Purification of Intensive Shrimp Farming Effluent by Gracilaria Coupled with Oysters
Abstract
1. Introduction
2. Materials and Methods
2.1. Purification of Nitrogen and Phosphorus in Intensive Shrimp Farming Effluent by Gracilaria Under Different Environmental Conditions
2.1.1. Experimental Materials
Gracilaria
Experimental Water
2.1.2. Experimental Design
2.1.3. Water Quality Measurement
2.2. Purification of Intensive Shrimp Farming Effluent by the Coupling of Gracilaria and Oysters
2.2.1. Experimental Materials
Oysters
Experimental Water
2.2.2. Experimental Design
2.2.3. Water Quality Measurement
2.3. Deposition Effects of Nitrogen and Phosphorus in Effluent by Gracilaria–Oyster Coupling
2.3.1. Experimental Materials
2.3.2. Sample Processing and Indicator Detection
2.4. Data Analysis
3. Results
3.1. Purification of Nitrogen and Phosphorus in Intensive Shrimp Culture Effluent by Sargassum Under Different Environmental Conditions
3.1.1. Purification of Nitrogen and Phosphorus in Intensive Shrimp Farming Effluent by Gracilaria with Different Biomass
3.1.2. Purification of Nitrogen and Phosphorus in Intensive Shrimp Farming Effluent by Gracilaria Under Different Temperatures
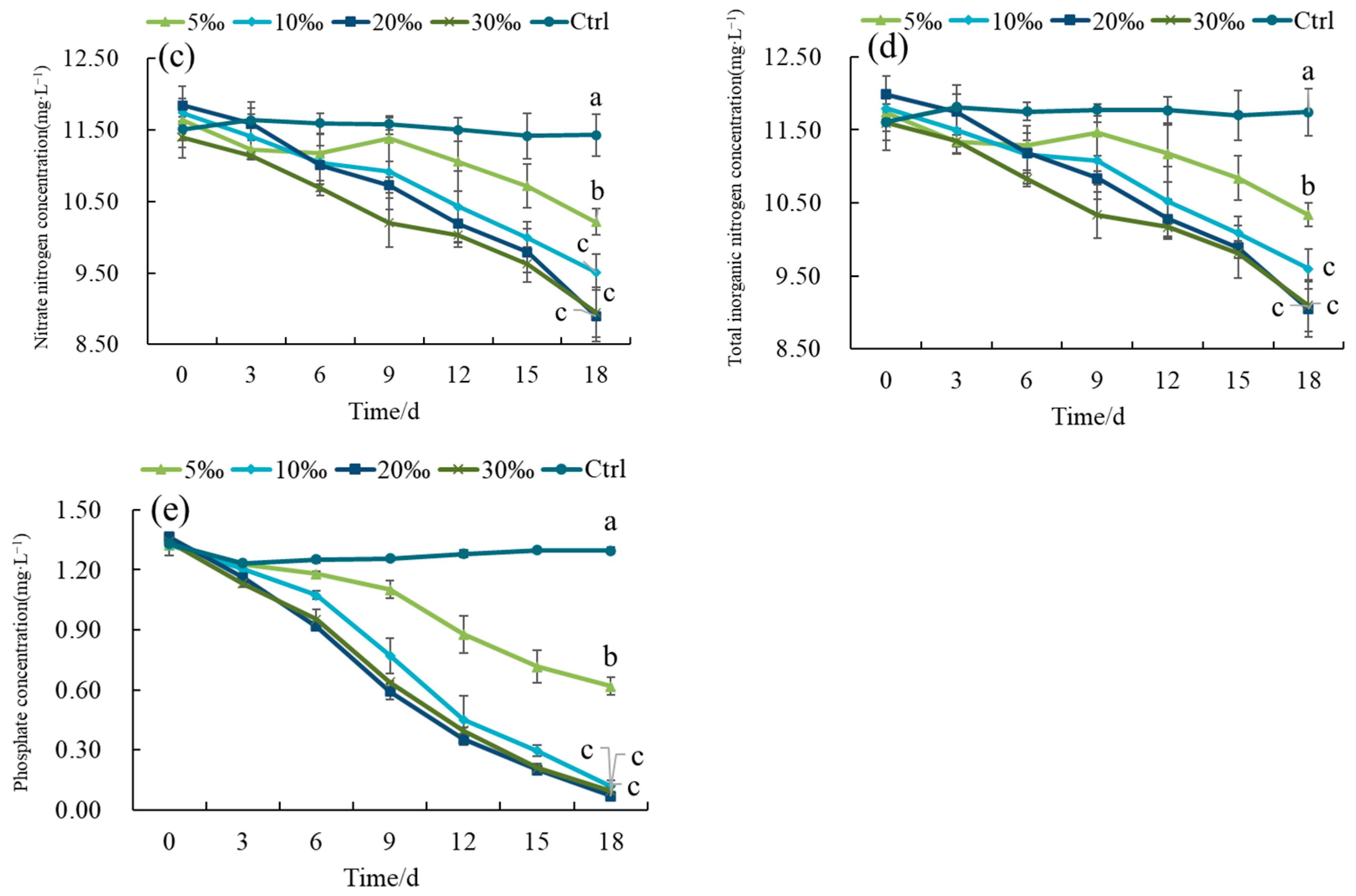
3.1.3. Purification of Nitrogen and Phosphorus in Intensive Shrimp Farming Effluent by Gracilaria Under Different Salinity Conditions
3.2. Purification of Intensive Shrimp Farming Effluent by Gracilaria Coupled with Oysters
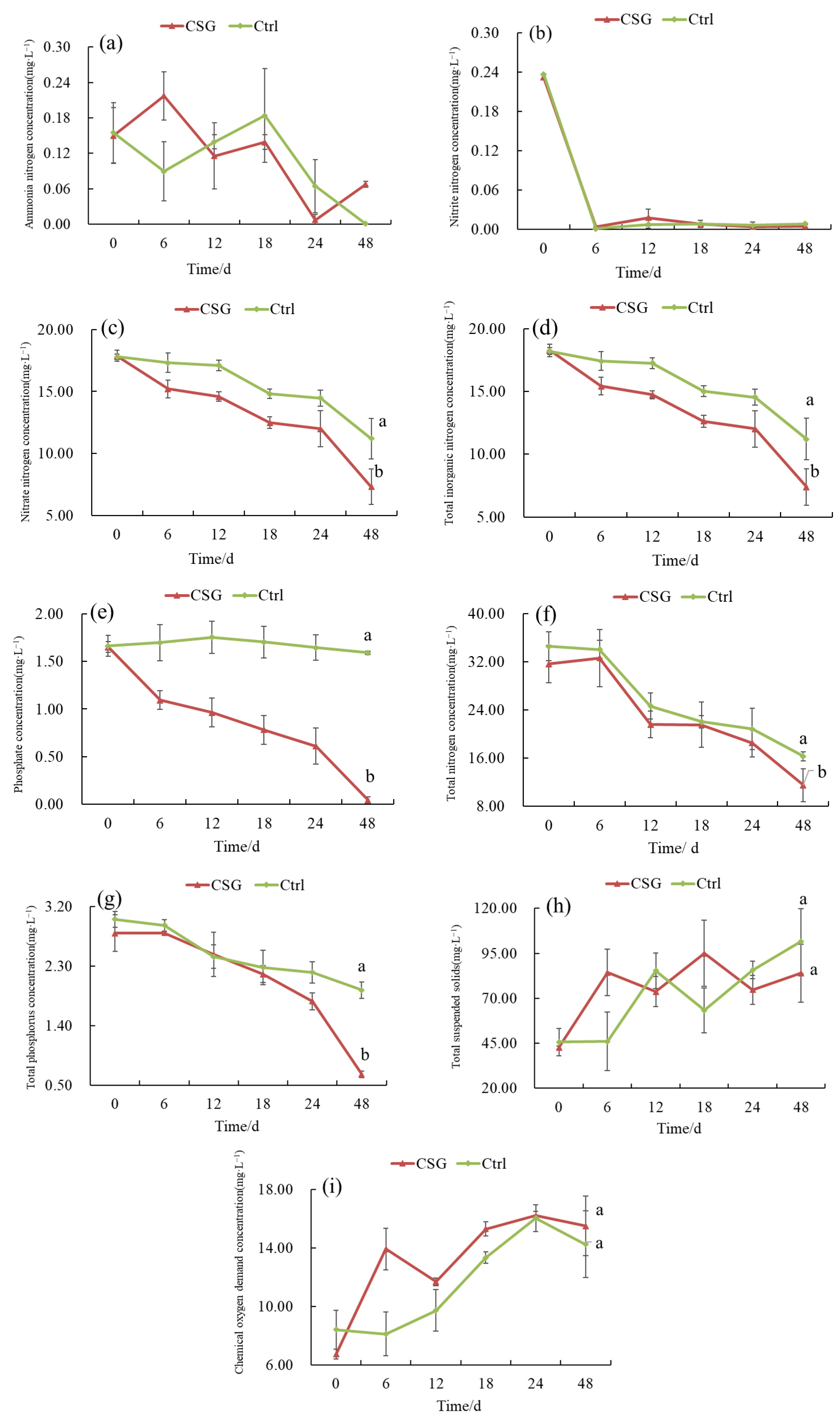
3.2.1. Changes in Water Quality Indicators of Intensive Shrimp Farming Effluent
3.2.2. Survival and Growth of Oysters
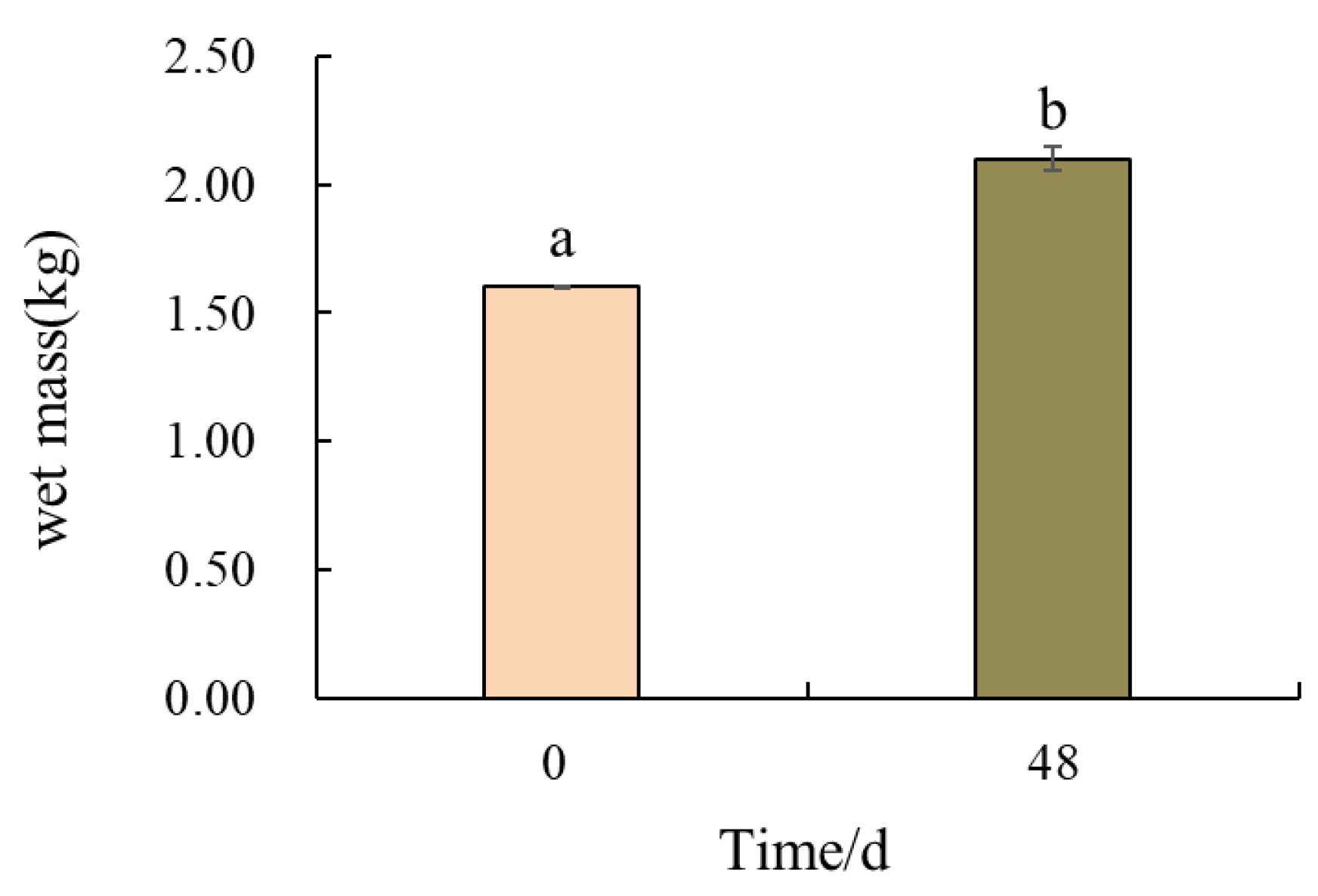
3.2.3. Changes in Wet Mass of Gracilaria
3.3. Deposition Effects of Nitrogen and Phosphorus in Effluent by Gracilaria Coupled with Oyster
3.3.1. Changes in Dry Weight, Content per Unit Dry Weight, and Net Increase per Unit Dry Weight of Gracilaria
3.3.2. Total Nitrogen and Phosphorus Content and Biological Deposition of Dry Matter of Gracilaria
4. Discussion
4.1. Impact of Biomass Parameters on the Purification of Intensive Shrimp Farming Effluent by Gracilaria
4.2. Impact of Temperature Conditions on the Purification of Intensive Shrimp Farming Effluent by Gracilaria
4.3. Impact of Salinity Conditions on the Purification of Intensive Shrimp Farming Effluent by Gracilaria
4.4. Purification Effects of Gracilaria Coupled with Oysters on Effluent
4.5. Deposition Effects of Gracilaria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.J.; Xu, Y.; Su, H.C.; Hu, X.J.; Yang, K.; Wen, G.L.; Cao, Y.C. Characteristics of Ammonia Removal and Nitrifying Microbial Communities in a Hybrid Biofloc-RAS for Intensive Litopenaeus vannamei Culture: A Pilot-Scale Study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Han, J.M.; Su, S.Q. Research progress and application overview of biofloc technology. Aquat. Sci. 2022, 41, 491–503. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, J.; Rivas-Vega, M.E.; de los Ángeles Mariscal-López, M.; Emerenciano, M.G.; Martínez-Porchas, M.; Miranda-Baeza, A. Reusing water in a biofloc culture system favors the productive performance of the Nile tilapia (Oreochromis niloticus) without affecting the health status. Aquaculture 2022, 558, 738363. