative of M. hubbsi as well as a smaller centrum for articulation with the Weberian complex. Moxostoma hubbsi currently occupies an area around Montreal, Canada, that was glaciated at the time the fossils were laid down, and the area was later occupied by the Champlain Sea and Lampsilis Lake before becoming riverine about 6000–5000 years ago, meaning that M. hubbsi had to have arrived at its current distribution relatively recently and been extirpated from elsewhere.
Key Contribution:
The endangered Copper Redhorse is only known from the St. Lawrence River around Montreal, Canada, an area that was covered in ice during the last glacial cycle. However, based on fossil evidence, the distribution of the species once included Indiana and Alabama in the Mississippi River basin.
1. Introduction
Fishes of the family Catostomidae (suckers) are found mostly in North America, but one species, Myxocyprinus asiaticus, is found only in Asia and one species (Catostomus catostomus) has populations that recolonized Asia from North America [1]. Castostomids, as members of the order Cypriniformes, lack oral teeth but do have well developed pharyngeal teeth. Cypriniformes have a thickened, muscularized palatal organ supported by the pharyngobranchials, epibranchials, and the basioccipital pharyngeal process (a bony extension of the basioccipital that extends below and posterior to the occipital condyle). The palatal organ functions to separate food from inorganic materials. In cyprinids and catostomids, there is also the presence of a chewing pad, which is a keratinized portion of the palatal organ (catostomids) or the basioccipital (cyprinids) [2,3].
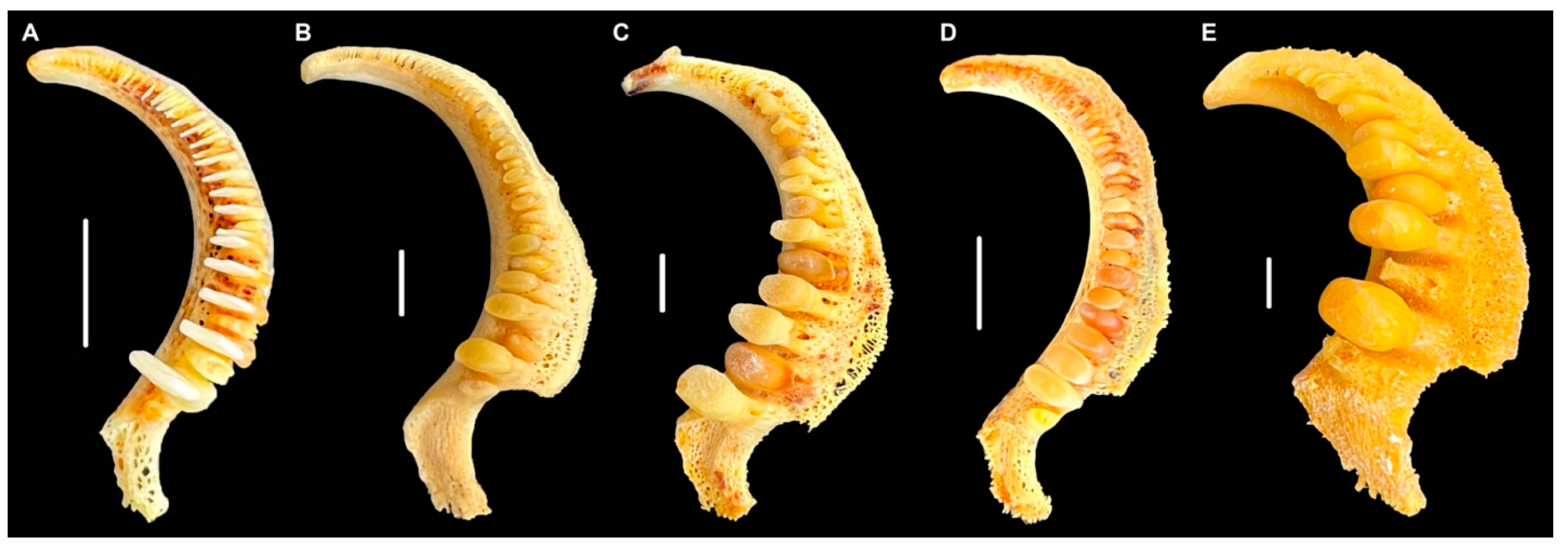
In eastern North America, the most speciose genus of suckers is Moxostoma or Redhorses and Jumprocks. Most Moxostoma have narrow pharyngeal teeth arranged in a comb-like fashion (Figure 1A), but four species of Redhorse have molariform teeth that occlude against the chewing pad for crushing mollusk shells [2,3,4]: M. ugidatli Jenkins, Favrot, Freeman, Albanese, and Armbruster (Sicklefin Redhorse, Figure 1B), M. carinatum (Cope) (River Redhorse, Figure 1C), M. robustum (Cope) (Robust Redhorse, Figure 1D), and M. hubbsi Legendre (Copper Redhorse, Figure 1E). Based on the most complete phylogeny to date for catostomids [5], it is likely that molariform pharyngeal teeth evolved three times with the Sicklefin and River Redhorse as sister species. Today, M. carinatum is found throughout the Great Lakes, Mississippi River, Gulf of Mexico coastal drainages from the Escambia River to the Pearl River, and is sympatric with M. hubbsi in the St. Lawrence River; M. robustum occurs along the south Atlantic slope from North Carolina to Georgia; M. ugidatli is in the Little Tennessee and Hiwassee of North Carolina and Georgia; and the distribution of M. hubbsi is only in the Saint Lawrence River basin around Montreal, Canada in Quebec and Ottawa ([6]; Figure 2). No other freshwater fish species has a distribution even remotely like that of M. hubbsi [7], and the St. Lawrence was covered by glaciers that did not begin to recede until about 14,500 years ago and was later occupied by the Champlain Sea [8,9]. Among the molariform Moxostoma, M. hubbsi represents an extreme of tooth development (Figure 1D), and because of this, it is likely possible to identify the species from its pharyngeal teeth.
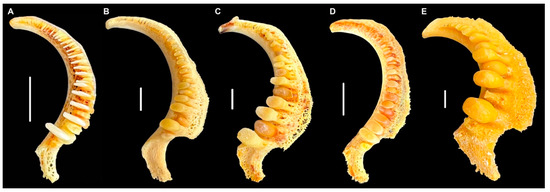
Figure 1.
Left pharyngeal jaws, occlusal view, of (A) Moxostoma breviceps Cope, AUM uncataloged isolated jaw from Swain Co., NC; (B) Moxostoma ugidatli, AUM 86400; (C) M. carinatum, AUM 86333; (D) M. robustum, AUM 86420, and (E) M. hubbsi, CU 52912. Scale = 1 cm. Photos by J.W. Armbruster.
