The Combined Effects of Cadmium and Microplastic Mixtures on the Digestion, Energy Metabolism, Oxidative Stress Regulation, Immune Function, and Metabolomes in the Pearl Oyster (Pinctada fucata martensii)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Sample Collection
2.3. Biochemical Measurements
2.4. Metabolite Evaluation
2.4.1. Extraction of Metabolites
2.4.2. LC-MS/MS Analysis
2.5. Data Analysis
2.5.1. Metabolomic Analysis
2.5.2. Biochemical Analysis
3. Results
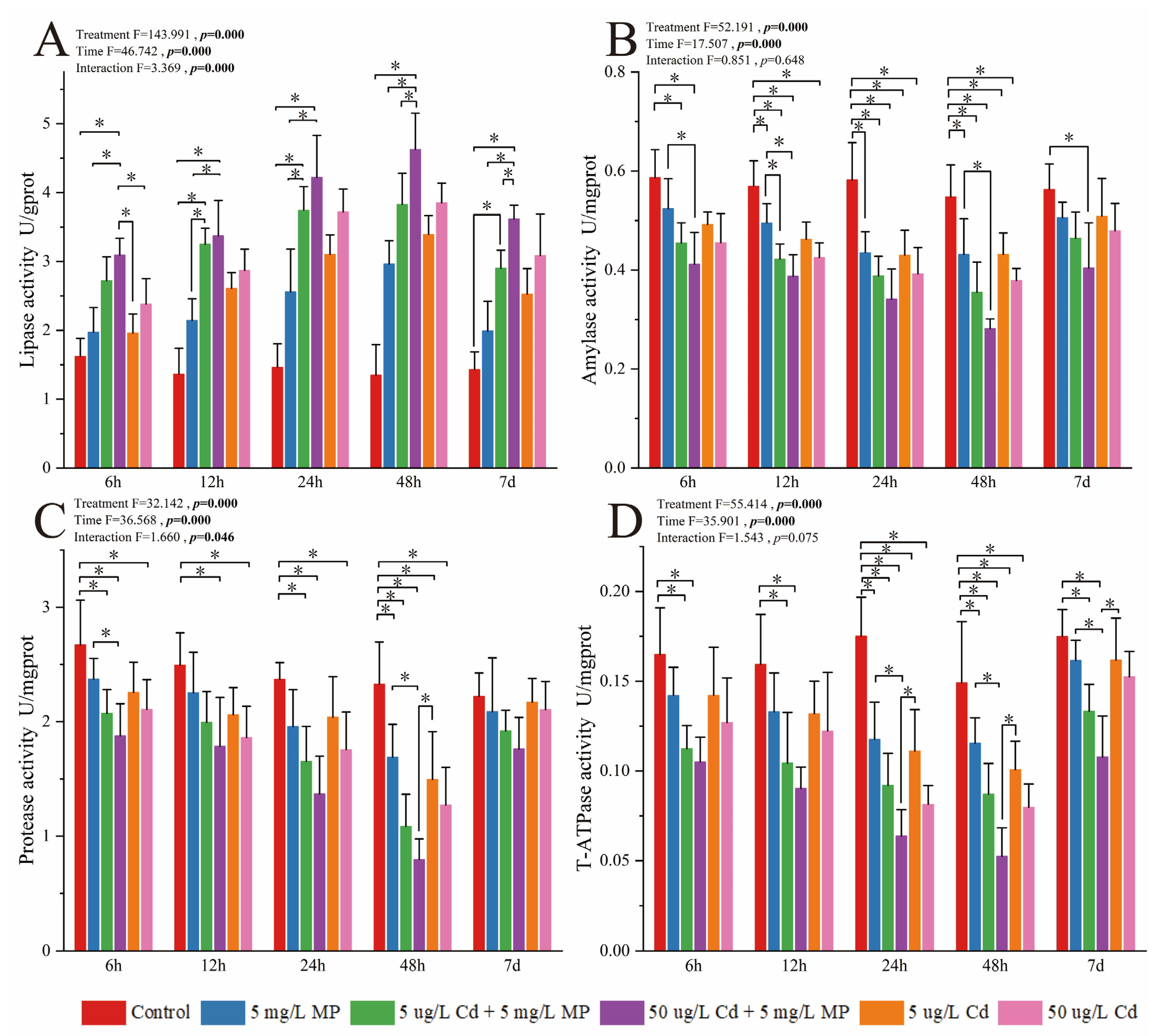
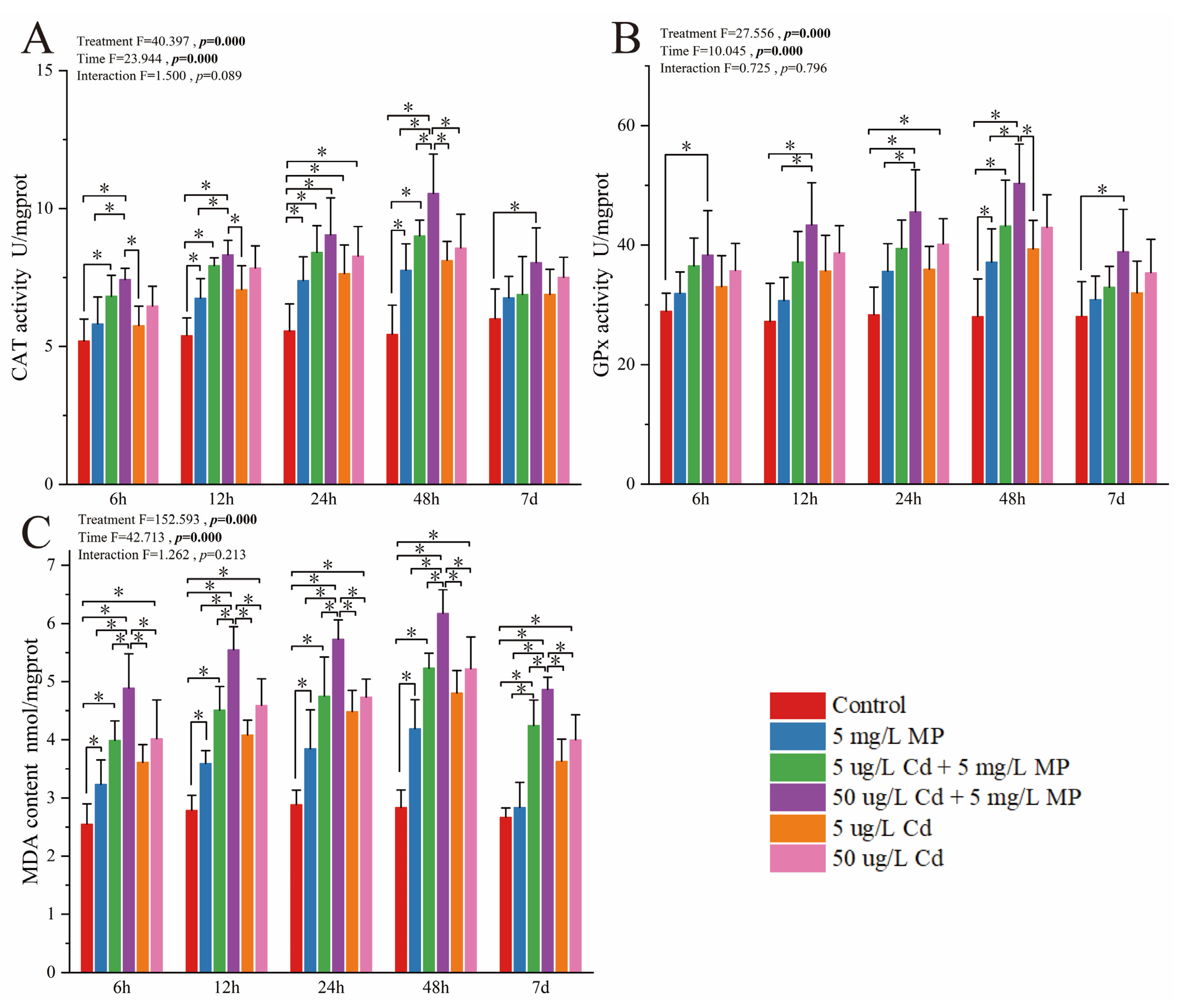
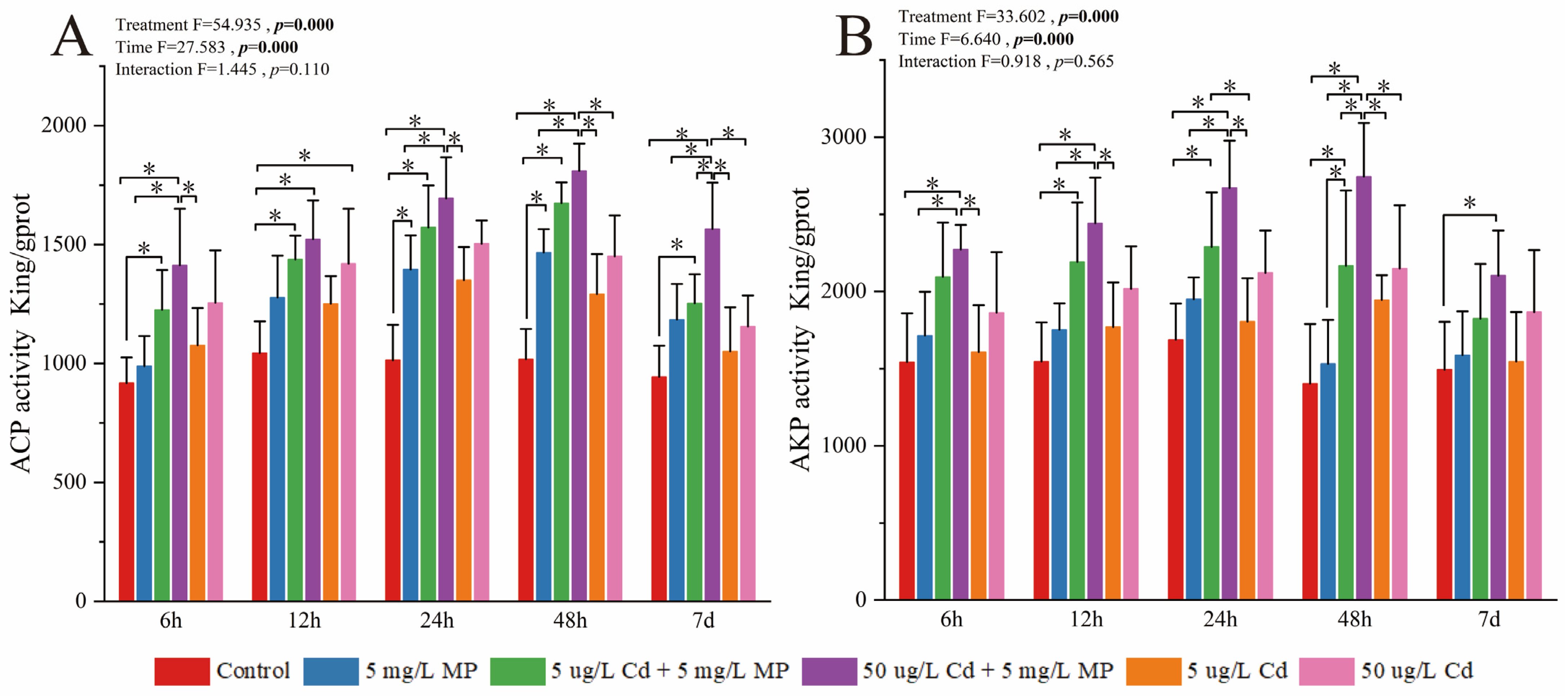
3.1. Detection and Analysis of Enzyme Activity in Pearl Oysters
3.1.1. Activity of Enzymes Involved in Digestion and Energy Metabolism
3.1.2. Activity of Enzymes and Oxidants Involved in Oxidative Stress Regulation
3.1.3. Activity of Enzymes Involved in Immune Function
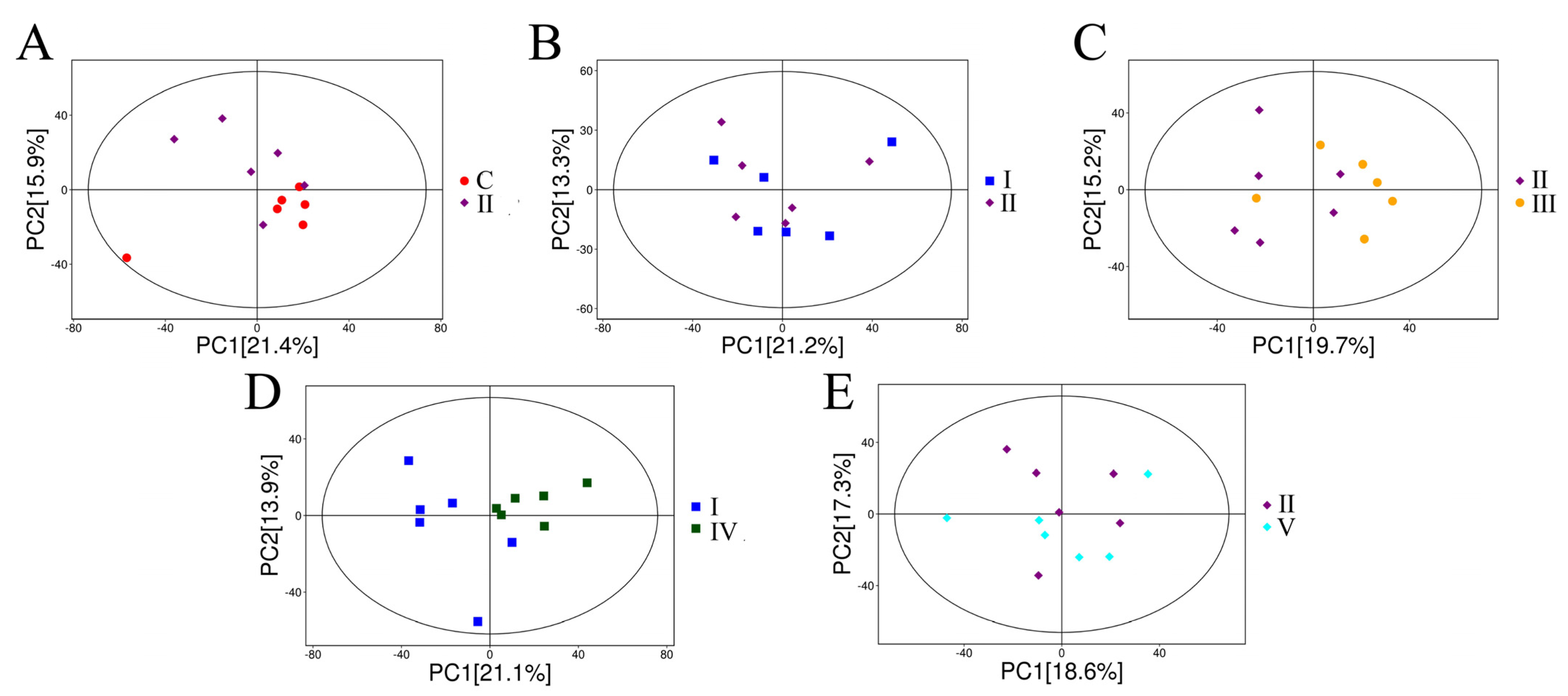
3.2. Analysis of the Pearl Oyster Metabolome
3.2.1. Multivariate Analysis of Metabolites
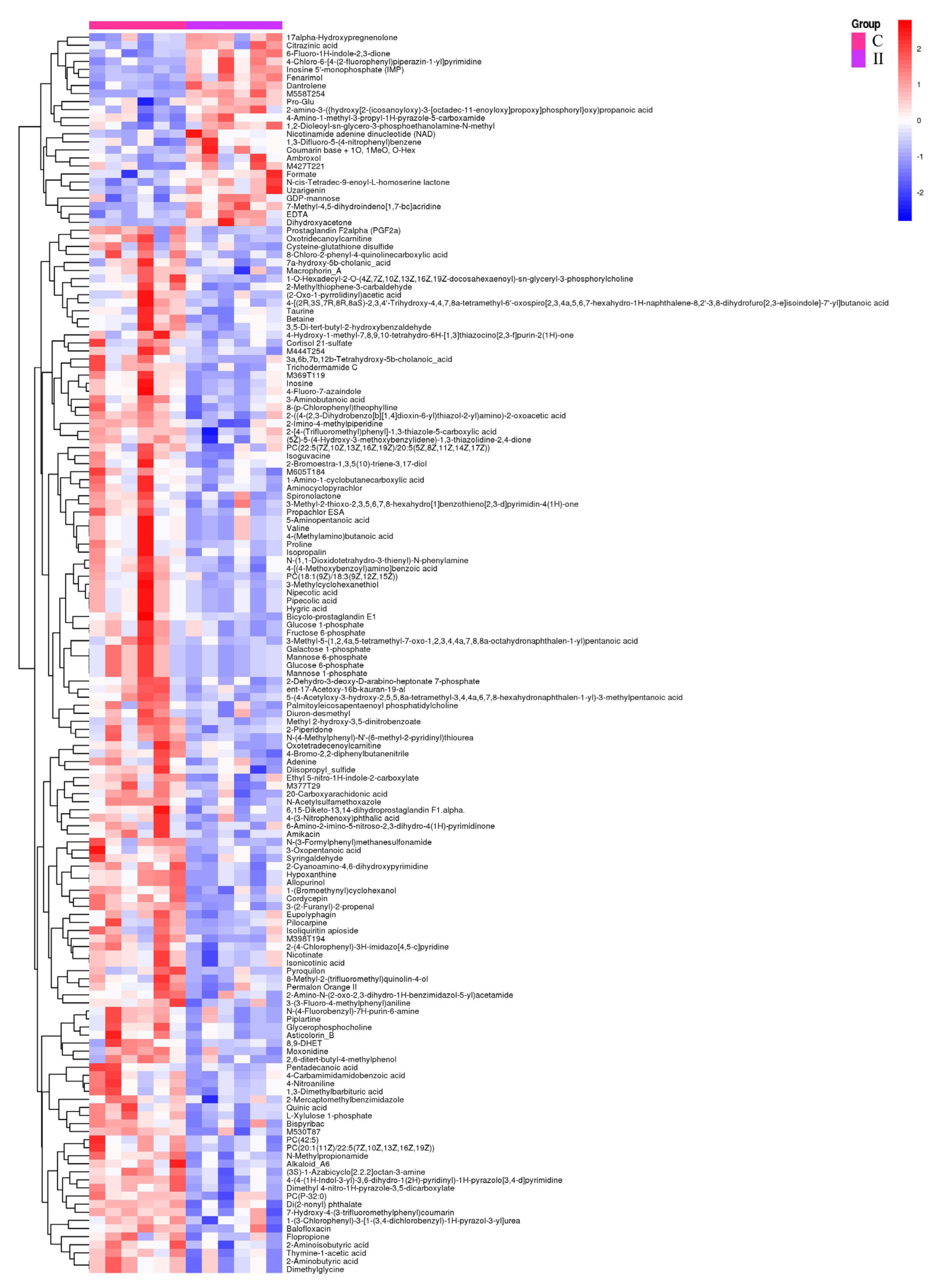
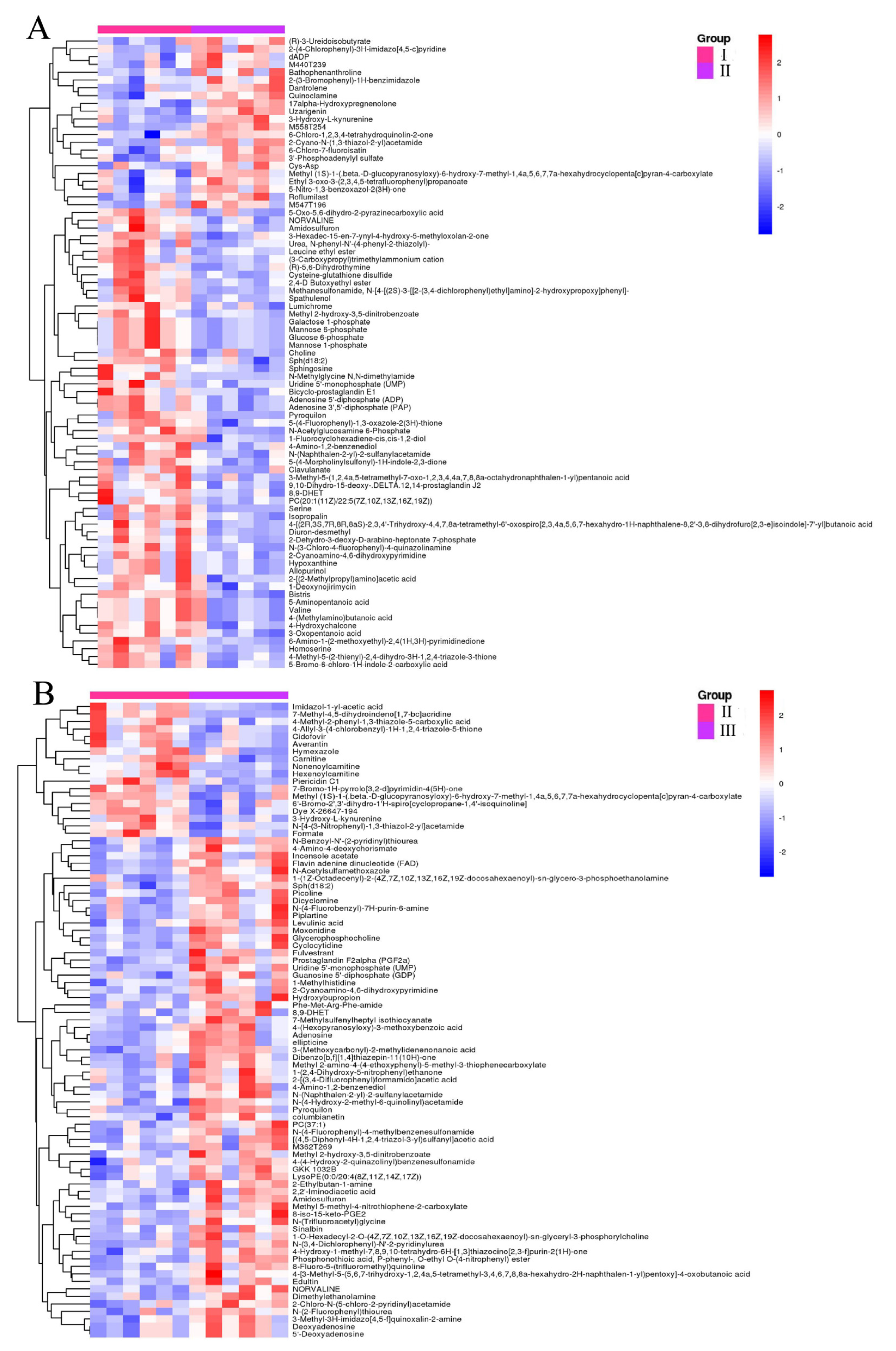
3.2.2. Identification of Significantly Different Metabolites (SDMs)
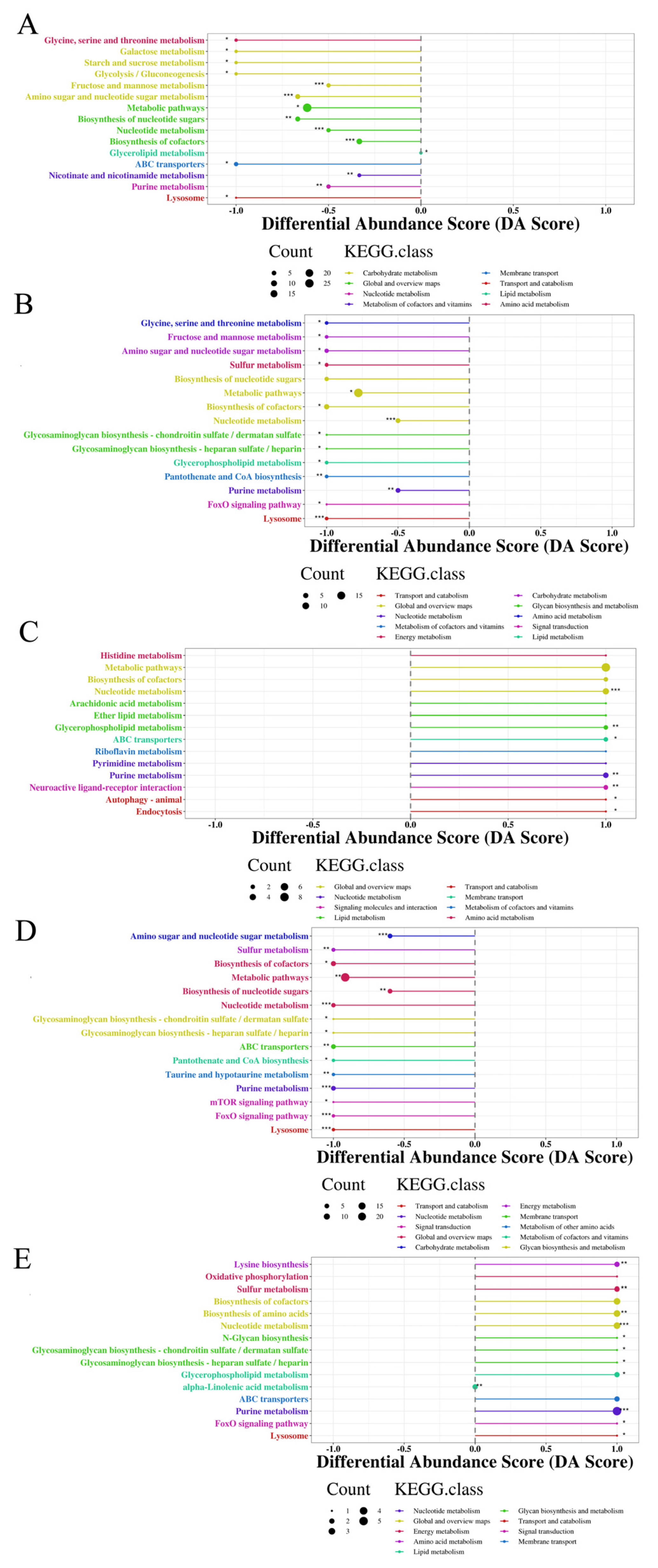
3.2.3. Characterization and Functional Analysis of Metabolic Pathways
4. Discussion
4.1. Effects of MPs, Cd, and Combined MPs and Cd Exposure on Digestion and Energy Metabolism
4.2. Effects of MPs, Cd, and Combined Exposure to MPs and Cd on Oxidative Stress
4.3. Effects of MPs, Cd, and Combined Exposure to MPs and Cd on Immune Activity
4.4. Effects of MPs, Cd, and Combined Exposure to MPs and Cd on Lipid Metabolism
4.5. Effects of MPs, Cd, and Combined Exposure to MPs and Cd on Neuronal Excitability
4.6. Effects of Short-Term Recovery on the Pearl Oyster
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Thompson, C.R.; Olsen, Y.; Mitchell, P.R.; Davis, A.; Rowland, J.S.; John, G.W.A.; McGonigle, D.; Russell, E.A. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Sebille, V.E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, D.B.; Franeker, V.J.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, L.K. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Nadia, M.V.; Patricia, B.; Angela, K. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar]
- Fackelmann, G.; Sommer, S. Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Mar. Pollut. Bull. 2019, 143, 193–203. [Google Scholar] [CrossRef]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, K.; Li, B.; Chen, Q.; Su, L.; Wu, C.; Hollert, H.; Shi, H. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 2018, 213, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Xia, S.D.; Ning, Y.; Pan, X.; Qu, J.H.; Xu, Y.J. Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus kuda Bleeker. Mar. Pollut. Bull. 2019, 149, 110510. [Google Scholar]
- Gardon, T.; Reisser, C.; Soyez, C.; Quillien, V.; Moullac, L.G. Microplastics affect energy balance and gametogenesis in the pearl oyster Pinctada margaritifera. Environ. Sci. Technol. 2018, 52, 5277–5286. [Google Scholar] [CrossRef]