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Kasan, N.A.; Manan, H.; Ismail, T.I.T.; Salam, A.I.A.; Rahim, A.I.A.; Kamarruzan, A.S.; Ishak, A.N.; Deraman, S.; Nasrin, Z.; Chik, C.E.N.C.E. Effect of Biofloc product-Rapid BFT TM vs. clear water system in improving the water quality and growth performances of Pacific Whiteleg shrimp, P. vannamei, cultured in indoor aquaculture system. Aquac. Res. 2021, 52, 6504–6513. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, S.L.; Wang, F.; Gao, Q.F.; Tian, X.L. Carbon dioxide and methane fluxes from feeding and no-feeding mariculture ponds. Environ. Pollut. 2016, 212, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, J.Y.; Gou, J.W.; Hou, J.; Li, D.P.; He, X.G. The effect of different carbon sources on water quality, microbial community and structure of biofloc systems. Aquaculture 2018, 482, 103–110. [Google Scholar] [CrossRef]
- Muguirrima, P.V.M.; Chirinza, N.P.; Zerpa, F.L.; Pino, C.A.M. Treatment of domestic effluents using sustainable biofilter methods. Desalin. Water Treat. 2024, 317, 100266. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Reddy, M.A. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Lu, S.H.; Fang, Y.X.; Yang, K.X.; Ding, J.F.; Ye, X.P.; Zhang, H.J. Technology for Upgrading the Tailwater of Municipal Sewage Treatment Plants: The Efficacy and Mechanism of Microbial Coupling for Nitrogen and Carbon Removal. Water 2021, 13, 2850. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdullah, S.R.S.; Abu Hasan, H.; Othman, A.R.; Ismail, N.I. Aquaculture industry: Supply and demand, best practices, effluent and its current issues and treatment technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yuan, J.L.; Ni, M.; Lian, Q.P. Assessment of the effectiveness of a field-scale combined ecological treatment system at removing water pollutants, after optimization using a system dynamic model: A case study of rural inland ponds in China. Environ. Sci. Pollut. Res. Int. 2022, 29, 30169–30183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.W.; Li, Q.; Xu, C.X. Growth, survival and gonad development of two new types of reciprocal triploid hybrids between Crassostrea gigas and C. angulata. Aquaculture 2022, 559, 738451. [Google Scholar] [CrossRef]
- Zirino, A.; Elwany, H.; Facca, C.; Maicu’, F.; Neira, C.; Mendoza, G. Nitrogen to phosphorus ratio in the Venice (Italy) Lagoon (2001–2010) and its relation to macroalgae. Mar. Chem. 2016, 180, 33–41. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yang, J.X.; Qi, C.; Du, Q.R.; Xu, D. Effects of reproduction characteristics and environmental factors on the fertilization efficiency of Gracilaria vermiculophylla. Period. Ocean. Univ. China 2024, 54, 70–75. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chai, Z.Y.; Wang, Q.; Chen, W.Z.; He, Z.L.; Jiang, S.J. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Mawi, S.; Krishnan, S.; Din, M.F.M.; Arumugam, N.; Chelliapan, S. Bioremediation potential of macroalgae Gracilaria edulis and Gracilaria changii co-cultured with shrimp wastewater in an outdoor water recirculation system. Environ. Technol. Innov. 2020, 17, 100571. [Google Scholar] [CrossRef]
