Long-Term Heavy Metal Bioaccumulation in Sprat (Sprattus sprattus) from the Romanian Black Sea: Ecological and Human Health Risks in the Context of Sustainable Fisheries
Abstract
:1. Introduction
2. Materials and Methods
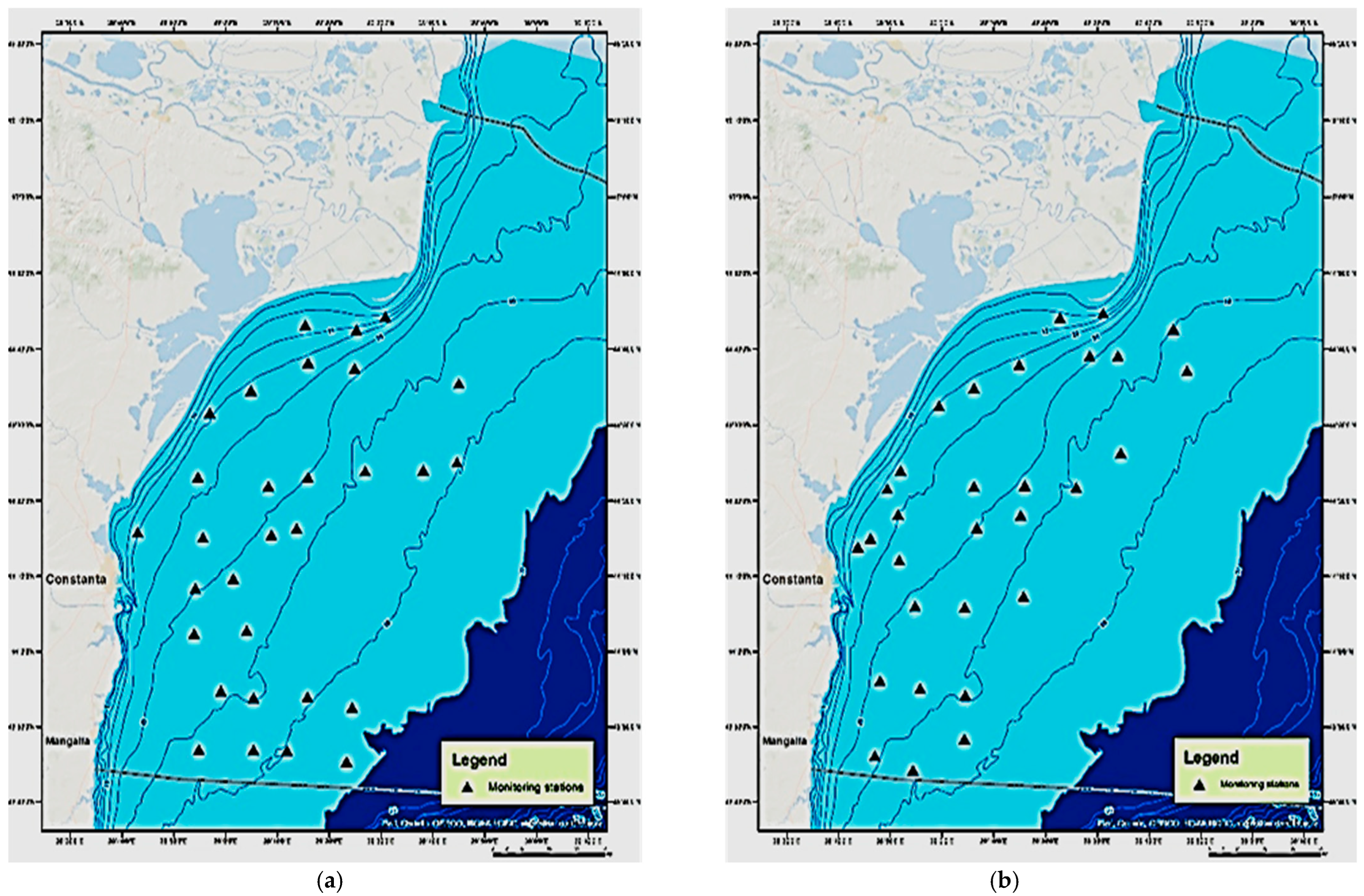
2.1. Study Area
2.2. Sampling Procedure
2.3. HMs Analysis
2.4. Health Risk Indices
3. Results
3.1. Distribution of HM Concentrations
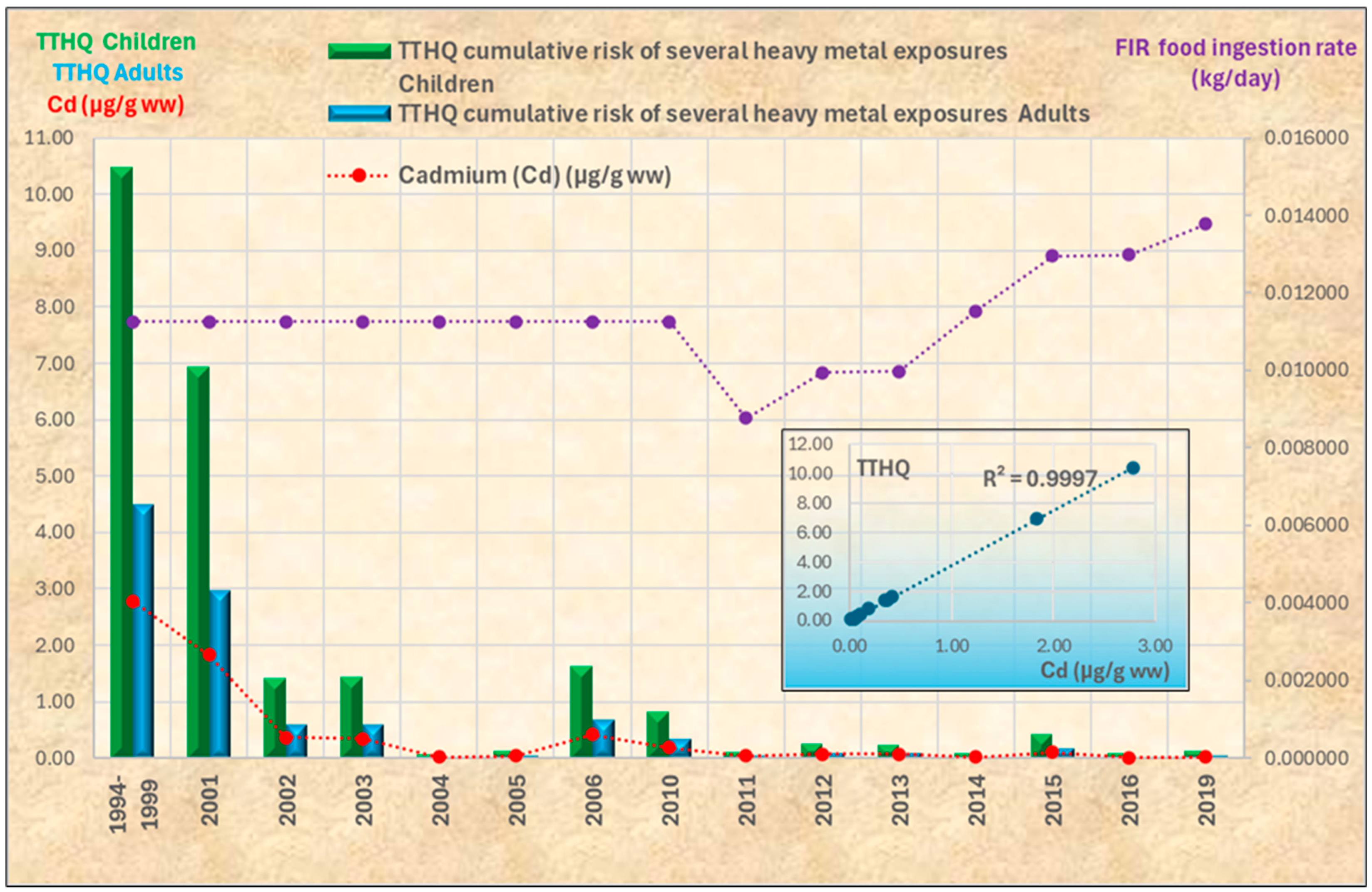
3.2. Temporal Trends
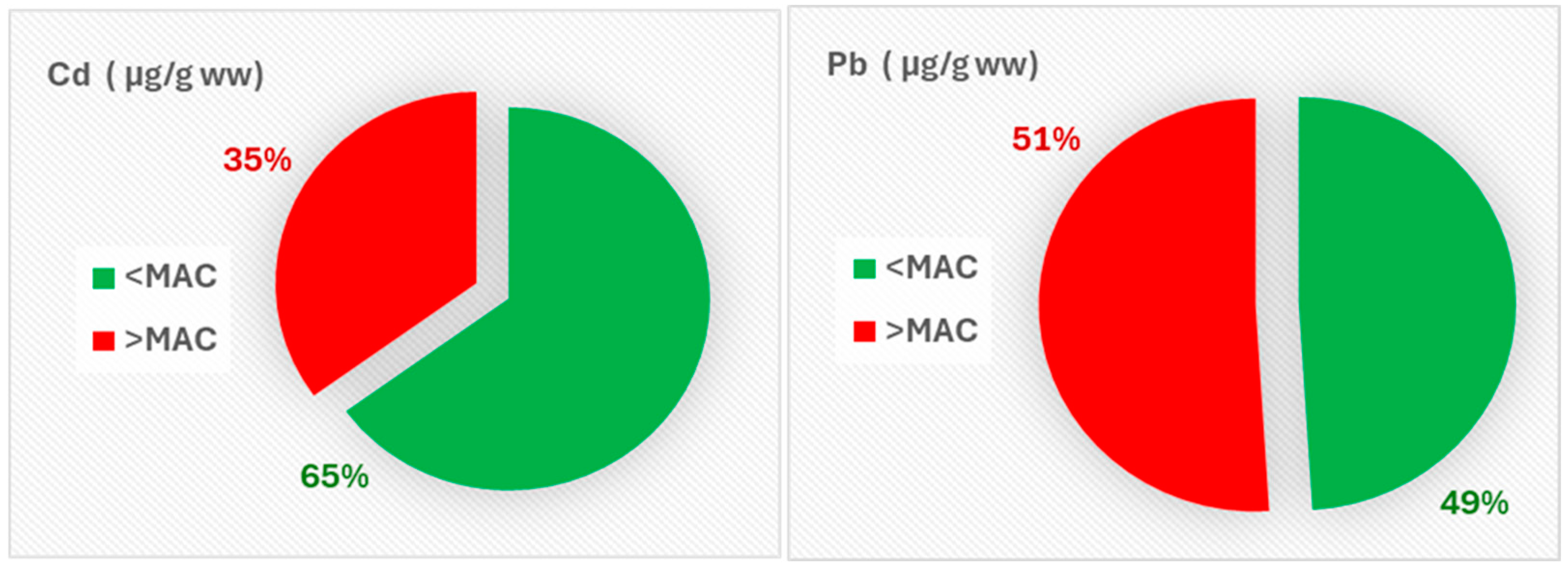
3.3. Exceedances of Safety Limits
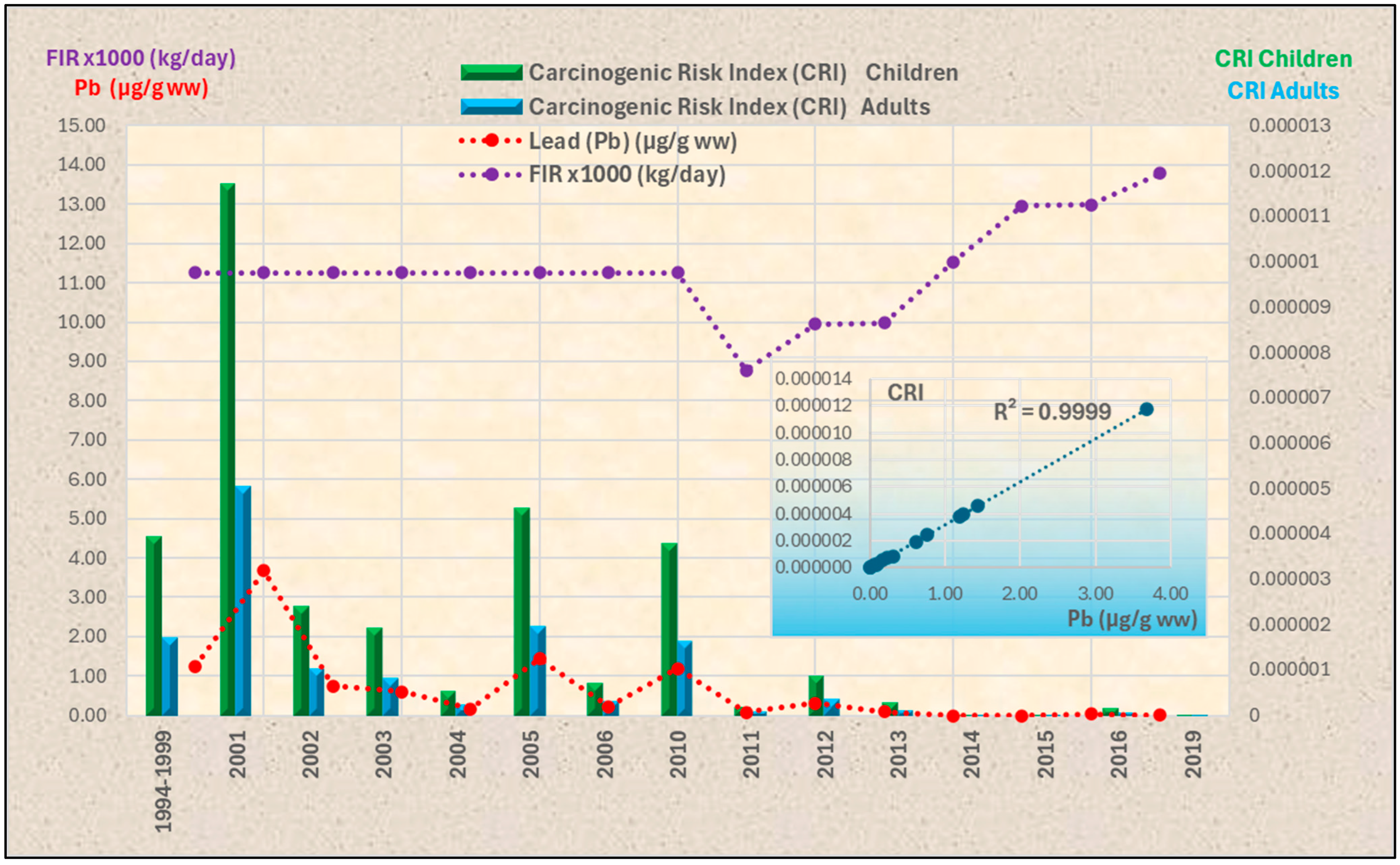
3.4. Health Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Țoțoiu, A.; Galațchi, M.; Danilov, C.; Radu, G. Evolution of the Sprat (Sprattus Sprattus, Linnaeus 1758) Population at the Romanian Littoral during 2008–2016. Cercet. Mar. Rech. Mar. 2017, 47, 205–221. [Google Scholar]
- Maximov, V.; Radu, G.; Tiganov, G. Pelagic Surveys at the Romanian Black Sea (GSA 29) in 2018. Cercet. Mar. Rech. Mar. 2019, 49, 102–115. [Google Scholar]
- Galaţchi, M.; Ţiganov, G.; Păun, C.; Danilov, C.; Roşioru, D.; Maximov, V.; Costache, M. Studies on the Migration Behavior of the Species Engraulis Encrasicolus from the Romanian Black Sea Coast. Cercet. Mar. Rech. Mar. 2019, 49, 125–132. [Google Scholar]
- Polak-Juszczak, L. Temporal Trends in the Bioaccumulation of Trace Metals in Herring, Sprat, and Cod from the Southern Baltic Sea in the 1994–2003 Period. Chemosphere 2009, 76, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, N.; Sun, S.; An, Q.; Li, P.; Li, X.; Li, Z.; Zhang, W. Trends and Health Risk of Trace Metals in Fishes in Liaodong Bay, China, From 2015 to 2020. Front. Mar. Sci. 2022, 8, 789572. [Google Scholar] [CrossRef]
- Bat, L.; Arici, E.; Öztekin, A. Metal Levels in Commercial Pelagic Fishes and Their Contribution to Their Exposure in Turkish People of the Black Sea. J. Fish. Res. 2017, 1, 2. [Google Scholar] [CrossRef]
- Bat, L.; Sezgin, M.; Ustun, F.; Sahin, F. Heavy Metal Concentrations in Ten Species of Fishes Caught in Sinop Coastal Waters of the Black Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2012, 12, 371–376. [Google Scholar] [CrossRef]
- Nita, V.; Nenciu, M.; Galatchi, M.; Diaconu, D. Fish Species of the Romanian Coast: Updated Atlas; CD Press: Bucharest, Romania, 2024; ISBN 978-606-528-757-0. [Google Scholar]
- Bișinicu, E.; Harcotă, G.; Lazar, L.; Niță, V.; Țoțoiu, A.; Țiganov, G. Fish Abundance and Mesozooplankton Resource: A Study on Sprattus Sprattus (Linnaeus, 1758) (Actinopterygii: Clupeidae) in the Romanian Black Sea Waters. Acta Zool. Bulg. 2024, 76, 215–224. [Google Scholar] [CrossRef]
- Radu, G.; Totoiu, A.; Galatchi, M.; Spanu, A. Evolution of the Sprat Fishery at the Romania. Cercet. Mar. Rech. Mar. 2016, 46, 128–143. [Google Scholar]