Two main collections suggest that M. hubbsi may have had a broader distribution in the Pleistocene. The first is a collection from Prairie Creek, Indiana, in Zone D. The discovery of mastodon bones in 1972 brought researchers to study the Prairie Creek Area of Daviess Co., Washington, IN, USA [10]. Zone D of Prairie Creek was believed to have been a lake in the Late Pleistocene that preserved some aquatic life as it drained ~14,000 years ago [10,11,12]. Among the fossils found were some fish microfossils [10] including a relatively large pharyngeal jaw of a sucker, a smaller pharyngeal jaw, and some loose teeth. The large pharyngeal jaw (left side) is a fragment from the second tooth from the ventral end to about the sixth tooth, and it bears two full, molariform teeth (teeth 2 and 3) and the eroded bases of teeth 4–6 (Figure 3A). One of us (REJ) had borrowed the specimens believing that the large jaw and teeth could be of the Copper Redhorse (Moxostoma hubbsi).
The second collection is from Bell Cave, Alabama, and was collected in 1987 (note that the cave is now closed and illegal to enter). The herpetofauna of Bell Cave has been described [13] and fish specimens were mentioned as having been found. Fish specimens were eventually described including specimens purportedly of M. carinatum, M. macrolepidotum (Lesueur) (Shorthead Redhorse), and the extinct M. lacerum (Jordan and Brayton) (Harelip Sucker) as well as other sucker genera [14]. A pharyngeal tooth examined from Bell Cave was thought to be that of M. carinatum. Unavailable for the Jacquemin et al. [14] study was a set of Moxostoma pharyngeal teeth collected from Zone 1/2, 3 in Bell Cave. Amongst the teeth is the largest pharyngeal tooth we have observed (Figure 4). Zone 1/2 was dated as 11,820 +480/−500 BP, so roughly the same time as the Indiana specimens with both near the end of the Pleistocene (end at 11,700 BP). Some fossil teeth at Bell Cave were also found in Zone 4, which is 26,500 +870/−990 BP [14]. Also found was an isolated basioccipital pharyngeal process from Zone 3 (which is not dated; Figure 5C) that can be identified to species and a fragment of a basioccipital pharyngeal process from Zone 1/2. Our objective was to determine the species identity of these pharyngeal teeth and basioccipitals to have a better picture of catostomid biodiversity in what is now the Mississippi River basin.
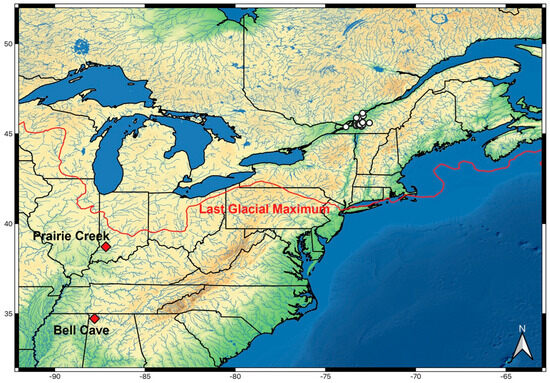
Figure 2.
Distribution of M. hubbsi (white dots) and fossil localities (red diamonds). Maximum extent of last glacier indicated (~20.7–18.0 Ka). Glacial extent from Dalton et al. [15].
Figure 2.
Distribution of M. hubbsi (white dots) and fossil localities (red diamonds). Maximum extent of last glacier indicated (~20.7–18.0 Ka). Glacial extent from Dalton et al. [15].
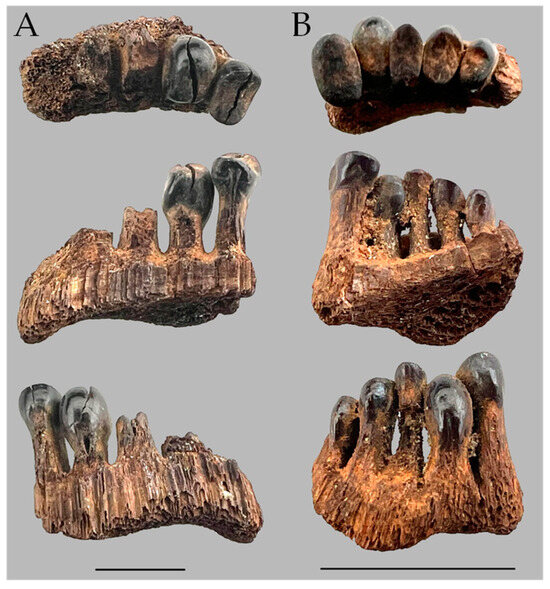
Figure 3.
Portions of pharyngeal jaws from Prairie Creek, Indiana. (A) Large specimen, INSM L-849; (B) small specimen INSM L-1188. Scale = 1 cm. Photos by J.W. Armbruster.
Figure 3.
Portions of pharyngeal jaws from Prairie Creek, Indiana. (A) Large specimen, INSM L-849; (B) small specimen INSM L-1188. Scale = 1 cm. Photos by J.W. Armbruster.