- Mkuye, R.; Gong, S.; Zhao, L.; Masanja, F.; Ndandala, C.; Bubelwa, E.; Yang, C.; Deng, Y. Effects of microplastics on physiological performance of marine bivalves, potential impacts, and enlightening the future based on a comparative study. Sci. Total Environ. 2022, 838, 155933. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.N.; Liu, J.H.; Feng, X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Gao, J.W.; Wu, H.; Li, S.M.; Xie, M.; Li, W.X.; Song, R. Ammonia nitrogen and cadmium stress on antioxidant system and immune function of furong crucian carp (Cyprinus capio furong.♀× Carssius auratus red var.♂). Acta Hydrobiol. Sin. 2022, 46, 448–456. (In Chinese) [Google Scholar]
- Sun, R.; Wang, Z. Heavy metals contamination and shellfish poison pollution in marine shellfish in Shenzhen coastal waters. J. Appl. Oceanogr. 2017, 36, 575–579. (In Chinese) [Google Scholar]
- Vaseem, H.; Banerjee, T.K. Evaluation of pollution of Ganga River water using fish as bioindicator. Environ. Monit. Assess. 2016, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jerome, F.C.; Hassan, A.; Omoniyi-Esan, G.O.; Odujoko, O.O.; Chukwuka, A.V. Metal uptake, oxidative stress and histopathological alterations in gills and hepatopancreas of Callinectes amnicola exposed to industrial effluent. Ecotoxicol. Environ. Saf. 2017, 139, 179–193. [Google Scholar] [CrossRef]
- Brown, C. Arsenic and cadmium are contaminants of concern. CMAJ 2016, 188, E5. [Google Scholar] [CrossRef]
- Ding, T.T.; Du, S.L.; Zhang, Y.H.; Wang, H.; Zhang, Y.; Cao, Y.; He, L. Hardness-dependent water quality criteria for cadmium and an ecological risk assessment of the Shaying River Basin, China. Ecotoxicol. Environ. Saf. 2020, 198, 110666. [Google Scholar] [CrossRef]
- Borgmann, U.; Norwood, W.P.; Babirad, I.M. Relationship between chronic toxicity and bioaccumulation of cadmium in Hyalella azteca. Can. J. Fish. Aquat. Sci. 1991, 48, 1055–1060. [Google Scholar] [CrossRef]
- Eimers, M.C.; Evans, R.D.; Welbourn, P.M. Partitioning and bioaccumulation of cadmium in artificial sediment systems: Application of a stable isotope tracer technique. Chemosphere 2002, 46, 543–551. [Google Scholar] [CrossRef]
- Mebane, C.A.; Schmidt, T.S.; Miller, J.L.; Balistrieri, L.S. Bioaccumulation and toxicity of cadmium, copper, nickel, and zinc and their mixtures to aquatic insect communities. Environ. Toxicol. Chem. 2020, 39, 812–833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dahms, H.U.; Dong, F.; Jing, W.; Wang, L. Immune-associated parameters and antioxidative responses to cadmium in the freshwater crab Sinopotamon henanense. Ecotoxicol. Environ. Saf. 2016, 129, 235–241. [Google Scholar] [CrossRef]
- Lei, W.W.; Xu, T.; Wang, L. Effects of cadmium on apoptosis of cardiomyocyte in the freshwater crab Sinopotamon yangtsekiense. Oceanol. Et Limnol. Sin. 2012, 43, 299–305. (In Chinese) [Google Scholar]
- Bai, S.J.; Xu, Z.R. Effects of cadmium on mitochondrion structure and energy metabolism of Pelteobagrus fulvidraco gill. Chin. J. Appl. Ecol. 2006, 7, 1213–1217. (In Chinese) [Google Scholar]
- Shi, H.Q.; Zhang, L.J.; Wan, X.Y.; Peng, H.; Zhao, J.; Peng, S.Q. Toxic Effects of Cadmium Chloride Exposure on Neurobehavior of Zebrafish Larvae. Asian J. Ecotoxicol. 2013, 8, 374–380. (In Chinese) [Google Scholar]
- Wang, T.; Yang, C.Y.; Wang, C.; Liao, Y.; Mkuye, R.; Deng, Y. Bacterial community profiling associated with pearl culture facilities of Liusha Bay, the largest marine pearl culture base on the western Guangdong coast, South China. Mar. Environ. Res. 2023, 189, 106063. [Google Scholar] [CrossRef] [PubMed]