- Gücü, A.C.; Genç, Y.; Dağtekin, M.; Sakınan, S.; Ak, O.; Ok, M.; Aydın, İ. On Black Sea Anchovy and Its Fishery. Rev. Fish. Sci. Aquac. 2017, 25, 230–244. [Google Scholar] [CrossRef]
- Ţiganov, G.; Danilov, C.; Paun, C.; Galatchi, M. Preliminary Data on the Ichtyofauna Structure from the Northern Part of the Romanian Black Sea Coast. Ann. Acad. Rom. Sci. Ser. Biol. Sci. 2021, 10, 5–11. [Google Scholar] [CrossRef]
- Rana, V.; Milke, J.; Gałczyńska, M. Inorganic and Organic Pollutants in Baltic Sea Region and Feasible Circular Economy Perspectives for Waste Management: A Review. In Handbook of Solid Waste Management: Sustainability Through Circular Economy; Springer: Singapore, 2022; pp. 1743–1777. [Google Scholar] [CrossRef]
- Eid, M.H.; Eissa, M.; Mohamed, E.A.; Ramadan, H.S.; Tamás, M.; Kovács, A.; Szűcs, P. New Approach into Human Health Risk Assessment Associated with Heavy Metals in Surface Water and Groundwater Using Monte Carlo Method. Sci. Rep. 2024, 14, 1008. [Google Scholar] [CrossRef]
- Kalipci, E.; Cüce, H.; Ustaoğlu, F.; Dereli, M.A.; Türkmen, M. Toxicological Health Risk Analysis of Hazardous Trace Elements Accumulation in the Edible Fish Species of the Black Sea in Türkiye Using Multivariate Statistical and Spatial Assessment. Environ. Toxicol. Pharmacol. 2023, 97, 104028. [Google Scholar] [CrossRef]
- Mansouri, B.; Ebrahimpour, M.; Babaei, H. Bioaccumulation and Elimination of Nickel in the Organs of Black Fish (Capoeta Fusca). Toxicol. Ind. Health 2012, 28, 361–368. [Google Scholar] [CrossRef]
- Jamil Emon, F.; Rohani, M.F.; Sumaiya, N.; Tuj Jannat, M.F.; Akter, Y.; Shahjahan, M.; Abdul Kari, Z.; Tahiluddin, A.B.; Goh, K.W. Bioaccumulation and Bioremediation of Heavy Metals in Fishes-A Review. Toxics 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Han, J.L.; Pan, X.D.; Chen, Q.; Huang, B.F. Health Risk Assessment of Heavy Metals in Marine Fish to the Population in Zhejiang, China. Sci. Rep. 2021, 11, 11079. [Google Scholar] [CrossRef]
- Hashempour-baltork, F.; Jannat, B.; Tajdar-oranj, B.; Aminzare, M.; Sahebi, H.; Mirza Alizadeh, A.; Hosseini, H. A Comprehensive Systematic Review and Health Risk Assessment of Potentially Toxic Element Intakes via Fish Consumption in Iran. Ecotoxicol. Environ. Saf. 2023, 249, 114349. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, W.Q.; da Fonseca Alves, B.S.; Dantas, K.D. Health Risk Assessment Attributed the Consumption of Fish and Seafood in Belém, Pará, Brazil. J. Trace Elem. Miner. 2023, 6, 100103. [Google Scholar] [CrossRef]
- Pokorska-Niewiada, K.; Witczak, A.; Protasowicki, M.; Cybulski, J. Estimation of Target Hazard Quotients and Potential Health Risks for Toxic Metals and Other Trace Elements by Consumption of Female Fish Gonads and Testicles. Int. J. Environ. Res. Public Health 2022, 19, 2762. [Google Scholar] [CrossRef]