- Jia, Z.; She, Z.C.; Peng, Y.S.; Feng, B.; Kang, Z.J.; Yang, B.; Yu, D.H. Effects of Hongkong oyster Crassostrea hongkongensis fattening in onshore ponds and in offshore sea. Fish. Sci. 2019, 38, 514–520. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, Y.; Li, J.; Wang, Z.P.; Yan, X.W.; Yu, Z.N. Incomplete sterility of hybrids produced by Crassostrea hongkongensis female × Crassostrea gigas male crosses. Aquac. Res. 2017, 48, 1351–1358. [Google Scholar] [CrossRef]
- Jones, A.B.; Dennison, W.C.; Preston, N.P. Integrated treatment of shrimp effluent by sedimentation, oyster filtration and macroalgal absorption: A laboratory scale study. Aquaculture 2001, 193, 155–178. [Google Scholar] [CrossRef]
- Tong, S.; Stocks, J.L.; Rodriguez-Gonzalez, L.C.; Feng, C.; Ergas, S.J. Effect of oyster shell medium and organic substrate on the performance of a particulate pyrite autotrophic denitrification (PPAD) process. Bioresour. Technol. 2017, 244, 296–303. [Google Scholar] [CrossRef]
- Chow, F.; Macchiavello, J.; Santa Cruz, S.; Fonck, E.; Olivares, J. Utilization of Gracilaria chilensis (Rhodophyta: Gracilariaceae) as a Biofilter in the Depuration of Effluents from Tank Cultures of Fish, Oysters, and Sea Urchins. J. World Aquac. Soc. 2001, 32, 215–220. [Google Scholar] [CrossRef]
- Prata Gaona, C.A.; Souza Almeida, M.; Viau, V.; Poersch, L.H.; Wasielesky, W.J. Effect of different total suspended solids levels on a Litopenaeus vannamei (Boone, 1931) BFT culture system during biofloc formation. Aquac. Res. 2017, 48, 1070–1079. [Google Scholar] [CrossRef]
- Ye, T.T.; Li, M.; Lin, Y.B.; Su, Z.J. An effective biological treatment method for marine aquaculture wastewater: Combined treatment of immobilized degradation bacteria modified by chitosan-based aerogel and macroalgae (Caulerpa lentillifera). Aquaculture 2023, 570, 739392. [Google Scholar] [CrossRef]
- Hao, J.M.; Zheng, J.; Li, Z.B.; Huang, Y.C.; Tu, Y.; Li, Y.B.; Lin, Y.J.; Zhou, W.H. Study on the growth of Spirulina platensis in shrimp aquaculture wastewater. J. Aquat. Ecol. 2011, 32, 149–152. [Google Scholar] [CrossRef]
- Beevi, U.S.; Singh, A. Biomass production and phycoremediation of microalgae cultivated in polluted river water. Bioresour. Technol. 2022, 351, 126948. [Google Scholar] [CrossRef]
- Bekkby, T.; Angeltveit, G.; Gundersen, H.; Tveiten, L.; Norderhaug, K.M. Red sea urchins (Echinus esculentus) and water flow influence epiphytic macroalgae density. Mar. Biol. Res. 2015, 11, 375–384. [Google Scholar] [CrossRef]
- Li, Y.D.; Zhou, F.L.; Huang, J.H.; Yang, L.S.; Jiang, S.; Yang, Q.B.; Jiang, S.G. Transcriptome and miRNA Profiles of Black Tiger Shrimp, Penaeus monodon, Under Different Salinity Conditions. Front. Mar. Sci. 2020, 7, 579381. [Google Scholar] [CrossRef]
- Gao, X.F.; Liu, W.; Zhong, Y.Y.; Xing, H.; Zhang, J.L.; Chang, J.N.; Sun, B.; He, P.M. Effect of different temperatures on the growth and photosynthetic physiology of Zostera marina. J. Appl. Environ. Biol. 2022, 28, 175–181. [Google Scholar] [CrossRef]
- Alberto, P.R.; Alexia, O.; Eduardo, Q.G.; Gabriela, M.C.; García Pérez, O.D.; Regina, E.G. Microbiome changes of an integrated aquaculture system of shrimp Litopenaeus vannamei and seaweed Ulva lactuca with different water exchanges. Aquacult. Int. 2023, 32, 1955–1973. [Google Scholar] [CrossRef]
- GB/T 12763.4-2007; The Specification for Marine Survey—Part 4: Investigation of Seawater Chemical Elements. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2007.