- Mkuyea, R.; Yang, C.Y.; Masanjaa, F.; Ibrahim, S.; Yang, X.Y.; Mwemi, H.; Mrope, P.; Salman, M.; Alfatat, A.; Deng, Y.W. Omics insights in responses of bivalves exposed to plastic pollution. Aquat. Toxicol. 2025, 279, 107224. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, M.; Zhang, H.; Yin, Y.; Cai, Y.; Zhu, Z.J. Metabolite annotation from knowns to unknowns through knowledge-guided multi-layer metabolic networking. Nat. Commun. 2022, 13, 6656. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Liu, S.; Liu, X. Combining transcriptomic and metabolomic analyses to investigate the acute effects of microcystin-LR and nanoplastics of asian clams. Water 2023, 15, 3519. [Google Scholar] [CrossRef]
- Salgado-García, R.L.; Kraffe, E.; Tripp-Valdez, M.A.; Ramírez-Arce, J.L.; Artigaud, S.; Flye-Sainte-Marie, J.; Mathieu-Resuge, M.; Sicard, T.M.; Arellano-Martínez, M.; Racotta, I.S. Energy metabolism of juvenile scallops Nodipecten subnodosus under acute increased temperature and low oxygen availability. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 278, 111373. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, W. Gradual effects of gradient concentrations of polystyrene nanoplastics on metabolic processes of the razor clams. Environ. Pollut. 2021, 287, 117631. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Ortiz, J.M.; Courant, F.; Gomez, E.; García-Pimentel, M.D.M.; León, V.M.; Campillo, J.A.; Santos, L.H.M.L.M.; Barceló, D.; Rodríguez-Mozaz, S. Combined exposure of the bivalve Mytilus galloprovincialis to polyethylene microplastics and two pharmaceuticals (citalopram and bezafibrate): Bioaccumulation and metabolomic studies. J. Hazard. Mater. 2023, 458, 131904. [Google Scholar] [CrossRef]
- Zhou, L.; Li, M.; Zhong, Z.; Chen, H.; Wang, X.; Wang, M.; Xu, Z.; Cao, L.; Lian, C.; Zhang, H.; et al. Biochemical and metabolic responses of the deep-sea mussel Bathymodiolus platifrons to cadmium and copper exposure. Aquat. Toxicol. 2021, 236, 105845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Liu, J.; Liu, X.; Tu, Z.; Zheng, Y.; Xu, J.; Fan, H.; Wang, Y.; Hu, M. Multi-omics reveals response mechanism of liver metabolism of hybrid sturgeon under ship noise stress. Sci. Total Environ. 2022, 851, 158348. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Wu, H.; Yang, H.; Zhao, J.; Lv, J. Gill damage and neurotoxicity of ammonia nitrogen on the clam Ruditapes philippinarum. Ecotoxicology 2017, 26, 459–469. [Google Scholar] [CrossRef]
- Yang, C.; Zeng, Y.; Liao, Y.; Deng, Y.; Du, X.; Wang, Q. Integrated GC–MS-and LC–MS-Based untargeted metabolomics studies of the effect of vitamin D3 on pearl production traits in pearl oyster Pinctada fucata martensii. Front. Mol. Biosci. 2021, 8, 614404. [Google Scholar] [CrossRef]
- Adeyemi, J.A.; Deaton, L.E. The effect of cadmium exposure on digestive enzymes in the eastern oyster Crassostrea virginica. J. Shellfish Res. 2012, 31, 631–634. [Google Scholar] [CrossRef]
- Wu, H.; Xuan, R.; Li, Y.; Zhang, X.; Wang, Q.; Wang, L. Effects of cadmium exposure on digestive enzymes, antioxidant enzymes, and lipid peroxidation in the freshwater crab Sinopotamon henanense. Environ. Sci. Pollut. Res. 2013, 20, 4085–4092. [Google Scholar] [CrossRef]