- Almafrachi, H.A.A.; Gümüş, N.E.; Çorak Öcal, İ. Heavy Metal Bioaccumulation in Fish: Implications for Human Health Risk Assessment in Ten Commercial Fish Species from Konya, Türkiye. Int. J. Environ. Sci. Technol. 2025, 22, 4065–4074. [Google Scholar] [CrossRef]
- Bošković, N.; Joksimović, D.; Bajt, O. Content of Trace Elements and Human Health Risk Assessment via Consumption of Commercially Important Fishes from Montenegrin Coast. Foods 2023, 12, 762. [Google Scholar] [CrossRef]
- Copat, C.; Conti, G.O.; Signorelli, C.; Marmiroli, S.; Sciacca, S.; Vinceti, M.; Ferrante, M. Risk Assessment for Metals and PAHs by Mediterranean Seafood. Food Nutr. Sci. 2013, 4, 10–13. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kasiyan, O.; Kamiński, P.; Kurhaluk, N. Assessing the Human Health Risk of Baltic Sea Sea Trout (Salmo Trutta L.) Consumption. Fish. Aquat. Life 2022, 30, 27–43. [Google Scholar] [CrossRef]
- Tuomisto, J.T.; Asikainen, A.; Meriläinen, P.; Haapasaari, P. Health Effects of Nutrients and Environmental Pollutants in Baltic Herring and Salmon: A Quantitative Benefit-Risk Assessment. BMC Public Health 2020, 20, 64. [Google Scholar] [CrossRef]
- Szefer, P. Safety Assessment of Seafood with Respect to Chemical Pollutants in European Seas. Oceanol. Hydrobiol. Stud. 2013, 42, 110–118. [Google Scholar] [CrossRef]
- Mikolajczyk, S.; Warenik-Bany, M.; Pajurek, M. PCDD/Fs and PCBs in Baltic Fish—Recent Data, Risk for Consumers. Mar. Pollut. Bull. 2021, 171, 112763. [Google Scholar] [CrossRef]
- Lin, H.; Luo, X.; Yu, D.; He, C.; Cao, W.; He, L.; Liang, Z.; Zhou, J.; Fang, G. Risk Assessment of As, Cd, Cr, and Pb via the Consumption of Seafood in Haikou. Sci. Rep. 2024, 14, 19549. [Google Scholar] [CrossRef]
- Zhuzzhassarova, G.; Azarbayjani, F.; Zamaratskaia, G. Fish and Seafood Safety: Human Exposure to Toxic Metals from the Aquatic Environment and Fish in Central Asia. Int. J. Mol. Sci. 2024, 25, 1590. [Google Scholar] [CrossRef]
- Zhao, R.; Yan, S.; Liu, M.; Wang, B.; Hu, D.; Guo, D.; Wang, J.; Xu, W.; Fan, C. Seafood Consumption among Chinese Coastal Residents and Health Risk Assessment of Heavy Metals in Seafood. Environ. Sci. Pollut. Res. 2016, 23, 16834–16844. [Google Scholar] [CrossRef]
- Pandion, K.; Arunachalam, K.D.; Rajagopal, R.; Ali, D.; Alarifi, S.; Chang, S.W.; Ravindran, B. Health Risk Assessment of Heavy Metals in the Seafood at Kalpakkam Coast, Southeast Bay of Bengal. Mar. Pollut. Bull. 2023, 189, 114766. [Google Scholar] [CrossRef]
- Karsli, B. Determination of Metal Content in Anchovy (Engraulis Encrasicolus) from Turkey, Georgia and Abkhazia Coasts of the Black Sea: Evaluation of Potential Risks Associated with Human Consumption. Mar. Pollut. Bull. 2021, 165, 112108. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, T. Heavy Metal Concentrations in the Edible Tissues of Some Commercial Fishes Caught along the Eastern Black Sea Coast of Turkey and the Health Risk Assessment. Spectrosc. Lett. 2021, 54, 437–445. [Google Scholar] [CrossRef]