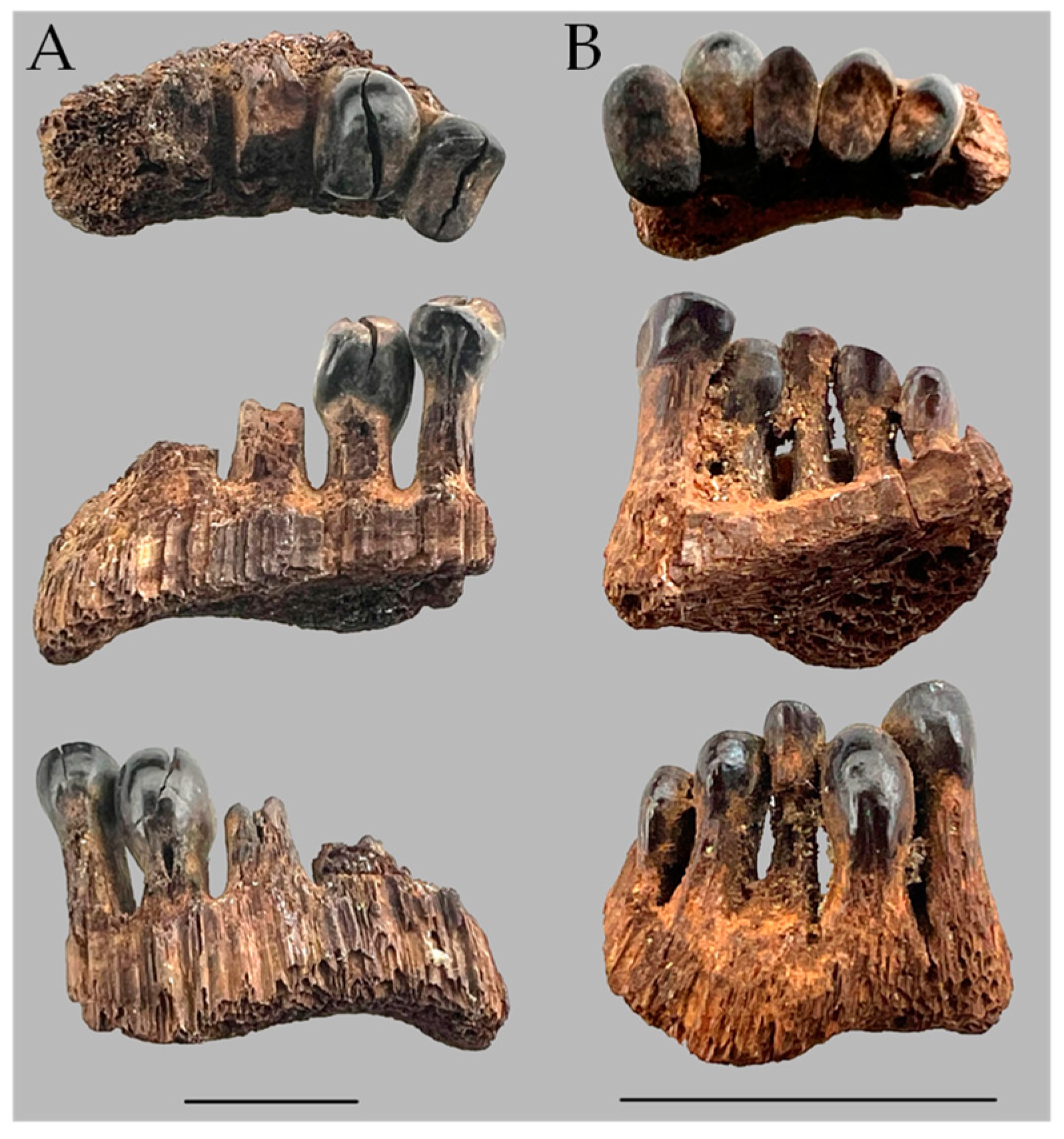

Figure 4.
Pharyngeal teeth from Bell Cave, Al, side (bottom) and occlusal (top) views. MSC 51132. Scale = 1 cm. Photos by J.W. Armbruster.
Figure 4.
Pharyngeal teeth from Bell Cave, Al, side (bottom) and occlusal (top) views. MSC 51132. Scale = 1 cm. Photos by J.W. Armbruster.

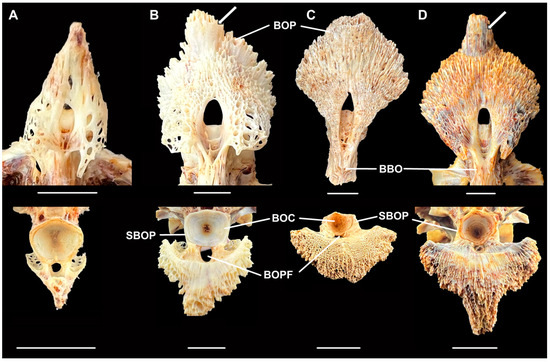
Figure 5.
Basioccipital pharyngeal processes in ventral (top) and posterior (bottom) views of (A) Moxostoma breviceps, AUM 86339; (B) M. carinatum, AUM 86330; (C) M. hubbsi, Bell Cave, AL, fossil, MSC 45256; and (D) M. hubbsi, AUM 86391. BBO: body of basioccipital, BOC: basioccipital centrum, BOP: basioccipital pharyngeal process, BOPF: basioccipital pharyngeal process foramen, and SBOP: strut of basioccipital pharyngeal process. Scale = 1 cm. Photos by J.W. Armbruster.
Figure 5.
Basioccipital pharyngeal processes in ventral (top) and posterior (bottom) views of (A) Moxostoma breviceps, AUM 86339; (B) M. carinatum, AUM 86330; (C) M. hubbsi, Bell Cave, AL, fossil, MSC 45256; and (D) M. hubbsi, AUM 86391. BBO: body of basioccipital, BOC: basioccipital centrum, BOP: basioccipital pharyngeal process, BOPF: basioccipital pharyngeal process foramen, and SBOP: strut of basioccipital pharyngeal process. Scale = 1 cm. Photos by J.W. Armbruster.