- Trestrail, C.; Walpitagama, M.; Miranda, A.; Nugegoda, D.; Shimeta, J. Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021, 779, 146418. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, P.; Cao, B.; Wu, M.; Li, X.; Wang, H.; Chai, L. Intestinal response characteristic and potential microbial dysbiosis in digestive tract of Bufo gargarizans after exposure to cadmium and lead, alone or combined. Chemosphere 2021, 271, 129511. [Google Scholar] [CrossRef]
- Xie, D.; Li, Y.; Liu, Z.; Chen, Q. Inhibitory effect of cadmium exposure on digestive activity, antioxidant capacity and immune defense in the intestine of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 222, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Vlahović, M.; Ilijin, L.; Lazarević, J.; Mrdaković, M.; Gavrilović, A.; Matić, D.; Mataruga, V.P. Cadmium-induced changes of gypsy moth larval mass and protease activity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 160, 9–14. [Google Scholar] [CrossRef]
- Lu, F.L.; Guo, C.A.; Mkuye, R.; Chen, W.; Yang, X.; Zhou, Z.; He, Y.; Yang, C.Y.; Deng, Y.W. Effects of polyvinyl chloride microplastic on pearl oyster (Pinctada fucata martensii). Reg. Stud. Mar. Sci. 2024, 69, 103313. [Google Scholar] [CrossRef]
- Chen, Q.L.; Gong, Y.; Luo, Z.; Zheng, J.L.; Zhu, Q.L. Differential effect of waterborne cadmium exposure on lipid metabolism in liver and muscle of yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 2013, 142, 380–386. [Google Scholar] [CrossRef]
- Fu, L.; Xi, M.; Nicholaus, R.; Wang, Z.; Wang, X.; Kong, F.; Yu, Z. Behaviors and biochemical responses of macroinvertebrate Corbicula fluminea to polystyrene microplastics. Sci. Total Environ. 2022, 813, 152617. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, F.; Fu, L.; Li, Y.; Li, M.; Yu, Z. Responses of Asian clams (Corbicula fluminea) to low concentration cadmium stress: Whether the depuration phase restores physiological characteristics. Environ. Pollut. 2021, 284, 117182. [Google Scholar] [CrossRef]
- Lu, L.; Huang, W.; Han, Y.; Tong, D.; Sun, S.; Yu, Y.; Liu, G.; Shi, W. Toxicity of microplastics and triclosan, alone and in combination, to the fertilisation success of a broadcast spawning bivalve Tegillarca granosa. Environ. Toxicol. Pharmacol. 2023, 101, 104208. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tian, D.; Han, Y.; Huang, L.; Tang, Y.; Zhang, W.; Zhou, W.; Shi, W.; Yu, Y.; Liu, G. Impacts of microplastics and carbamazepine on the shell formation of thick-shell mussels and the underlying mechanisms of action. Sci. Total Environ. 2022, 838, 156442. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.H.; Sun, Z.X.; Feng, L.S.; Jin, T.; Xing, J.C.; Wen, X.L. Algal density affects the influences of polyethylene microplastics on the freshwater rotifer Brachionus calyciflorus. Chemosphere 2021, 270, 128613. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of different exercise modalities on oxidative stress: A systematic review. BioMed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]