- Bat, L.; Şahin, F.; Öztekin, A.; Arici, E. Toxic Metals in Seven Commercial Fish from the Southern Black Sea: Toxic Risk Assessment of Eleven-Year Data Between 2009 and 2019. Biol. Trace Elem. Res. 2022, 200, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Bat, L.; Yardım, Ö.; Öztekin, A.; Arıcı, E. Assessment of Heavy Metal Concentrations in Scophthalmus Maximus (Linnaeus, 1758) from the Black Sea Coast: Implications for Food Safety and Human Health. J. Hazard. Mater. Adv. 2023, 12, 100384. [Google Scholar] [CrossRef]
- Jitar, O.; Teodosiu, C.; Oros, A.; Plavan, G.; Nicoara, M. Bioaccumulation of Heavy Metals in Marine Organisms from the Romanian Sector of the Black Sea. N. Biotechnol. 2015, 32, 369–378. [Google Scholar] [CrossRef]
- Galaţchi, M.; Oros, A.; Coatu, V.; Costache, M.; Coprean, D.; Galaţchi, L.-D. Pollutant Bioaccumulation in Anchovy (Engraulis Encrasicolus) Tissue, Fish Species of Commercial Interest at the Romanian Black Sea Coast. Ovidius Univ. Ann. Chem. 2017, 28, 11–17. [Google Scholar] [CrossRef]
- Damir, N.; Danilov, D.; Oros, A.; Lazăr, L.; Coatu, V. Chemical Status Evaluation of the Romanian Black Sea Marine Environment Based on Benthic Organisms’ Contamination. Cercet. Mar. Rech. Mar. 2022, 52, 52. [Google Scholar] [CrossRef]
- Oros, A.; Pantea, E.-D.; Ristea, E. Heavy Metal Concentrations in Wild Mussels Mytilus Galloprovincialis (Lamarck, 1819) during 2001–2023 and Potential Risks for Consumers: A Study on the Romanian Black Sea Coast. Science 2024, 6, 45. [Google Scholar] [CrossRef]
- Damir, N.; Coatu, V.; Danilov, D.; Lazăr, L.; Oros, A. From Waters to Fish: A Multi-Faceted Analysis of Contaminants’ Pollution Sources, Distribution Patterns, and Ecological and Human Health Consequences. Fishes 2024, 9, 274. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Strungaru, S.-A.; Petrea, S.-M.; Cristea, V.; Nicoara, M.; Mogodan, A.; Oprica, L.; Costin, D.; Nica, A. Heavy Metals Accumulation in Fish Reared in a Pond Ecosystems and Health Risk Evaluation on Romanian Consumers. In Proceedings of the 2020 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 29 October 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Simionov, I.-A.; Cristea, D.S.; Petrea, Ș.-M.; Mogodan, A.; Jijie, R.; Ciornea, E.; Nicoară, M.; Turek Rahoveanu, M.M.; Cristea, V. Predictive Innovative Methods for Aquatic Heavy Metals Pollution Based on Bioindicators in Support of Blue Economy in the Danube River Basin. Sustainability 2021, 13, 8936. [Google Scholar] [CrossRef]
- Galatchi, L.-D.; Mihalache, D.-L. Food Chain Security in Romania. In Food Chain Security. NATO Science for Peace and Security Series C: Environmental Security; Alpas, H., Çırakoğlu, B., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 59–78. [Google Scholar]