- GB/T 17378.4-2007; The Specification for Marine Monitoring—Part 4: Seawater Analysis. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2007.
- GB/T 17378.5-2007; The Specification for Marine Monitoring—Part 5: Sediment Analysis. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2007.
- Brichette, I.; Reyero, R.I.; García, C. A genetic analysis of intraspecific competition for growth in mussel cultures. Aquaculture 2001, 192, 155–169. [Google Scholar] [CrossRef]
- Chen, Z.T.; Tong, C.F. Intraspecific competition of Chiromantes dehaani and influencing factors in a salt marsh of the yangtze Estuary. J. Ecol. 2021, 40, 3228–3233. [Google Scholar] [CrossRef]
- Ullah, M.R.; Islam, M.A.; Khan, A.B.S.; Bosu, A.; Farhana, Y.; Hasan, M.M.; Islam, M.M.; Rahman, M.A.; Yahia, M. Effect of stocking density and water depth on the growth and production of red seaweed, Gracilaria tenuistipitata in the Kuakata coast of Bangladesh. Aquacult. Rep. 2023, 29, 101509. [Google Scholar] [CrossRef]
- Banik, U.; Mohiuddin, M.; Wahab, M.A.; Rahman, M.M.; Nahiduzzaman, M.; Sarker, S.; Wong, L.L.; Asaduzzaman, M. Comparative performances of different farming systems and associated influence of ecological factors on Gracilaria sp. seaweed at the south-east coast of the Bay of Bengal, Bangladesh. Aquaculture 2023, 574, 739675. [Google Scholar] [CrossRef]
- Sawant, O.; Jaiswar, S. Assessing the nitrate and phosphate uptake kinetics potential and growth performance of Gracilaria corticata var. cylindrica in shrimp farm water (SFW). Discov. Ocean. 2024, 1, 7. [Google Scholar] [CrossRef]
- Zhou, W.; Sui, Z.H.; Wang, J.G.; Chang, L.P. An orthogonal design for optimization of growth conditions for all life history stages of Gracilariopsis lemaneiformis (Rhodophyta). Aquaculture 2013, 392, 98–105. [Google Scholar] [CrossRef]
- Huang, Y.J.; Cui, J.J.; Wang, S.P.; Chen, X.Y.; Liao, J.W.; Guo, Y.Y.; Xin, R.; Huang, B.W.; Xie, E.Y. Transcriptome analysis reveals the molecular mechanisms of adaptation to high temperatures in Gracilaria bailinae. Front. Plant Sci. 2023, 14, 1125324. [Google Scholar] [CrossRef]
- Dawes, C.J.; Orduña-Rojas, J.; Robledo, D. Response of the tropical red seaweed Gracilaria cornea to temperature, salinity and irradiance. J. Appl. Phycol. 1998, 10, 419–425. [Google Scholar] [CrossRef]
- Geniane, S.; Figueroa, F.L.; Vega, J.; Avilés, A.; Horta Antunes, P.; Korbee, N.; Bonomi-Barufi, J. Effects of UV–visible radiation on growth, photosynthesis, pigment accumulation and UV-absorbing compounds in the red macroalga Gracilaria cornea (Gracilariales, Rhodophyta). Algal Res. 2022, 64, 102702. [Google Scholar] [CrossRef]