- Geret, F.; Serafim, A.; Barreira, L.; Bebianno, M.J. Effect of cadmium on antioxidant enzyme activities and lipid peroxidation in the gills of the clam Ruditapes decussatus. Biomarkers 2002, 7, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Géret, F.; Jouan, A.; Turpin, V.; Bebianno, M.J.; Cosson, R.P. Influence of metal exposure on metallothionein synthesis and lipid peroxidation in two bivalve mollusks: The oyster (Crassostrea gigas) and the mussel (Mytilus edulis). Aquat. Living Resour. 2002, 15, 61–66. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Liu, G.; Wang, Q. Enzyme responses and lipid peroxidation in gills and hepatopancreas of clam Mactra vereformis, following cadmium exposure. Chin. J. Oceanol. Limnol. 2011, 29, 981–989. [Google Scholar] [CrossRef]
- Arasu, S.M.; Reddy, P.S. Changes in lipid peroxidation in the gill and muscle of the marine bivalve (Perna viridis) during exposure to cadmium and copper. Chem. Ecol. 1995, 11, 105–112. [Google Scholar] [CrossRef]
- Yan, B.; Liu, X.; Zhao, X.; Tian, S. Single and joint oxidative stress of cadmium and phenanthrene on the Bivalve Anadara subcrenata. J. Environ. Sci. Health Part A 2020, 55, 448–456. [Google Scholar] [CrossRef]
- Lu, J.; Yao, T.; Yu, G.; Ye, L. Adaptive response of triploid Fujian oyster (Crassostrea angulata) to nanoplastic stress: Insights from physiological, metabolomic, and microbial community analyses. Chemosphere 2023, 341, 140027. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Li, Y.; Jiang, H.; Miao, A.; Liao, Y.; Pan, K. Effects of nanoplastics on clam Ruditapes philippinarum at environmentally realistic concentrations: Toxicokinetics, toxicity, and gut microbiota. J. Hazard. Mater. 2023, 456, 131647. [Google Scholar] [CrossRef]
- Boudjema, K.; Kourdali, S.; Bounakous, N.; Meknachi, A.; Badis, A. Catalase activity in brown mussels (Perna perna) under acute cadmium, lead, and copper exposure and depuration tests. J. Mar. Sci. 2014, 2014, 830657. [Google Scholar] [CrossRef]
- Company, R.; Serafim, A.; Cosson, R.; Camus, L.; Shillito, B.; Fiala-Médioni, A.; Bebianno, M.J. The effect of cadmium on antioxidant responses and the susceptibility to oxidative stress in the hydrothermal vent mussel Bathymodiolus azoricus. Mar. Biol. 2006, 148, 817–825. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Yuan, H.; Xu, Y.; He, Z. Concentrations of cadmium and zinc in seawater of Bohai Bay and their effects on biomarker responses in the bivalve Chlamys farreri. Arch. Environ. Contam. Toxicol. 2010, 59, 120–128. [Google Scholar] [CrossRef]
- Adzigbli, L.; Yu, W.M.; Li, J.H.; Yang, C.; Deng, Y. Influence of age on pearl production performance, enzymatic activity, and immune-related gene expression of the pearl oyster Pinctada fucata martensii. N. Am. J. Aquac. 2019, 81, 430–437. [Google Scholar] [CrossRef]
- Li, F.F.; Xie, Y.F.; Yang, C.Y.; Ye, Q.; Wang, F.; Liao, Y.; Mkuye, R.; Deng, Y.W. The physiological responses to titanium dioxide nanoparticles exposure in pearl oysters (Pinctada fucata martensii). Mar. Environ. Res. 2024, 195, 106345. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Eom, H.J.; Nam, S.E.; Shin, Y.K.; Rhee, J.S. Chlorothalonil induces oxidative stress and reduces enzymatic activities of Na+/K+-ATPase and acetylcholinesterase in gill tissues of marine bivalves. PLoS ONE 2019, 14, e0214236. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Teng, J.; Wang, D.; Li, J.; Wang, X.; Zhao, J.; Shan, E.; Wang, Q. Potential threats of microplastics and pathogenic bacteria to the immune system of the mussels Mytilus galloprovincialis. Aquat. Toxicol. 2024, 272, 106959. [Google Scholar] [CrossRef]