- Ministry Of Agriculture and Rural Development/National Agency For Fisheries and Aquaculture. [ROU] Annual Report on Data Collection in the Fisheries and Aquaculture Sectors. 2023. Available online: https://datacollection.jrc.ec.europa.eu/documents/d/dcf/romania_annual_report_2023_text. (accessed on 28 January 2025).
- Oros, A.; Gomoiu, M.-T. A Review of Metal Bioaccumulation Levels in the Romanian Black Sea Biota during the Last Decade -a Requirement for Implementing Marine Strategy Framework Directive (Descriptors 8 and 9). J. Environ. Prot. Ecol. 2012, 13, 1730. [Google Scholar]
- Oros, A.; Coatu, V.; Tolun, L.-G.; Atabay, H.; Denga, Y.; Damir, N.; Danilov, D.; Aslan, E.; Litvinova, M.; Oleinik, Y.; et al. Hazardous Substances Assessment in Black Sea Biota. Cercet. Mar. Rech. Mar. 2021, 51, 27–48. [Google Scholar] [CrossRef]
- Lazar, L. (Ed.) ANEMONE Deliverable 2.1 “Impact of the Rivers on the Black Sea Ecosystem”; Editura CD Press: Bucharest, Romania, 2021; ISBN 978-606-528-528-6. [Google Scholar]
- Lazar, L.; Spanu, A.; Boicenco, L.; Oros, A.; Damir, N.; Bisinicu, E.; Abaza, V.; Filimon, A.; Harcota, G.; Marin, O.; et al. Methodology for Prioritizing Marine Environmental Pressures under Various Management Scenarios in the Black Sea. Front. Mar. Sci. 2024, 11, 1388877. [Google Scholar] [CrossRef]
- Bisinicu, E.; Abaza, V.; Boicenco, L.; Adrian, F.; Harcota, G.-E.; Marin, O.; Oros, A.; Pantea, E.; Spinu, A.; Timofte, F.; et al. Spatial Cumulative Assessment of Impact Risk-Implementing Ecosystem-Based Management for Enhanced Sustainability and Biodiversity in the Black Sea. Sustainability 2024, 16, 4449. [Google Scholar] [CrossRef]
- Oros, A.; Coatu, V.; Damir, N.; Danilov, D.; Ristea, E. Recent Findings on the Pollution Levels in the Romanian Black Sea Ecosystem: Implications for Achieving Good Environmental Status (GES) Under the Marine Strategy Framework Directive (Directive 2008/56/EC). Sustainability 2024, 16, 9785. [Google Scholar] [CrossRef]
- UNEP/FAO/IOC/IAEA. Guidelines for Monitoring Chemical Contaminants in the Sea Using Marine Organisms. In References Methods for Marine Pollution Studies No 6; United Nations Environment Program, Regional Seas: Nairobi, Kenya, 1993. [Google Scholar]
- IAEA-MEL. Training Manual on the Measurement of Heavy Metals in Environmental Samples; IAEA-MEL: Quai Antoine 1er, Monaco, 1999. [Google Scholar]
- Ullah, A.K.M.A.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Dietary Intake of Heavy Metals from Eight Highly Consumed Species of Cultured Fish and Possible Human Health Risk Implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Mendelsohn, R.; Deziel, N.C. Health Risks of Heavy Metals in Food and Their Economic Burden in Armenia. Environ. Int. 2023, 172, 107794. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 4 June 2024).