2. Materials and Methods
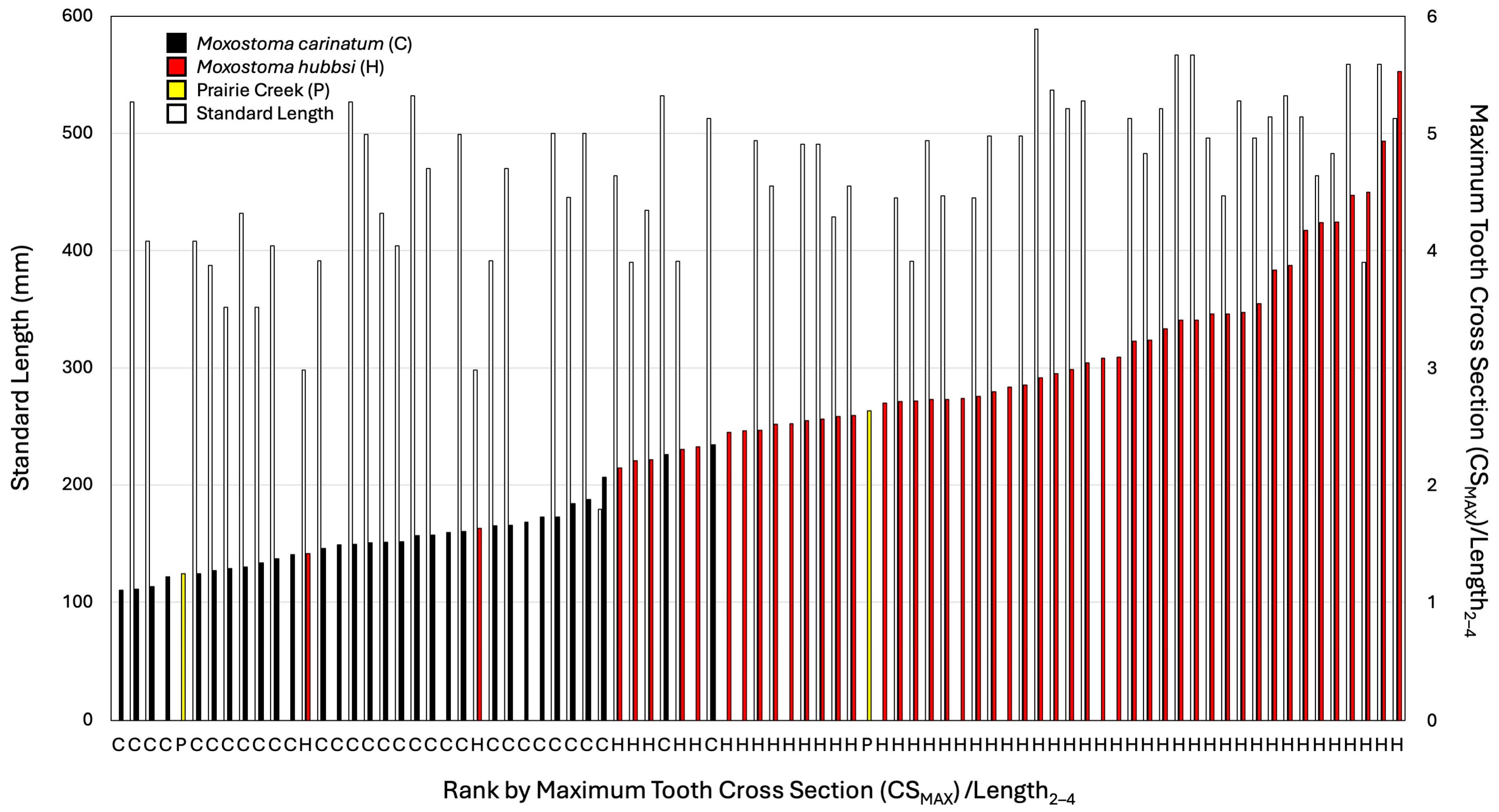
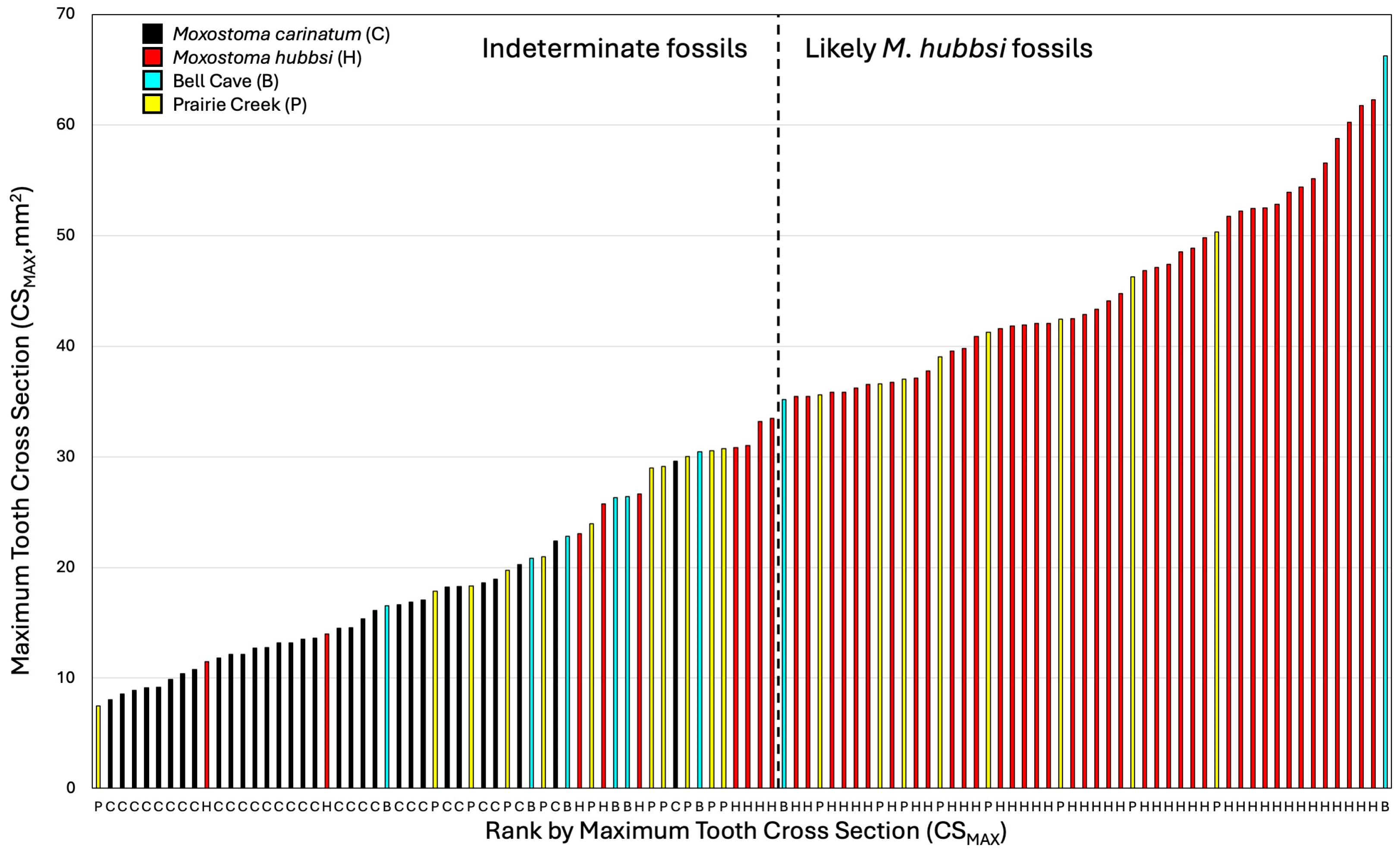
The first three pharyngeal teeth (from ventral side) were examined for their length (perpendicular to the long axis of the pharyngeal jaw, L), their greatest width (parallel to the long axis of the pharyngeal jaw, W), and their height (H, measured on the lateral side from the jaw to the maximum height). In addition, the length of the pharyngeal base encompassing teeth 2 and 4 (L2–4) was measured as was the greatest width of the pharyngeal jaw (WP). Measurements were taken with Mitutoyo digital calipers to the nearest 0.1 mm. Ratios were made of W/L, and both W and L to L2–4, and WP. In addition, the greatest cross-sectional area of the tooth (CS) was approximated as an oval (1/2W * 1/2L * pi). Ratios were calculated of CS to L2–4 and WP. In addition, loose pharyngeal teeth were measured for W and L with area calculated as above. Maximum tooth cross-sectional area (CSMAX) was also calculated as the tooth with the greatest CS and compared against L2–4 and WP. Measurements were taken for the jaws that REJ had available for M. carinatum, M. robustum, and M. hubbsi as well as the fossil specimens. Standard lengths (SL) of whole specimens were taken from the tip of the snout to the end of the vertebral column along the midline. The Sicklefin Redhorse and Robust Redhorse were not further examined here because their teeth are considerably smaller than the other molariform species, and the fossil samples are clearly not Sicklefin or Robust Redhorse. Plots were made by ranking the specimens based on CSMAX/L2–4 and CSMAX creating bar graphs with those measurements and SL (Figure 6 and Figure 7). An estimate is provided based on the distribution of CSMAX on which fossils are likely M. hubbsi and which are inconclusive, given that it is impossible to standardize measurements on isolated teeth and that it is unclear if the isolated teeth are among the top three widest teeth.