- Weng, N.; Meng, J.; Huo, S.; Wu, F.; Wang, W.X. Hemocytes of bivalve mollusks as cellular models in toxicological studies of metals and metal-based nanomaterials. Environ. Pollut. 2022, 312, 120082. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, S.; Mohandas, A. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicol. Environ. Saf. 2005, 62, 140–143. [Google Scholar] [CrossRef]
- Jayakumar, P.; Jothivel, N.; Paul, V. Heavy metals induced alterations in the acid phosphatase activity in the edible freshwater mussel Lamellidens marginalis (Lamarck). Internet J. Toxicol. 2008, 5, 1–8. [Google Scholar]
- Evtushenko, Z.S.; Belcheva, N.N.; Lukyanova, O.N. Cadmium accumulation in organs of the scallop Mizuhopecten yessoensis—I. Activities of phosphatases and composition and amount of lipids. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1986, 83, 371–376. [Google Scholar] [CrossRef]
- Métais, I.; Latchere, O.; Roman, C.; Perrein-Ettajani, H.; Mouloud, M.; Georges, D.; Audroin, T.; Catrouillet, C.; Gigault, J.; Agnès-Feurtet-Mazel; et al. Continuum from microplastics to nanoplastics: Effects of size and source on the estuarine bivalve Scrobicularia plana. Environ. Sci. Pollut. Res. 2023, 30, 45725–45739. [Google Scholar] [CrossRef]
- Zhang, J.; Jie, W.; Cheng, G.; Gu, Z.; Liu, X. Transcriptome analysis of response mechanism to Microcystin-LR and microplastics stress in Asian clam (Corbicula fluminea). Fish Shellfish Immunol. 2023, 139, 108875. [Google Scholar] [CrossRef]
- Roman, C.; Mahé, P.; Latchere, O.; Catrouillet, C.; Gigault, J.; Métais, I.; Châtel, A. Effect of size continuum from nanoplastics to microplastics on marine mussel Mytilus edulis: Comparison in vitro/in vivo exposure scenarios. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109512. [Google Scholar] [CrossRef]
- Chen, J.Y.; Huang, J.; Peng, J.Q.; Yang, C.; Liao, Y.; Li, J.; Deng, Y.; Du, X. Effects of hypoxic stress on the digestion, energy metabolism, oxidative stress regulation, and immune function of the pearl oyster (Pinctada fucata martensii). Aquac. Rep. 2022, 25, 101246. [Google Scholar] [CrossRef]
- Regoli, F.; Principato, G. Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: Implications for the use of biochemical biomarkers. Aquat. Toxicol. 1995, 31, 143–164. [Google Scholar] [CrossRef]
- Rui, X.; Ping, L.X.; Yu, J.L.; Yang, C.; Liao, Y.; Li, J.; Deng, Y.W.; Du, X.D. Purification and enzymatic characterization of alkaline phosphatase from Pinctada fucata. J. Mol. Catal. B Enzym. 2002, 17, 65–74. [Google Scholar]
- He, Y.; Zhou, L.; Wang, M.; Zhong, Z.; Chen, H.; Lian, C.; Zhang, H.; Wang, H.; Cao, L.; Li, C. Integrated transcriptomic and metabolomic approaches reveal molecular response and potential biomarkers of the deep-sea mussel Gigantidas platifrons to copper exposure. J. Hazard. Mater. 2024, 473, 134612. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Zhang, X.; Gong, X.; Han, D.; Zhang, H.; Tian, X.; Xu, Y. Metabolomics comparison of metabolites and functional pathways in the gills of Chlamys farreri under cadmium exposure. Environ. Toxicol. Pharmacol. 2021, 86, 103683. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Yang, C.Y.; Liao, Y.S.; Wang, Q.; Deng, Y. Transcriptomic and metabolomic analyses reveal sex-related differences in the gonads of Pinctada fucata martensii. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101304. [Google Scholar] [CrossRef]