- Regional Screening Levels (RSLs)—Generic Tables|US EPA. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 4 June 2024).
- The Risk Assessment Information System. Available online: https://rais.ornl.gov/cgi-bin/tools/TOX_search (accessed on 4 June 2024).
- Rajan, S.; Ishak, N.S. Estimation of Target Hazard Quotients and Potential Health Risks for Metals by Consumption of Shrimp (Litopenaeus Vannamei) in Selangor, Malaysia. Sains Malays. 2017, 46, 1825–1830. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Rayasam, S.D.G.; Axelrad, D.A.; Bennett, D.H.; Brown, P.; Carignan, C.C.; Chartres, N.; Diamond, M.L.; Joglekar, R.; Shamasunder, B.; et al. Addressing Systemic Problems with Exposure Assessments to Protect the Public’s Health. Environ. Health 2023, 21, 121. [Google Scholar] [CrossRef]
- Shetty, B.R.; Pai, B.J.; Salmataj, S.A.; Naik, N. Assessment of Carcinogenic and Non-Carcinogenic Risk Indices of Heavy Metal Exposure in Different Age Groups Using Monte Carlo Simulation Approach. Sci. Rep. 2024, 14, 30319. [Google Scholar] [CrossRef]
- Nag, R.; Cummins, E. Human Health Risk Assessment of Lead (Pb) through the Environmental-Food Pathway. Sci. Total Environ. 2022, 810, 151168. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software, Inc. TIBCO Statistica, Version 14.0.1.25; TIBCO Software, Inc.: Palo Alto, CA, USA, 2023.
- Bakan, G.; Büyükgüngör, H. The Black Sea. Mar. Pollut. Bull. 2000, 41, 24–43. [Google Scholar] [CrossRef]
- Mee, L. Reviving Dead Zones. Sci. Am. 2006, 295, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lazar, L.; Vlas, O.; Pantea, E.; Boicenco, L.; Marin, O.; Abaza, V.; Filimon, A.; Bisinicu, E. Black Sea Eutrophication Comparative Analysis of Intensity between Coastal and Offshore Waters. Sustainability 2024, 16, 5146. [Google Scholar] [CrossRef]
- Rudneva, I.; Petzold-Bradley, E. Environment and Security Challenges in the Black Sea Region. In Responding to Environmental Conflicts: Implications for Theory and Practice; Springer: Dordrecht, The Netherlands, 2001; pp. 189–207. [Google Scholar]
- Martinovic-Vitanovic, V.; Kalafatic, V. Ecological Impact on the Danube After NATO Air Strikes. In Environmental Consequences of War and Aftermath; Springer: Berlin/Heidelberg, Germany, 2009; pp. 253–282. [Google Scholar]
- Surubaru, N.-C.; Nitoiu, C. One Decade Onwards: Assessing the Impact of European Union Membership on Bulgaria and Romania. Eur. Politics Soc. 2021, 22, 161–166. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. OJ L 119, 5.5.2023 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915. (accessed on 5 June 2024).
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Update of the Risk Assessment of Nickel in Food and Drinking Water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Raymond Boobis, A.; Ceccatelli, S.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Statement on Tolerable Weekly Intake for Cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Bogalecka, M. The Baltic Sea under Anthropopressure—The Sea of Paradoxes. Water 2022, 14, 3772. [Google Scholar] [CrossRef]
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Durán Valsero, J.J.; Morales García, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and Review of Heavy Metals Pollution in Sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- Reckermann, M.; Omstedt, A.; Soomere, T.; Aigars, J.; Akhtar, N.; Bełdowska, M.; Bełdowski, J.; Cronin, T.; Czub, M.; Eero, M.; et al. Human Impacts and Their Interactions in the Baltic Sea Region. Earth Syst. Dyn. 2022, 13, 1–80. [Google Scholar] [CrossRef]
- Zitoun, R.; Marcinek, S.; Hatje, V.; Sander, S.G.; Völker, C.; Sarin, M.; Omanović, D. Climate Change Driven Effects on Transport, Fate and Biogeochemistry of Trace Element Contaminants in Coastal Marine Ecosystems. Commun. Earth Environ. 2024, 5, 560. [Google Scholar] [CrossRef]
- Radakovitch, O.; Roussiez, V.; Ollivier, P.; Ludwig, W.; Grenz, C.; Probst, J.-L. Input of Particulate Heavy Metals from Rivers and Associated Sedimentary Deposits on the Gulf of Lion Continental Shelf. Estuar. Coast. Shelf Sci. 2008, 77, 285–295. [Google Scholar] [CrossRef]
- Whelan, M.J.; Linstead, C.; Worrall, F.; Ormerod, S.J.; Durance, I.; Johnson, A.C.; Johnson, D.; Owen, M.; Wiik, E.; Howden, N.J.K.; et al. Is Water Quality in British Rivers “Better than at Any Time since the End of the Industrial Revolution”? Sci. Total Environ. 2022, 843, 157014. [Google Scholar] [CrossRef] [PubMed]
- Heim, S.; Schwarzbauer, J. Pollution History Revealed by Sedimentary Records: A Review. Environ. Chem. Lett. 2013, 11, 255–270. [Google Scholar] [CrossRef]