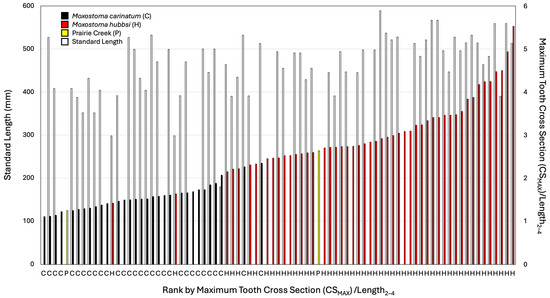
Figure 6.
A graphical ranking of maximum tooth area (CSMAX) to length of arch between second and fourth tooth (L2–4) ratio. Left bar is standard length (when available, white bars), right bar is maximum tooth cross section to length of the base from tooth 2 to 4 (species or population colored per legend). Species/population also indicated along the x axis per the legend.
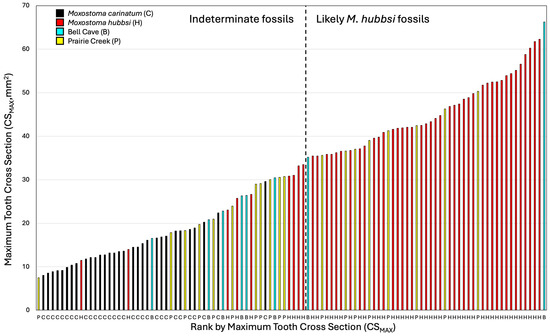
Figure 7.
A graphical ranking of maximum tooth area in specimens examined (species or population colored per legend). Species/population also indicated along the x axis per the legend. The dashed line separates fossil specimens to the right that are likely specimens of M. hubbsi because they are outside the known distribution of M. carinatum from fossils whose identities are uncertain to the left.
The basioccipital pharyngeal process was compared to the large skeletal collection that was assembled by REJ and that is now mostly cataloged at the Auburn University Museum of Natural History (AUM). Gross comparisons were made and the maximum width of the fossil basioccipital was compared against the largest M. carinatum and M. hubbsi to qualitatively determine differences in basioccipital centrum shape. Measurements taken were basioccipital condyle width and height and basioccipital pharyngeal process maximum width. Ratios were made of condyle height to width and basioccipital pharyngeal process maximum width to condyle width.
Specimens Examined
Institutional acronyms follow Sabaj [16] with the exception that UT is referring to the Zooarchaeological Collection of the McClung Museum at the University of Tennessee and not the fish collection and TU refers to the osteological collection at Tulane University. Numbers of specimens include the number of full skeletal specimens and then the total number of pharyngeal jaws (some collections only had pharyngeal jaws). County is abbreviated as Co. and Regional County Municipality as RCM. Moxostoma carinatum: AUM 86330 (1 specimen, 2 pharyngeal jaws), Indiana, Pulaski Co., Tippecanoe River, 6.25 miles SSW of Winamac, 40.9754, −86.65636; AUM 86333 (2, 4), Virginia, Russel Co., Clinch River at Nash Ford, 7.3 miles NNE of junction of 19 and 82 in Lebanon, 36.96758, −82.07946; AUM 86334 (2, 4), North Carolina, Macon Co., Little Tennessee River, 3.4 miles NW of Iotla, 35.26012, −83.44573; AUM 86335 (0, 1), Quebec, unknown locality; AUM 86336 (1, 2), Quebec, Montérégie RCM, Bassin de Chambly, Richelieu River, at Chambly, 43.44985, −73.28924; AUM 86338 (1, 2), Arkansas, Marion Co., Bull Shoals River, in reservoir, ~18 miles W of Mountain Home, 33.44027, −92.6891; AUM 86428 (1, 3), Alabama, Cherokee Co., Coosa River, below Sewell’s Ferry, 34.19746, −85.58526; AUM 86465 (0, 1), Kentucky, Franklin Co., Kentucky River, at mouth and lower 500 m of Elkhorn Creek, 38.31913, −84.8569; AUM 86593 (0, 2), North Carolina, Macon Co., Iotla Creek, ~40 m above mouth, at Rt. 28 bridge, at Iotla, 35.23449, −83.39623; AUM Uncataloged (1, 1), GMNH 549 (2, 2), Alabama Cherokee Co., Coosa River, 1 mile downstream from Sewell’s Ferry, ~34.2083, ~−85.6185; GMNH 550 (1, 2), Alabama Cherokee Co., Coosa River, at first bend below Alexis’ Ferry, ~34.2021, ~−85.5677; TU Osteology 646 (1, 2), Alabama, Wilcox Co., Alabama River at Black Bluff Landing, river mile 123.2, 32.0010, −87.47210; UT Zooarchaeology 140 (1, 1), Missouri, Jefferson and St. Louis Co., Meramec River, Times Beach, 38.50176, −90.59122; UT Zooarchaeology 4087 (1, 2), Illinois, Kankakee Co., Kankakee River.