- Borlongan, I.A.; Suzuki, S.; Nishihara, G.N.; Kozono, J.; Terada, R. Effects of light quality and temperature on the photosynthesis and pigment content of a subtidal edible red alga Meristotheca papulosa (Solieriaceae, Gigartinales) from Japan. J. Appl. Phycol. 2020, 32, 1329–1340. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Shi, M.; Xu, J.; Wu, R.; Xu, J.; Wang, J.; Zhou, W. Synergistic effects of warming and low salinity enhanced the production of Gracilariopsis lemaneiformis in the warming drainage zone of Tianwan Nuclear Power Plant, China. Aquaculture 2023, 577, 739961. [Google Scholar] [CrossRef]
- Martins, I.; Azevedo, A.; Goméz, I.; Valente, L.M.P. Variation on the standing stock of Gracilaria sp. in a temperate estuary under single-stressor and multiple-stressor climate change scenarios. Algal Res. 2020, 51, 102079. [Google Scholar] [CrossRef]
- She, T.T.; Zhong, C.H.; Lin, Q.; Tang, L.C.; Huan, Z.Y.; Zhou, W.F. Effects of different hypotonic salinity stress treatments on regeneration capacity and photosynthetic physiology of Undaria pinnatifida cuttings. J. Fish. Sci. 2023, 47, 49–59. [Google Scholar] [CrossRef]
- Li, K.; Jiang, R.T.; Qiu, J.Q.; Liu, J.L.; Shao, L.; Zhang, J.H.; Liu, Q.G.; Jiang, Z.J.; Wang, H.; He, W.H.; et al. How to control pollution from tailwater in large scale aquaculture in China: A review. Aquaculture 2024, 590, 741085. [Google Scholar] [CrossRef]
- Jackson, C.J.; Preston, N.; Burford, M.A.; Thompson, P.J. Managing the development of sustainable shrimp farming in Australia: The role of sedimentation ponds in treatment of farm discharge water. Aquaculture 2003, 226, 23–34. [Google Scholar] [CrossRef]
- Zhao, H.R.; Zou, L.P.; Jiang, M.T.; Li, Y.Y.; Liu, J.Y. Ozone pretreatment combined with partial denitrification-anammox process for efficient nitrogen removal from nanofiltration concentrate of landfill leachate. Chem. Eng. J. 2023, 471, 144641. [Google Scholar] [CrossRef]
- Yadav, S.; Suantak, K. A review of electrochemical methods for treatment of wastewater. Mater. Today Proc. 2023, 78, 36–39. [Google Scholar] [CrossRef]
- Sindilariu, P.D.; Schulz, C.; Reiter, R. Treatment of flow-through trout aquaculture effluents in a constructed wetland. Aquaculture 2007, 270, 92–104. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, Y.S.; Meng, Z.Q.; Yang, Y.Z.; Xu, Z.F.; Ma, H.R. A bio-enhanced treatment strategy for tannery wastewater based on progressive activation of microbial agents. J. Water Process Eng. 2024, 67, 106087. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Choi, S.K.; Zhu, Q.; Lee, S.I. Nitrification of low concentration ammonia nitrogen using zeolite biological aerated filter (ZBAF). Environ. Eng. Res. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Chen, X.J.; Chang, L.D.; Liu, W.W.; Wang, S.W.; Xiao, J.N.; Xu, G.J. Research progress on resource utilization of shell waste. Shandong Chem. Ind. 2023, 52, 82–84+88. [Google Scholar] [CrossRef]