- Peck, M.A.; Baumann, H.; Bernreuther, M.; Clemmesen, C.; Herrmann, J.-P.; Haslob, H.; Huwer, B.; Kanstinger, P.; Köster, F.W.; Petereit, C.; et al. The Ecophysiology of Sprattus Sprattus in the Baltic and North Seas. Prog. Oceanogr. 2012, 103, 42–57. [Google Scholar] [CrossRef]
- Chen, C.-T.; Carlotti, F.; Harmelin-Vivien, M.; Lebreton, B.; Guillou, G.; Vassallo, L.; Le Bihan, M.; Bănaru, D. Diet and Trophic Interactions of Mediterranean Planktivorous Fishes. Mar. Biol. 2022, 169, 119. [Google Scholar] [CrossRef]
- Möllmann, C.; Kornilovs, G.; Fetter, M.; Köster, F.W. Feeding Ecology of Central Baltic Sea Herring and Sprat. J. Fish. Biol. 2004, 65, 1563–1581. [Google Scholar] [CrossRef]
- Raab, K.; Nagelkerke, L.; Boerée, C.; Rijnsdorp, A.; Temming, A.; Dickey-Collas, M. Dietary Overlap between the Potential Competitors Herring, Sprat and Anchovy in the North Sea. Mar. Ecol. Prog. Ser. 2012, 470, 101–111. [Google Scholar] [CrossRef]
- Sofoulaki, K.; Kalantzi, I.; Zeri, C.; Machias, A.; Pergantis, S.; Tsapakis, M. Sardine and Anchovy as Bioindicators of Metal Content in Greek Coastal Waters. Mediterr. Mar. Sci. 2022, 23, 546–560. [Google Scholar] [CrossRef]
- Bera, T.; Kumar, S.V.; Devi, M.S.; Kumar, V.; Behera, B.K.; Das, B.K. Effect of Heavy Metals in Fish Reproduction: A Review. J. Environ. Biol. 2022, 43, 631–642. [Google Scholar] [CrossRef]
- Dey, S.; Rajak, P.; Sen, K. Bioaccumulation of Metals and Metalloids in Seafood: A Comprehensive Overview of Mobilization, Interactive Effects in Eutrophic Environments, and Implications for Public Health Risks. J. Trace Elem. Miner. 2024, 8, 100141. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Khan, M. Status of Heavy Metal Pollution of Water and Fishes in Balu and Brahmaputra Rivers. Progress. Agric. 2017, 27, 444–452. [Google Scholar] [CrossRef]
- Copat, C.; Bella, F.; Castaing, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy Metals Concentrations in Fish from Sicily (Mediterranean Sea) and Evaluation of Possible Health Risks to Consumers. Bull. Environ. Contam. Toxicol. 2012, 88, 78–83. [Google Scholar] [CrossRef]
- Copat, C.; Grasso, A.; Fiore, M.; Cristaldi, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. Trace Elements in Seafood from the Mediterranean Sea: An Exposure Risk Assessment. Food Chem. Toxicol. 2018, 115, 13–19. [Google Scholar] [CrossRef]
- Filazi, A.; Baskaya, R.; Kum, C.; Hismiogullari, S.E. Metal Concentrations in Tissues of the Black Sea Fish Mugil Auratus from Sinop-Icliman, Turkey. Hum. Exp. Toxicol. 2003, 22, 85–87. [Google Scholar] [CrossRef]
- Turan, C.; Dural, M.; Oksuz, A.; Öztürk, B. Levels of Heavy Metals in Some Commercial Fish Species Captured from the Black Sea and Mediterranean Coast of Turkey. Bull. Environ. Contam. Toxicol. 2009, 82, 601–604. [Google Scholar] [CrossRef]
- Korkmaz Görür, F.; Keser, R.; Akçay, N.; Dizman, S. Radioactivity and Heavy Metal Concentrations of Some Commercial Fish Species Consumed in the Black Sea Region of Turkey. Chemosphere 2012, 87, 356–361. [Google Scholar] [CrossRef]
- Plavan, G.; Jitar, O.; Teodosiu, C.; Nicoara, M.; Micu, D.; Strungaru, S.-A. Toxic Metals in Tissues of Fishes from the Black Sea and Associated Human Health Risk Exposure. Environ. Sci. Pollut. Res. 2017, 24, 7776–7787. [Google Scholar] [CrossRef] [PubMed]
- Bat, L.; Yardim, Ö.; Öztekin, A. Metal Levels in Trachurus Mediterraneus Ponticus Aleev, 1956 and Their Health Risk Appraisal For Consumers. Sinop Üniversitesi Bilim. Derg. 2023, 8, 19–29. [Google Scholar] [CrossRef]
- Bat, L.; Yardım, Ö.; Öztekin, A.; Arıcı, E. Assessing Health Risks from Metal Contamination in Engraulis Encrasicolus (Linnaeus, 1758) along the Black Sea. J. Food Compos. Anal. 2024, 132, 106309. [Google Scholar] [CrossRef]
- Bisinicu, E.; Lazar, L. Assessing the Black Sea Mesozooplankton Community Following the Nova Kakhovka Dam Breach. J. Mar. Sci. Eng. 2025, 13, 67. [Google Scholar] [CrossRef]
- Jiang, D.; Khokhlov, V.; Tuchkovenko, Y.; Kushnir, D.; Ovcharuk, V.; Spyrakos, E.; Stanica, A.; Slabakova, V.; Tyler, A. The Biogeochemical Response of the North-Western Black Sea to the Kakhovka Dam Breach. Commun. Earth Environ. 2025, 6, 185. [Google Scholar] [CrossRef]
- Suami, R.B.; Sivalingam, P.; Al Salah, D.M.; Grandjean, D.; Mulaji, C.K.; Mpiana, P.T.; Breider, F.; Otamonga, J.-P.; Poté, J. Heavy Metals and Persistent Organic Pollutants Contamination in River, Estuary, and Marine Sediments from Atlantic Coast of Democratic Republic of the Congo. Environ. Sci. Pollut. Res. 2020, 27, 20000–20013. [Google Scholar] [CrossRef]
- European Parliament. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). OJ L 164, 25.6.2008 2008. Available online: https://eur-lex.europa.eu/eli/dir/2008/56/oj. (accessed on 6 September 2024).