Moxostoma hubbsi: All collections Quebec. AUM 86391 (1, 2), D’Autray RCM, St. Lawrence River. At Lavaltrie, N side, 45.89713, −73.26318; AUM 86392 (1, 2), Montérégie RCM, Bassin Chambly, Richelieu River at Chambly, 45.44985, −73.28924; AUM 86393 (1, 2), Pierre-De Saurel RCM, Richelieu River, at Saint-Ours, left side of river about 300 m below the lock, 45.86582, −73.14904; CUMV 25512 (0, 3), Rouville RCM, Yamaska River near St. Césaire, 45.41211, −72.99993; CUMN 47986 (0, 1), Les Maskoutains RCM, Yamaska River above Saint Hyacinthe, Station 53, 45.43652, −72.98712; CUMV 52911 (0, 2), Montérégie RCM, Bassin de Chambly at St. Mathias, at E of channel between lines 17 and 19, 45.47341, −73.27233; CUMV 52912 (0, 1), Rouville RCM, Yamaska River, upstream from mouth of Noire River (Station 75), 45.40747, −72.99971; CUMN 52913 (0, 2), Les Maskoutains RCM, Yamaska River in region (town of) Saint Hyacinthe, 45.62388, −72.93806; CUMV 52915 (0, 2), Les Maskoutains RCM, Noire River, ~0.3 and 1.2 miles downstream from dam at Saint-Pie (Stations 20 and 34), 45.51248, −72.91945. Loose pharyngeal jaws from Canadian institutions may not be cataloged and are in the process of being repatriated. Assumptions on the origins of these specimens and their identification numbers are: FAPAQ 291B127B639E (0, 2), FAPAQ 291B284B8577 (0, 1), FAPAQ 291B306CDF3A (0, 2), FAPAQ 291B31B8E3DA (0, 1), FAPAQ 291B32D4DB8E (0, 2), FAPAQ 291B504E89DD (0, 2), and FAPAQ 291B5F7BABAC (0, 2), Pierre-De Saurel RCM, Richelieu River, Saint-Ours, ~45.8838, ~−73.1536; FAPAQ 291B1C063056 (0, 2), Montérégie RCM, Bassin de Chambly, Richelieu River; QPM 17915 (0, 2), locality unknown; QPM 29336 (0, 2), 29354 (0, 1), and 29564 (0, 2), Montérégie RCM, Bassin de Chambly; QPM Uncataloged 1999 (0, 8), Quebec, St. Lawrence River?, locality unknown; QPM Uncataloged 2005, Quebec, dead at Montreal Biodome, locality unknown.
Fossil material. All collections Pleistocene, Wisconsinan, Bell Cave, Colbert Co., Alabama, Acb-2 (zone 1–2 11,820 +48−/−500 BP, zone 3 unknown, zone 4 26,500 +870/−990 BP) [13]: MSC 4254, zone 1/2, pit 2, museum expedition, July 1984, 2 loose pharyngeal teeth; MSC 4839, zone 4, pit 1, museum expedition, July 1984, 1 loose pharyngeal tooth; MSC 4942, zone 3, pit 3, museum expedition, July 1984, partial basioccipital pharyngeal process; MSC 5179, zone 1/2, pit 4, museum expedition, July 1984, 2 loose pharyngeal teeth; MSC 45256, zone 3, pit 3, museum expedition, July 1984, basioccipital pharyngeal process; MSC 51132, 11 June 1987, 3 loose pharyngeal teeth. Prairie Creek Site, Daviess Co., Washington, IN, USA, ~3 mi. N of Washington, Zone D, Pleistocene, Wisconsinan (14,010 BP) [10]: INSM L-200, 4 loose pharyngeal teeth; INSM L-354, 4 loose pharyngeal teeth; INSM L-849, large pharyngeal jaw fragment (2 teeth measured); INSM L1188 2 loose pharyngeal teeth; INSM L-1188, small pharyngeal jaw fragment (3 teeth measured); INSM L-1632, 4 loose pharyngeal teeth; INSM L-2693, 2 loose pharyngeal teeth; and INSM L-2703, 1 loose pharyngeal tooth.
3. Results
Comparisons
Tooth height was an unreliable measurement as teeth are clearly lost and replaced through life. The reason for including the first three teeth is that the largest tooth is among these three, but it is not always the same tooth. New teeth grow from between the bases of the remaining teeth after being lost, and they will expand to fit the available area, ensuring a relatively continuous edge. Jaws were seen in the process of replacing one or two of these teeth, but it was rare to have all three fully developed. The surfaces of the teeth were clearly worn from abrasion.
When scaled with L2–4, the CSMAX of the large pharyngeal jaw from Prairie Creek (INSM L-849) was greater than all M. carinatum with the closest M. carinatum being a 512.7 mm SL specimen (AUM 84645; Figure 6). When scaled with WP, INSM L-849 falls well within the range of the largest M. hubbsi examined; however, it is possible that the width of the fossil has been eroded. The smaller Prairie Creek specimen is among the smallest measured, leaving two possibilities: that it is a juvenile M. hubbsi or that it is M. carinatum.
With just CSMAX, the only specimen of M. carinatum to overlap with the larger fossil specimens examined and M. hubbsi was one of the largest specimens of M. carinatum examined (AUM 84645, 512.7 mm SL; Figure 7). Body size and tooth size are certainly correlated, but the largest specimens examined of the two species did not have the largest teeth. The smallest M. hubbsi (CU 52911 #13, 298 mm SL) had teeth from both left and right sides falling out within M. carinatum; however, the specimen is considerably smaller than similarly ranked M. carinatum (Figure 5 and Figure 6). The large Bell Cave tooth with CSMAX of 66.3 mm2 was the largest tooth examined with the second largest a tooth from a 512.7 mm SL specimen of M. hubbsi (AUM 86392, right jaw, CSMAX = 62.3 mm2). The largest specimen of M. hubbsi (559.2 mm SL) had CSMAX of 61.8 mm2 (left) and 60.3 mm2 (right). The smaller pharyngeal jaw from Prairie Creek (ISM L-1188; Figure 3B) had the smallest CSMAX (7.47 mm2), making its identification inconclusive.