- Zhou, C.; Song, H.; Feng, J.; Hu, Z.; Yang, M.J.; Shi, P.; Li, Y.R.; Guo, Y.J.; Li, H.Z.; Zhang, T. Metabolomics and biochemical assays reveal the metabolic responses to hypo-salinity stress and osmoregulatory role of cAMP-PKA pathway in Mercenaria mercenaria. Comput. Struct. Biotechnol. J. 2022, 20, 4110–4121. [Google Scholar] [CrossRef]
- Liang, J.; Iqbal, S.; Wen, F.; Tong, M.; Liu, J. Phosphorus-induced lipid class alteration revealed by lipidomic and transcriptomic profiling in oleaginous microalga Nannochloropsis sp. PJ12. Mar. Drugs 2019, 17, 519. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, C.; Liu, W.; Liu, Y.; Ding, L.; Wang, P. Investigating the impact of disrupting the glutamine metabolism pathway on ammonia excretion in crucian carp (Carassius auratus) under carbonate alkaline stress using metabolomics techniques. Antioxidants 2024, 13, 170. [Google Scholar] [CrossRef]
- Jing, H.; Zhou, L.; Gao, Y.; Liu, Z.; Wu, B.; Sun, X.; Tu, K. Transcriptomics and metabolomics reveal the molecular and metabolic adaptation to heat stress in Manila clam Ruditapes philippinarum. Front. Mar. Sci. 2023, 10, 1204598. [Google Scholar] [CrossRef]
- García-Sevillano, M.Á.; García-Barrera, T.; Navarro-Roldán, F.; Montero-Lobato, Z.; Gómez-Ariza, J.L. A combination of metallomics and metabolomics studies to evaluate the effects of metal interactions in mammals. Application to Mus musculus mice under arsenic/cadmium exposure. J. Proteom. 2014, 104, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Leroux, N.; Hosseinzadeh, M.; Katsumiti, A.; Porte, C.; Cajaraville, M.P. Lipidomic analysis of mussel hemocytes exposed to polystyrene nanoplastics. Environ. Res. 2022, 214, 113763. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Kong, A.; Guo, C.; Liu, J.; Li, K.; Ren, Z.; Zhou, Y.; Tang, M.; Shi, H. Cadmium perturbed lipid profile and induced liver dysfunction in mice through phosphatidylcholine remodeling and promoting arachidonic acid synthesis and metabolism. Ecotoxicol. Environ. Saf. 2022, 247, 114254. [Google Scholar] [CrossRef]
- Fanelli, G.; Belardo, A.; Savino, R.; Rinalducci, S.; Zolla, L. Testosterone replacement therapy in insulin-sensitive hypogonadal men restores phosphatidylcholine levels by regulation of arachidonic acid metabolism. J. Cell. Mol. Med. 2020, 24, 8266–8269. [Google Scholar] [CrossRef]
- Ramirez, D.C.; Gimenez, M.S. Lipid modification in mouse peritoneal macrophages after chronic cadmium exposure. Toxicology 2002, 172, 1–12. [Google Scholar] [CrossRef]
- Yang, C.Y.; Du, X.D.; Hao, R.J.; Wang, Q.; Deng, Y.; Sun, R. Effect of vitamin D3 on immunity and antioxidant capacity of pearl oyster Pinctada fucata martensii after transplantation: Insights from LC–MS-based metabolomics analysis. Fish Shellfish Immunol. 2019, 94, 271–279. [Google Scholar] [CrossRef]
- Fokina, N.N.; Vasil’eva, O.B.; Ruokolainen, T.R.; Nemova, N.N. Changes in lipid composition and lipid peroxidation products content in the freshwater mussel Anodonta cygnea L. under cadmium effect. Limnol. Freshw. Biol. 2019, 5, 286–296. [Google Scholar] [CrossRef]