- Lee, S.-J.; Mamun, M.; Atique, U.; An, K.-G. Fish Tissue Contamination with Organic Pollutants and Heavy Metals: Link between Land Use and Ecological Health. Water 2023, 15, 1845. [Google Scholar] [CrossRef]
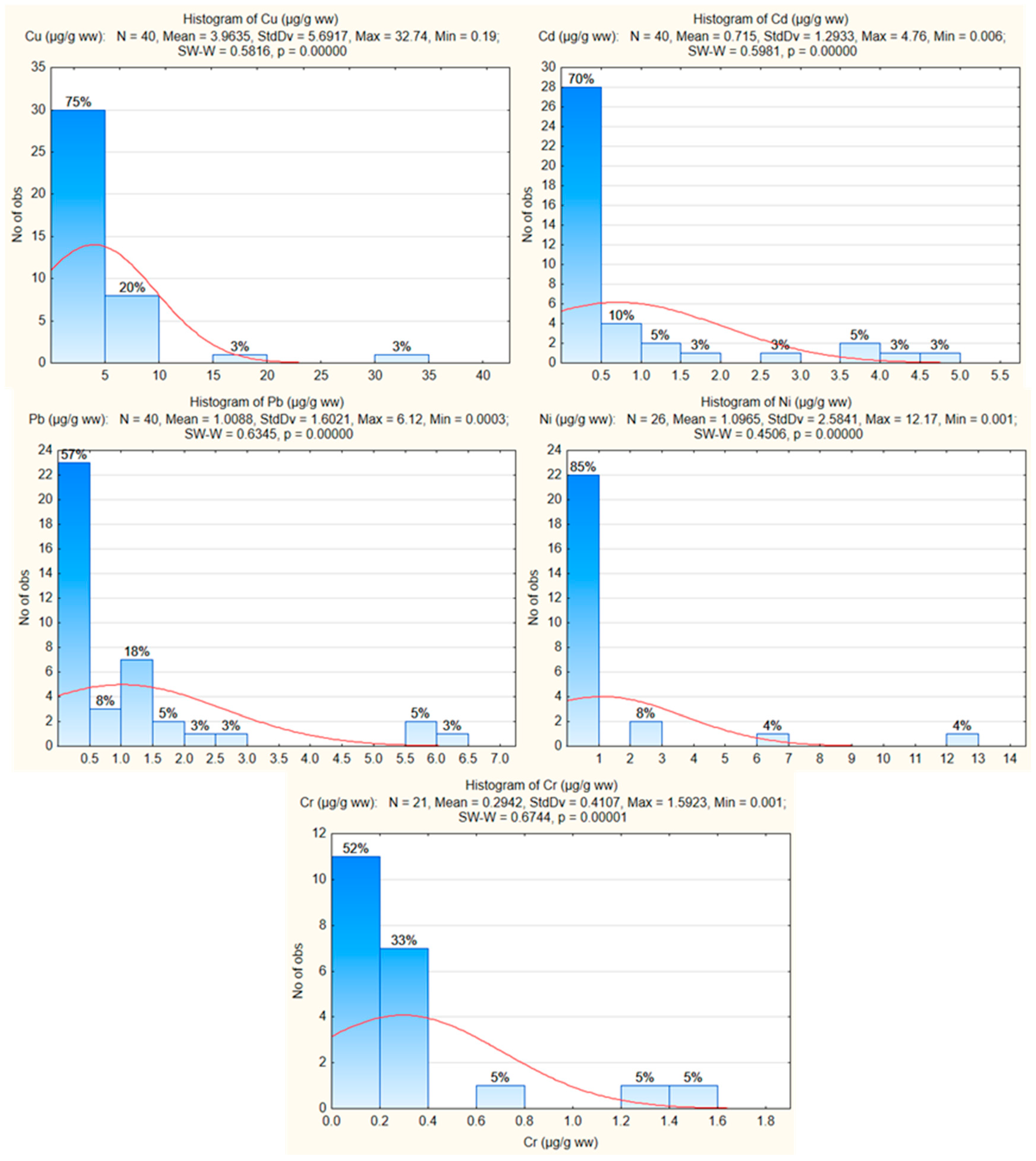

| Valid N | Mean | Median | Minimum | Maximum | 25th Percentile | 75th Percentile | Coef.Var. | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cu (µg/g ww) | 40 | 3.963 | 2.147 | 0.190 | 32.740 | 0.953 | 4.841 | 143.603 | 3.739 | 17.055 |
| Cd (µg/g ww) | 40 | 0.7149 | 0.093 | 0.005 | 4.760 | 0.029 | 0.581 | 180.883 | 2.159 | 3.597 |
| Pb (µg/g ww) | 40 | 1.008 | 0.321 | 0.003 | 6.120 | 0.050 | 1.390 | 158.812 | 2.396 | 5.361 |
| Ni (µg/g ww) | 26 * | 1.096 | 0.282 | 0.001 | 12.170 | 0.014 | 0.515 | 235.678 | 3.692 | 14.493 |
| Cr (µg/g ww) | 21 * | 0.2942 | 0.140 | 0.001 | 1.592 | 0.042 | 0.300 | 139.600 | 2.307 | 5.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oros, A.; Galatchi, M. Long-Term Heavy Metal Bioaccumulation in Sprat (Sprattus sprattus) from the Romanian Black Sea: Ecological and Human Health Risks in the Context of Sustainable Fisheries. Fishes 2025, 10, 178. https://doi.org/10.3390/fishes10040178
Oros A, Galatchi M. Long-Term Heavy Metal Bioaccumulation in Sprat (Sprattus sprattus) from the Romanian Black Sea: Ecological and Human Health Risks in the Context of Sustainable Fisheries. Fishes. 2025; 10(4):178. https://doi.org/10.3390/fishes10040178
Chicago/Turabian StyleOros, Andra, and Madalina Galatchi. 2025. "Long-Term Heavy Metal Bioaccumulation in Sprat (Sprattus sprattus) from the Romanian Black Sea: Ecological and Human Health Risks in the Context of Sustainable Fisheries" Fishes 10, no. 4: 178. https://doi.org/10.3390/fishes10040178
APA StyleOros, A., & Galatchi, M. (2025). Long-Term Heavy Metal Bioaccumulation in Sprat (Sprattus sprattus) from the Romanian Black Sea: Ecological and Human Health Risks in the Context of Sustainable Fisheries. Fishes, 10(4), 178. https://doi.org/10.3390/fishes10040178