Two sucker basioccipital pharyngeal processes were found, one of which is near complete (MSC 45256, Figure 5C). The near complete process appears to lack a posterior extension of the basioccipital present in M carinatum and M. hubbsi (extension indicated by arrows in Figure 5B,D) and some of the anterior and lateral portions. In comparison with other suckers, the basioccipital pharyngeal process of M. carinatum and particularly M. hubbsi are hypertrophied (Figure 5B,D), with the trabeculae fusing to form a more solid bone (vs. trabeculae forming a lattice, Figure 5A). Moxostoma hubbsi has very few holes piercing all the way through the basioccipital pharyngeal process while M. carinatum has several large holes anterolaterally and has a weaker, more crenulate posterior margin. MSC 45256 has the basioccipital pharyngeal process with few complete holes and a less crenulate posterior margin, as in M. hubbsi. In comparing skull size and the basioccipital centrum (the connection point of the skull to the vertebral column), the basioccipital centrum is comparatively smaller, given the width of the basioccipital pharyngeal process. The maximum width of the basioccipital pharyngeal process to centrum width ratio is 253.6–273.0% (N = 3) in M. hubbsi and 169.6–222.8% (N = 14) in M. carinatum while the Bell Cave basioccipital is within the range of M. hubbsi at 265.5%, and it appears to be eroded slightly. Only one specimen of M. carinatum has a wider basioccipital pharyngeal process than the Bell Cave fossil (37.6 vs. 37.5 mm), but the centrum width is much larger (19.7 vs. 14.1 mm). The basioccipital pharyngeal process envelops the basioccipital centrum with stout struts in M. hubbsi and the Bell Cave fossil that are clearly visible in posterior view through their entire height (about three fourths height of basioccipital centrum; Figure 5C,D) while the processes are thin and only their bases are visible in M. carinatum (Figure 3B). In addition, there is an opening below the basioccipital centrum for the dorsal aorta, and this opening is more parallel with the basioccipital pharyngeal process in the fossil and M. hubbsi than in M. carinatum (Figure 5B vs. Figure 5C,D). Given these characteristics, the Bell Cave fossil basioccipital is more similar to that of M. hubbsi than to M. carinatum. The second basioccipital (MSC 4942) is not complete but it is strongly ossified (no trabeculae) and consistent with M. hubbsi as well.
4. Discussion
Although an argument could be made that the Prairie Creek teeth could be from very large specimens of M. carinatum, the fact that there is only one specimen of M. carinatum that overlaps M. hubbsi in CSMAX makes it much more likely that several of these teeth represent something more like M. hubbsi. The large Bell Cave tooth is the largest tooth measured and would imply a ~560 mm SL+ specimen of M. hubbsi (largest M. hubbsi specimen examined is 559.2 mm SL) and something much larger than the measured maximum size of M. carinatum from the second author’s records of over 1100 specimens of M. carinatum examined. Although tooth area does correlate with size, this comparison is not perfect. The greatest CSMAX for M. carinatum (29.6 mm2, 512.7 mm SL) is only 44% the area of the Bell Cave specimen. The largest specimen of M. carinatum examined (uncataloged specimen form the Tippecanoe River, Indiana, 532 mm SL) is among the largest specimens in REJ’s records for morphometry of M. carinatum and it has CSMAX of 22.4 mm2 (right) and 19.0 mm2 (left). This leaves only M. hubbsi as an extant species as a possibility for the largest Bell Cave tooth at a minimum. For the teeth to be that of M. carinatum, the size of M. carinatum during the Pleistocene would have had to have been much greater than it is today. Some of the teeth were not measured from Bell Cave because they did not appear to be teeth within the first three from the ventral side, but smaller, dorsal teeth.
The fossil basioccipital (MSC 45256) also was more similar to that of M. hubbsi than to that of M. carinatum in the greater fusion of the trabeculae, stouter struts of the basioccipital pharyngeal process along the basioccipital centrum, as well as in overall shape. For their size, M. hubbsi have shorter skulls with smaller basioccipital condyles than M. carinatum, and this is seen in the fossil as well (Figure 5C). There may be some differences, though. The fossil has a longer body to the basioccipital when compared to M. hubbsi and the struts along the basioccipital condyle are much thicker.
It is possible that the fossils instead represent an extinct species, but it is unlikely that fragmentary evidence like this is sufficient to decide if a new species is warranted. Given that the current distribution of M. hubbsi is so unusual, it is certain that the current distribution does not match with its Late Pleistocene distribution. The maximum extent of the Wisconsin Glaciation event would have covered the current distribution of M. hubbsi until about 12,000 years ago (Figure 2), and the Prairie Creek locality is located just to the southwest of the maximum southern extent of the glacier. Moxostoma hubbsi’s current distribution was inundated after the glaciers began to melt by the Champlain Sea until about 10,000 years ago and then by Lampsilis Lake, which did have some marine incursions. The St. Lawrence probably did not become riverine until about 6000–5000 years ago, meaning that M. hubbsi would have had to have entered into their current distribution in the Holocene [8,9].
As the ice sheet began to melt, flow from the Great Lakes region was mostly to the southwest and into the Mississippi basin. As the glacier retreated northwards, flow in the lower Great Lakes began to shift to the east, and this period would have given access to M. hubbsi into the lower Great Lakes region. Six species in the Ottawa River (a tributary of the St. Lawrence) were thought to have entered the river through the Mississippi basin while only the Copper Redhorse was considered to have entered via the St. Lawrence [8]. It would seem more logical to suggest that M. hubbsi also gained entrance into the region through the Mississippi basin. Sympatric with the Copper Redhorse in the St. Lawrence is an apparently disjunct population of M. carinatum). River and Copper Redhorse may have become isolated in the St. Lawrence through similar geological processes except that Copper Redhorse became extirpated from the remainder of its distribution.
Bell Cave includes fossils of the Muskellunge (Esox masquinongy Mitchill) [13], which although relatively recently was found down the Appalachians, was not found as far down the Tennessee River as Bell Cave [16]. This suggests that the area of Bell Cave had cool waters suitable for M. hubbsi. With the unusual and clearly relictual distribution of M. hubbsi and the similarity of both the Indiana and Alabama teeth and the Alabama basioccipitals to M. hubbsi, we suggest that the distribution of M. hubbsi once included the current Mississippi River basin. Given the difficulty in identifying species of Redhorse, we would encourage curators to re-examine old specimens of M. carinatum to assure their species status. Bones of M. lacerum (Harelip Sucker) were also found in Bell Cave [14], and M. lacerum went from being discovered to its last specimen being collected in just about 30 years in the 1800s [1]. Perhaps M. hubbsi was missed from early collections in the Mississippi River basin.
The discovery of fossils of M. hubbsi is particularly important because the Copper Redhorse is considered endangered due to its small distribution and low population size [17,18]. Although there is no evidence for inbreeding [19], the species is in decline [20,21]. Perhaps learning the conditions M. hubbsi once inhabited and why it is no longer in that part of its range will aid fisheries managers who seek to propagate the species and increase population numbers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10030101/s1, Table S1: morphometric data and analyses.
Author Contributions
J.W.A. completed the work that was started by R.E.J. who died before formal writing on the manuscript could be implemented. All the comparative material was prepared by R.E.J. Conceptualization, J.W.A. and R.E.J.; methodology, J.W.A. and R.E.J.; validation, J.W.A.; formal analysis, J.W.A.; investigation, J.W.A. and R.E.J.; resources, J.W.A. and R.E.J.; data curation, J.W.A.; writing—original draft preparation, J.W.A.; writing—review and editing, J.W.A.; visualization, J.W.A.; project administration, J.W.A. and R.E.J.; funding acquisition, J.W.A. and R.E.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States National Science Foundation, grant number DEB-2428043.