- Li, M.; Callier, M.D.; Blancheton, J.P.; Gales, A.; Nahon, S.; Triplet, S.; Geoffroy, T.; Menniti, C.; Fouilland, E.; d’Orbcastel, E.R. Bioremediation of fishpond effluent and production of microalgae for an oyster farm in an innovative recirculating integrated multi-trophic aquaculture system. Aquaculture 2019, 504, 314–325. [Google Scholar] [CrossRef]
- Wang, S.L.; Ma, L. Experimental study on shellfish aquaculture for river water quality improvement. Rural. Sci. Technol. 2022, 13, 137–140. [Google Scholar] [CrossRef]
- Fu, J.X.; Pan, K.; Huang, L.F.; Lan, W.L.; Li, M.M.; Li, T.S. Effects of nitrogen and phosphorus levels on nitrogen and phosphorus uptake and growth of Gracilaria tenuistipitata. Guangxi Sci. 2022, 29, 522–531. [Google Scholar] [CrossRef]
- Samocha, T.M.; Fricker, J.; Ali, A.M.; Shpigel, M.; Neori, A. Growth and nutrient uptake of the macroalga Gracilaria tikvahiae cultured with the shrimp Litopenaeus vannamei in an Integrated Multi-Trophic Aquaculture (IMTA) system. Aquaculture 2015, 446, 263–271. [Google Scholar] [CrossRef]
- Yuan, X.T.; Yang, H.S.; Meng, L.M.; Wang, L.L.; Li, Y. Impacts of temperature on the scavenging efficiency by the deposit-feeding holothurian Apostichopus japonicus on a simulated organic pollutant in the bivalve-macroalage polyculture from the perspective of nutrient budgets. Aquaculture 2013, 406, 97–104. [Google Scholar] [CrossRef]
- Locher, B.; Hurst, N.R.; Walters, L.J.; Chambers, L.G. Juvenile Oyster (Crassostrea virginica) Biodeposits Contribute to a Rapid Rise in Sediment Nutrients on Restored Intertidal Oyster Reefs (Mosquito Lagoon, FL, USA). Estuaries Coasts 2021, 44, 1363–1379. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Dos Santos, D.Y.A.C. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.H.; Li, B.; Bai, Y.Y.; Qiao, H.J.; Feng, Y.W.; Cao, T.H.; Liu, Y.H. Effect of nitrogen and phosphorus concentrations on removal efficiency of NO3−-N and PO43−-P and growth in two species of seaweed Gracilaria at high water temperature. Aquat. Sci. 2019, 38, 19–25. [Google Scholar] [CrossRef]
- Checa, D.; Macey, B.M.; Bolton, J.J.; Hull, M.B.; O’Donohoe, P.; Cardozo, A.; Poersch, L.H.; Sánchez, I. Circularity assessment in aquaculture: The case of integrated multi-trophic aquaculture (IMTA) systems. Fishes 2024, 9, 165. [Google Scholar] [CrossRef]
- Huang, H.X.; Hu, X.Y.; Wu, W.P.; Li, J.Y. Deposition analysis of nitrogen and phosphorus in an integrated multi-trophic aquaculture pond. J. Jimei U. 2014, 19, 173–178. [Google Scholar] [CrossRef]