- Galle-Le Bastard, A.M.; Demandre, C.; Oursel, A.; Joseph, M.; Mazliak, P.; Kader, J.C. Phosphatidylcholine molecular species involved in Γ-linolenic acid biosynthesis in microsomes from borage seeds. Physiol. Plant. 2000, 108, 118–124. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, H.; Zhou, Z.; Zhang, H.; Liu, R.; Yi, Q.; Yang, C.; Gao, L.; Wang, L. Transcriptomic profile of oyster Crassostrea gigas hemocyte after short-term cadmium exposure and bacteria stimulation. Fish Shellfish Immunol. 2020, 98, 138–146. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Ji, C.; Wu, H. Toxicological mechanism of cadmium in the clam Ruditapes philippinarum using combined ionomic, metabolomic and transcriptomic analyses. Environ. Pollut. 2023, 323, 121286. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, F.; Liu, S.; Cheng, X.; Xu, J.; Liu, X.; Zhang, L. Response and adaptation mechanisms of Apostichopus japonicus to single and combined anthropogenic stresses of polystyrene microplastics or cadmium. Mar. Pollut. Bull. 2024, 204, 116519. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, F.; Di Dato, V.; Ianora, A.; Romano, G. Prostaglandins in marine organisms: A review. Mar. Drugs 2019, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Reddy, P.R.; Nagaraju GP, C. The synthesis and effects of prostaglandins on the ovary of the crab Oziotelphusa senex. Gen. Comp. Endocrinol. 2004, 135, 35–41. [Google Scholar] [CrossRef]
- Martínez, G.; Mettifogo, L.; Lenoir, R.; Campos, E.O. Prostaglandins and reproduction of the scallop Argopecten purpuratus: I. Relationship with gamete development. J. Exp. Zool. 1999, 284, 225–231. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Gao, Z.; Wang, Z.; Ge, Z.; Lin, Y.; Huang, L.; Liu, J.; Zou, H.; Yang, C.; Mkuye, R.; et al. The Combined Effects of Cadmium and Microplastic Mixtures on the Digestion, Energy Metabolism, Oxidative Stress Regulation, Immune Function, and Metabolomes in the Pearl Oyster (Pinctada fucata martensii). Fishes 2025, 10, 133. https://doi.org/10.3390/fishes10030133
Yao J, Gao Z, Wang Z, Ge Z, Lin Y, Huang L, Liu J, Zou H, Yang C, Mkuye R, et al. The Combined Effects of Cadmium and Microplastic Mixtures on the Digestion, Energy Metabolism, Oxidative Stress Regulation, Immune Function, and Metabolomes in the Pearl Oyster (Pinctada fucata martensii). Fishes. 2025; 10(3):133. https://doi.org/10.3390/fishes10030133
Chicago/Turabian StyleYao, Jiaying, Zixin Gao, Zhixiang Wang, Zhanbo Ge, Yujing Lin, Luomin Huang, Jiaen Liu, Heqi Zou, Chuangye Yang, Robert Mkuye, and et al. 2025. "The Combined Effects of Cadmium and Microplastic Mixtures on the Digestion, Energy Metabolism, Oxidative Stress Regulation, Immune Function, and Metabolomes in the Pearl Oyster (Pinctada fucata martensii)" Fishes 10, no. 3: 133. https://doi.org/10.3390/fishes10030133
APA StyleYao, J., Gao, Z., Wang, Z., Ge, Z., Lin, Y., Huang, L., Liu, J., Zou, H., Yang, C., Mkuye, R., & Deng, Y. (2025). The Combined Effects of Cadmium and Microplastic Mixtures on the Digestion, Energy Metabolism, Oxidative Stress Regulation, Immune Function, and Metabolomes in the Pearl Oyster (Pinctada fucata martensii). Fishes, 10(3), 133. https://doi.org/10.3390/fishes10030133