Data Availability Statement
Morphometric data and analyses used are in Supplementary Table S1.
Acknowledgments
The first author would like to thank J. Ebersole for his hospitality while visiting the McWane Center and for the loan of materials, J. Lamb for discussions on the Bell Cave locality, and D. Werneke for cataloging the large number of catostomid skeletons that were needed for comparisons. N. Vachon and A. Branchaud were instrumental to the second author’s work in Canada and for providing specimens necessary for the completion of this work. The first author would further like to thank the second for inspiring him to complete this study. Jenkins died before this study could be completed, but he lives on in the data and specimens that he collected throughout his career.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Harris, P.M.; Hubbard, G.; Sandel, M. Catostomidae: Suckers. In Freshwater Fishes of North America, Petromyzontidae to Catostomidae; Warren, M.L., Burr, B.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2014; Volume 1, pp. 451–502. [Google Scholar]
- Eastman, J.T. The pharyngeal bones and teeth of catostomid fishes. Am. Midl. Nat. 1977, 97, 68–88. Available online: https://www.jstor.org/stable/pdf/2424686.pdf (accessed on 1 January 2020). [CrossRef]
- Doosey, M.H.; Bart, H.L., Jr. Morphological variation of the palatal organ and chewing pad of Catostomidae (Teleostei: Cypriniformes). J. Morphol. 2011, 272, 1092–1108. [Google Scholar] [CrossRef]
- Bagley, J.C.; Mayden, R.L.; Harris, P.M. Phylogeny and divergence times of suckers (Cypriniformes: Catostomidae) inferred from Bayesian total-evidence analyses of molecules, morphology, and fossils. PeerJ 2018, 6, e5168. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.9bpcjn (accessed on 8 December 2023).
- Page, L.M.; Burr, B.M. Peterson Field Guide to Freshwater Fishes of North America North of Mexico, 2nd ed.; Houghton Mifflin Harcourt: Boston, MA, USA, 2011. [Google Scholar]
- Underhill, J.C. The fish fauna of the Laurentian Great Lakes, the St. Lawrence lowlands, Newfoundland and Labrador. In The Zoogeography of North American Freshwater Fishes; Hocutt, C.H., Wiley, E.O., Eds.; John Wiley & Sons: New York, NY, USA, 1986; pp. 105–136. [Google Scholar]
- Parent, M.; Occhietti, S. Late Wisconsinan deglaciation and Champlain Sea invasion in the St. Lawrence valley, Québec. Géogr. Phys. Quatern. 1988, 42, 215–246. [Google Scholar] [CrossRef]
- Tomak, C.H. Prairie Creek: A stratified site in southwestern Indiana. Proc. Indiana Acad. Sci. 1974, 84, 65–68. Available online: https://journals.indianapolis.iu.edu/index.php/ias/article/download/8139/8098 (accessed on 1 December 2024).
- Richards, R.L. Small mammals of the Prairie Creek Site, Daviess County, Indiana. Proc. Indiana Acad. Sci. 1992, 101, 245–278. Available online: https://journals.indianapolis.iu.edu/index.php/ias/article/download/7300/7302 (accessed on 1 December 2024).
- Holman, J.A.; Richards, R.L. Herpetofauna of the prairie creek site, Daviess County, Indiana. Proc. Indiana Acad. Sci. 1993, 102, 115–132. Available online: https://journals.iupui.edu/index.php/ias/article/download/7317/7323 (accessed on 1 December 2024).
- Holman, J.A.; Bell, G.; Lamb, J. A late Pleistocene herpetofauna from Bell cave, Alabama. Herpetol. J. 1990, 1, 521–529. [Google Scholar]
- Jacquemin, S.J.; Ebersole, J.A.; Dickinson, W.C.; Ciampaglio, C.N. Late Pleistocene fishes of the Tennessee River Basin: An analysis of a late Pleistocene freshwater fish fauna from Bell Cave (site ACb-2) in Colbert County, Alabama, USA. PeerJ 2016, 4, e1648. [Google Scholar] [CrossRef]
- Dalton, A.S.; Margold, M.; Stokes, C.R.; Tarasov, L.; Dyke, A.S.; Adams, R.S.; Allard, S.; Arends, H.E.; Atkinson, N.; Attig, J.W.; et al. An updated radiocarbon-based ice margin chronology for the last deglaciation of the North American Ice Sheet Complex. Quat. Sci. Rev. 2020, 234, 106223. [Google Scholar] [CrossRef]
- Sabaj, M.H. Codes for natural history collections in ichthyology and herpetology. Copeia 2020, 2020, 593–669. [Google Scholar] [CrossRef]
- Etnier, D.A.; Starnes, W.C. The Fishes of Tennessee; University of Tennessee Press: Knoxville, TN, USA, 1993. [Google Scholar]
- Mongeau, J.R.; Dumont, P.; Cloutier, L.; Clément, A.M. Le statut du suceur cuivré, Moxostoma hubbsi, au Canada. Can. Field Nat. 1988, 102, 132–139. [Google Scholar] [CrossRef]
- NatureServe. Moxostoma hubbsi. The IUCN Red List of Threatened Species; NatureServe: Arlington, VA, USA, 2014; p. e.T13917A58135857. [Google Scholar] [CrossRef]
- Lippé, C.; Dumont, P.; Bernatchez, L. High genetic diversity and no inbreeding in the endangered copper redhorse, Moxostoma hubbsi (Catostomidae, Pisces): The positive sides of a long generation time. Mol. Ecol. 2006, 15, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- De Lafontaine, Y.; Gilbert, N.L.; Dumouchel, F.; Brochu, C.; Moore, S.; Pelletier, P.; Bumont, P.; Branchaud, A. Is chemical contamination responsible for the decline of the copper redhorse (Moxostoma hubbsi), an endangered species in Canada? Sci. Total Environ. 2002, 298, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Vachon, N.; Chagnon, Y. Caractérisation de la population de cevalier cuivré (Moxostoma hubbsi) du fleuve Saint-Laurent (secteur Lavaltrie-Contrecoeur) à partir des captures fortuites d’un pêcheur commercial en 1999, 2000 et 2001. In Longueuil, Ministère des Ressources Naturelles, de la Faune et des Parcs du Québec, Technical Report; Gouvernement du Québec: Québec, QC, USA, 2004; Volume 16, pp. 1–83. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).