- Raut, Y.; Barr, C.R.; Paris, E.R.; Kapili, B.J.; Dekas, A.E.; Capone, D.G. Autochthonous carbon loading of macroalgae stimulates benthic biological nitrogen fixation rates in shallow coastal marine sediments. Front. Microbiol. 2024, 14, 1312843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Di, B.P.; Li, X.; Liu, D.Y. Environmental Indicative Role of Nitrogen Stable Isotopes in Intertidal Macroalgae. Mar. Environ. Sci. 2016, 35, 174–179. [Google Scholar] [CrossRef]
- Fernández-Aláez, M.; Fernández-Aláez, C.; Bécares, E. Nutrient content in macrophytes in Spanish shallow lakes. Hydrobiologia 1999, 408, 317–326. [Google Scholar] [CrossRef]
- Sun, Z.W.; Hu, X.J.; Xu, Y.; Wen, G.L.; Su, H.C.; Pan, Z.H.; Cao, Y.C. Purification Effect of Fish–Algae Coupling on Nitrogen and Phosphorus in Shrimp Aquaculture Effluent. Fishes 2024, 9, 490. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Moraes-Valenti, P.; Valenti, W.C. The budget of nitrogen in the grow-out of the Amazon river prawn (Macrobrachium amazonicum Heller) and tambaqui (Colossoma macropomum Cuvier) farmed in monoculture and in integrated multitrophic aquaculture systems. Aquacult. Res. 2019, 50, 3444–3461. [Google Scholar] [CrossRef]

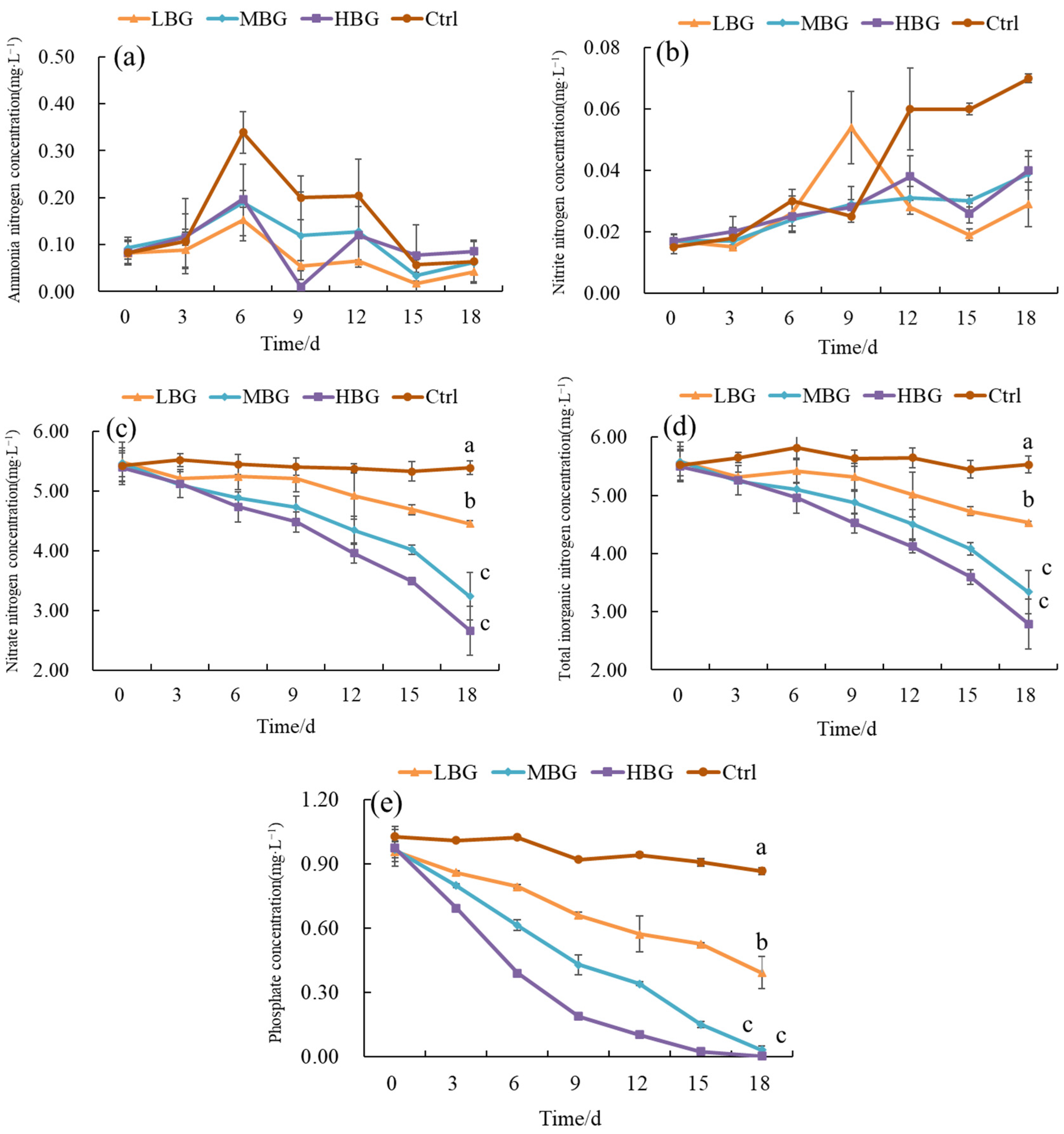
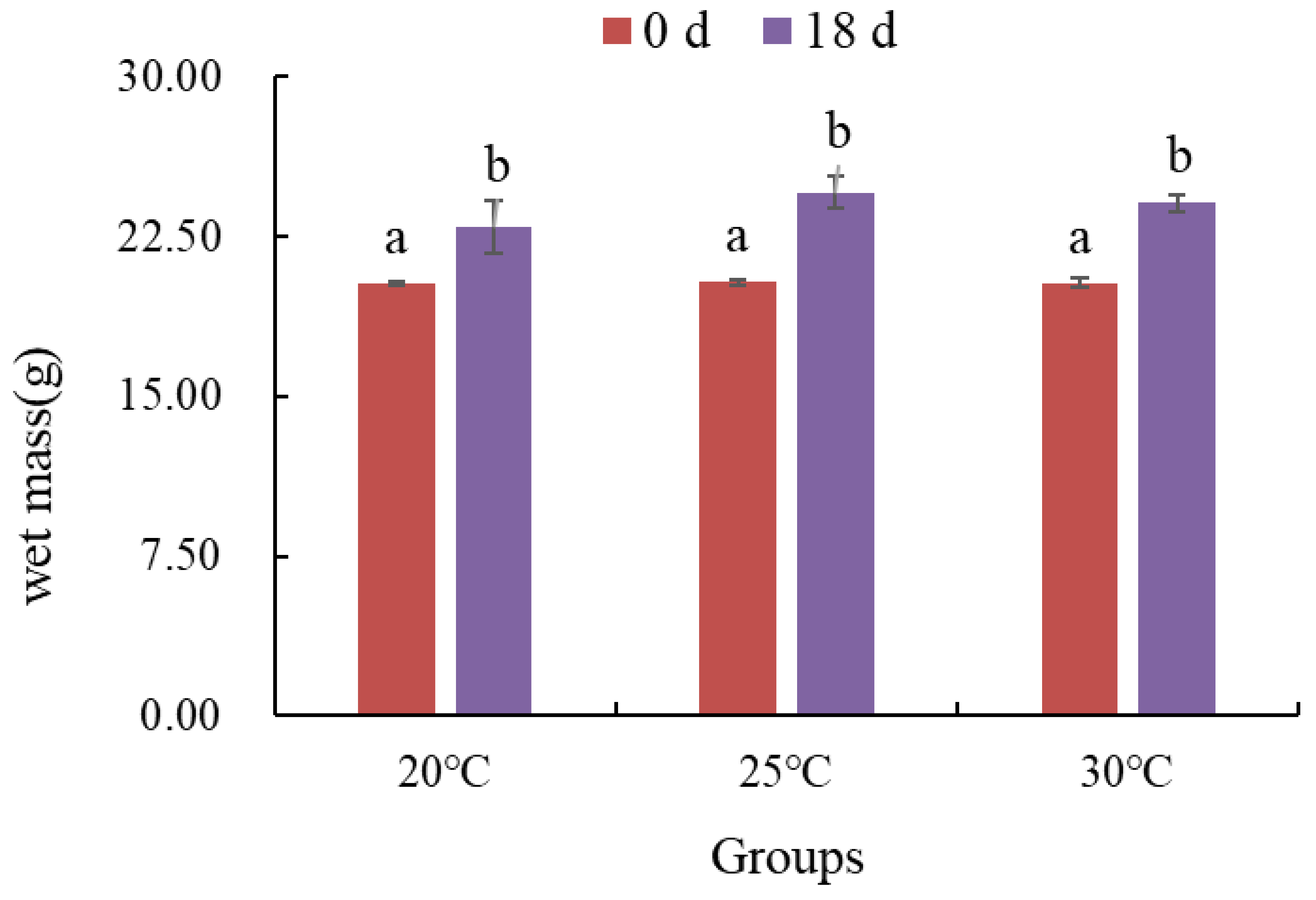


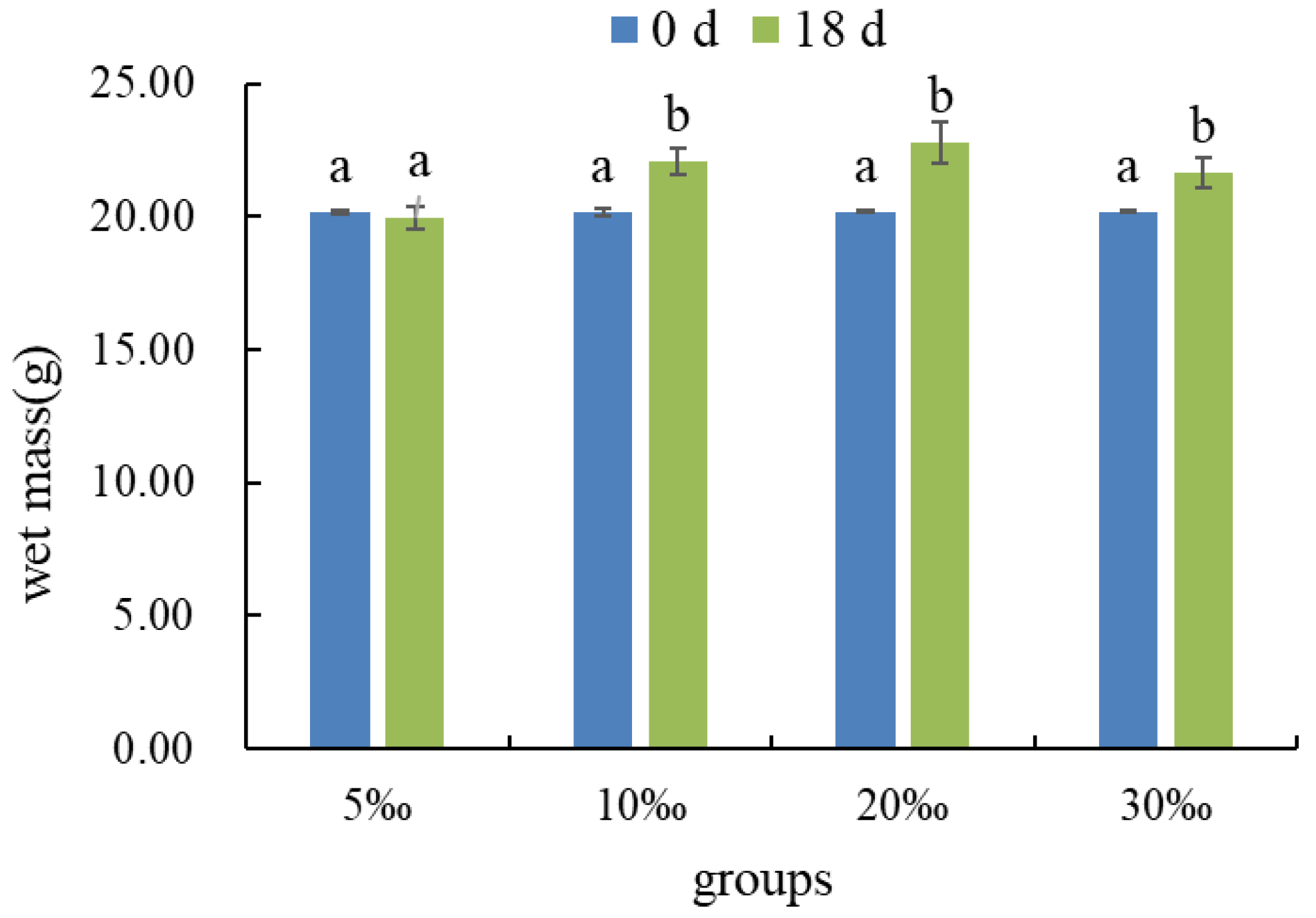
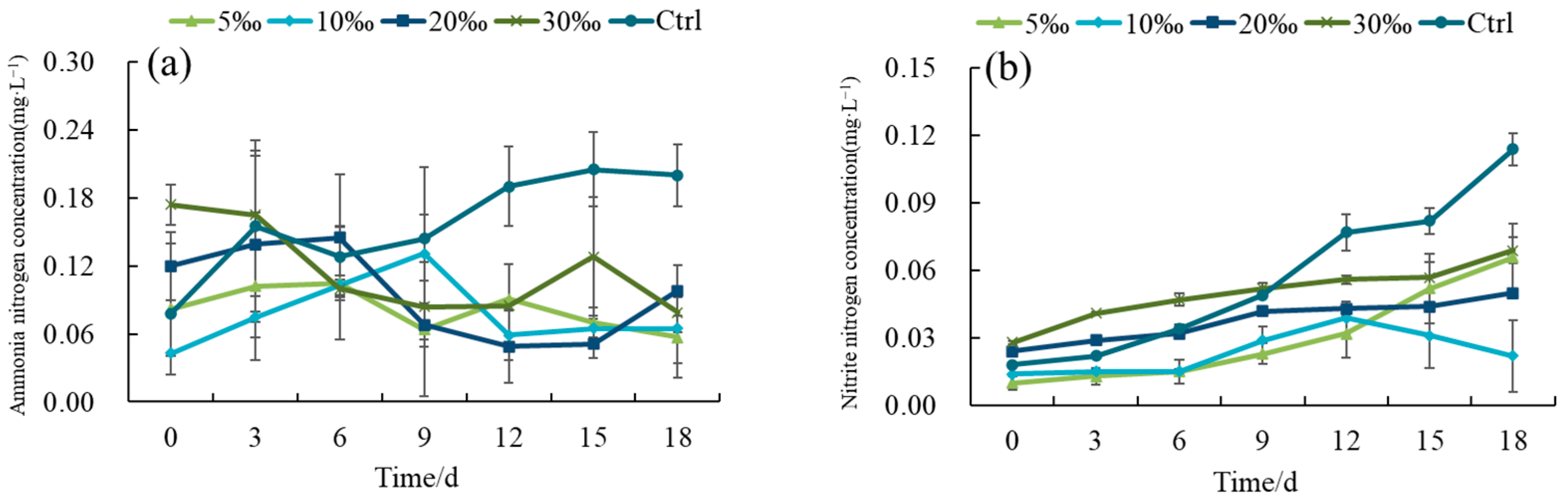

| Item | LBG | MBG | HBG | Ctrl |
|---|---|---|---|---|
| NO3−-N | 18.65 ± 3.15 b | 40.17 ± 10.87 c | 50.23 ± 9.95 c | 0.42 ± 4.05 a |
| TIN | 18.78 ± 3.63 b | 39.62 ± 9.94 c | 48.89 ± 10.24 c | −0.22 ± 3.48 a |
| PO43−-P | 59.22 ± 6.49 b | 96.66 ± 2.23 c | 99.56 ± 0.26 c | 15.54 ± 5.10 a |
| Item | 20 °C | 25 °C | 30 °C | Ctrl |
|---|---|---|---|---|
| NO3−-N | 18.75 ± 3.03 b | 19.35 ± 6.76 b | 21.50 ± 8.47 b | 3.54 ± 1.69 a |
| TIN | 18.18 ± 2.77 b | 19.18 ± 6.54 b | 19.22 ± 6.93 b | 1.70 ± 0.86 a |
| PO43−-P | 75.11 ± 1.95 b | 95.17 ± 3.70 c | 99.92 ± 0.00 d | 4.44 ± 0.32 a |
| Item | 5 | 10 | 20 | 30 | Ctrl |
|---|---|---|---|---|---|
| NO3−-N | 12.23 ± 2.56 b | 18.61 ± 2.99 bc | 24.78 ± 4.54 c | 21.51 ± 1.40 c | 0.61 ± 4.72 a |
| TIN | 11.88 ± 2.59 b | 18.26 ± 3.30 bc | 24.45 ± 4.54 c | 21.59 ± 0.97 c | −1.27 ± 4.97 a |
| PO43−-P | 53.33 ± 2.84 b | 91.13 ± 2.25 c | 95.00 ± 0.18 c | 92.95 ± 1.80 c | 2.40 ± 3.70 a |
| Item | CSG | Ctrl |
|---|---|---|
| TIN | 59.56 ± 7.54 a | 38.29 ± 9.68 b |
| PO43−-P | 97.43 ± 1.86 a | 3.47 ± 5.91 b |
| TN | 62.56 ± 11.66 a | 52.54 ± 5.34 a |
| TP | 75.90 ± 3.77 a | 29.85 ± 2.87 b |
| Groups | Dry Weight (g) | Content per Unit Dry Weight (mg·g−1) | Net Increase per Unit Dry Weight (mg·g−1) |
|---|---|---|---|
| BG0 | 1.75 ± 0.09 (20 g) | 86.97 ± 4.50 | |
| LBG18 | 1.36 ± 0.05 (10 g) | 101.79 ± 3.75 | 9.16 ± 3.75 a |
| MBG18 | 2.79 ± 0.47 (20 g) | 106.38 ± 5.86 | 19.66 ± 5.86 b |
| HBG18 | 3.67 ± 0.45 (40 g) | 90.45 ± 2.82 | 8.89 ± 2.82 a |
| T0 | 1.39 ± 0.04 | 69.06 ± 1.72 | |
| 20 °C18 | 1.72 ± 0.13 | 74.83 ± 2.94 | 5.74 ± 2.94 a |
| 25 °C18 | 1.87 ± 0.11 | 76.26 ± 3.05 | 7.17 ± 3.05 a |
| 30 °C18 | 2.14 ± 0.10 | 88.83 ± 2.78 | 19.74 ± 2.78 b |
| S0 | 1.60 ± 0.04 | 79.21 ± 2.00 | |
| 518 | 1.23 ± 0.05 | 61.93 ± 3.13 | −17.28 ± 3.13 a |
| 1018 | 1.87 ± 0.06 | 84.57 ± 2.50 | 5.36 ± 2.50 b |
| 2018 | 2.12 ± 0.12 | 93.21 ± 4.47 | 14.00 ± 4.47 c |
| 3018 | 2.15 ± 0.12 | 99.17 ± 4.67 | 19.96 ± 4.67 c |
| CSG0 | 2.72 ± 0.11 | 135.97 ± 6.39 | |
| CSG48 | 2.57 ± 0.21 | 128.20 ± 1.13 | −7.77 ± 1.13 a |
| Groups | Total Nitrogen Content (mg·g−1) | Increase in Total Nitrogen Content (mg·g−1) | Total Phosphorus Content (mg·g−1) | Increase in Total Phosphorus Content (mg·g−1) |
| BG0 | 12.42 ± 1.24 a (20 g) | 2.94 ± 0.26 a (20 g) | ||
| LBG18 | 19.35 ± 3.72 b | 6.17 ± 3.72 b | 5.38 ± 0.34 b | 2.48 ± 0.34 a |
| MBG18 | 17.57 ± 2.38 b | 6.90 ± 2.38 b | 5.87 ± 0.91 b | 2.97 ± 0.91 a |
| HBG18 | 12.55 ± 1.76 a | −0.84 ± 1.76 a | 5.58 ± 0.84 b | 2.68 ± 0.84 a |
| T0 | 16.47 ± 1.83 a | 3.07 ± 0.47 a | ||
| 20 °C18 | 16.56 ± 1.31 a | 0.09 ± 1.31 b | 4.27 ± 0.69 b | 1.20 ± 0.69 a |
| 25 °C18 | 20.01 ± 2.71 a | 3.54 ± 2.71 b | 5.74 ± 0.28 c | 2.67 ± 0.28 b |
| 30 °C18 | 15.89 ± 1.53 a | −0.58 ± 1.53 b | 5.92 ± 0.72 c | 2.85 ± 0.72 b |
| S0 | 16.75 ± 1.39 a | 2.39 ± 0.51 a | ||
| 518 | 13.95 ± 2.89 a | −2.80 ± 2.89 a | 5.56 ± 1.30 b | 3.17 ± 1.30 a |
| 1018 | 18.28 ± 3.33 a | 1.53 ± 3.33 a | 6.24 ± 0.39 b | 3.85 ± 0.39 a |
| 2018 | 17.02 ± 2.45 a | 0.27 ± 2.45 a | 5.96 ± 0.73 b | 3.57 ± 0.73 a |
| 3018 | 18.08 ± 1.42 a | 1.33 ± 1.42 a | 5.59 ± 0.55 b | 3.20 ± 0.55 a |
| CSG0 | 23.99 ± 2.15 a | 2.49 ± 0.21 a | ||
| CSG48 | 38.36 ± 10.37 b | 14.37 ± 10.37 | 8.54 ± 0.86 b | 6.29 ± 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, Y.; Deng, Y.; Hu, X.; Su, H.; Wen, G.; Cao, Y. Purification of Intensive Shrimp Farming Effluent by Gracilaria Coupled with Oysters. Fishes 2025, 10, 179. https://doi.org/10.3390/fishes10040179
Li J, Xu Y, Deng Y, Hu X, Su H, Wen G, Cao Y. Purification of Intensive Shrimp Farming Effluent by Gracilaria Coupled with Oysters. Fishes. 2025; 10(4):179. https://doi.org/10.3390/fishes10040179
Chicago/Turabian StyleLi, Junjing, Yu Xu, Yunlong Deng, Xiaojuan Hu, Haochang Su, Guoliang Wen, and Yucheng Cao. 2025. "Purification of Intensive Shrimp Farming Effluent by Gracilaria Coupled with Oysters" Fishes 10, no. 4: 179. https://doi.org/10.3390/fishes10040179
APA StyleLi, J., Xu, Y., Deng, Y., Hu, X., Su, H., Wen, G., & Cao, Y. (2025). Purification of Intensive Shrimp Farming Effluent by Gracilaria Coupled with Oysters. Fishes, 10(4), 179. https://doi.org/10.3390/fishes10040179






