Effects of Bacillus licheniformis Feeding on the Growth Performance, Blood Parameters and Intestinal Microbiota of Adult Hybrid Sturgeon
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacillus licheniformis and Diet Preparation
2.2. Fish and Fish Culture
2.3. Measurement of Growth Indicators
2.4. Measurement of Blood Biochemical Indicators
2.5. Diversity of the Gut Microbiota
2.6. Statistical Analysis
3. Results
3.1. Effects of B. licheniformis on the Growth Performance and Body Indices of Sturgeon
3.2. Effects of B. licheniformis on the Body Composition of Hybrid Sturgeon
3.3. Effects of B. licheniformis on the Blood Biochemical Parameters of Sturgeon
3.4. Effects of B. licheniformis on the Intestinal Microbiota of Sturgeon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pikitch, E.K.; Doukakis, P.; Lauck, L.; Chakrabarty, P.; Erickson, D.L. Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish. 2005, 6, 233–265. [Google Scholar] [CrossRef]
- Chen, X.H.; Li, C.J.; Yang, C.G.; Zhang, S.H.; Wu, J.P. Status and prospects of techniques in the sturgeon aquaculture industry in China. Freshw. Fish. 2017, 47, 108–112. [Google Scholar]
- Zhang, Y.; Sun, D.J.; Wang, B.; Xia, Y.T.; Wu, W.; Xu, S.J.; Liu, X.Y. Hybrid sturgeon “Xunlong no. 1”. China Fish. 2017, 5, 62–67. (In Chinese) [Google Scholar]
- Chen, X.; Wei, Z. Research progress on hybrid sturgeon in China. Sichuan Agric. Sci. Technol. 2023, 10, 122–124. [Google Scholar]
- Padeniya, U.; Larson, E.T.; Septriani, S.; Pataueg, A.; Kafui, A.R.; Hasan, E.; Brown, C.L. Probiotic treatment enhances pre-feeding larval development and early survival in zebrafish Danio rerio. J. Aquat. Anim. Health 2022, 34, 3–11. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Di, J.; Du, H.; Xu, Q.; Zhou, Q.; Wei, Q. The genome sequence of a new strain of Mycobacterium ulcerans ecovar Liflandii, emerging as a sturgeon pathogen. Aquaculture 2018, 489, 141–147. [Google Scholar] [CrossRef]
- Di, J.; Zhang, S.; Huang, J.; Du, H.; Zhou, Y.; Zhou, Q.; Wei, Q. Isolation and identification of pathogens causing haemorrhagic septicaemia in cultured Chinese sturgeon (Acipenser sinensis). Aquac. Res. 2018, 49, 3624–3633. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Ringø, E.; Hoseinifar, S.H.; Ghosh, K.; Doan, H.V.; Beck, B.R.; Song, S.K. Lactic acidbacteria in finfish—An update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, Z.R. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim. Feed. Sci. Technol. 2006, 127, 283–292. [Google Scholar]
- Ringø, E. Evaluation of probiotic strain Bacillus subtilis C-3102 as a feed supplement for koi carp (Cyprinus carpio). J. Aquac. Res. Dev. 2011, S1, 5. [Google Scholar]
- Samat, N.A.; Yusoff, F.M.; Rasdi, N.W.; Karim, M. The efficacy of Moina micrura enriched with probiotic Bacillus pocheonensis in enhancing survival and disease resistance of red hybrid tilapia (Oreochromis spp.) larvae. Antibiotics 2021, 10, 989. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic application for sustainable aquaculture. Rev. Aquac. 2019, 11, 907–924. [Google Scholar] [CrossRef]
- Onomu, A.J.; Okuthe, G.E. The role of functional feed additives in enhancing aquaculture sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.; Luo, L.; Tang, X.; Li, Y.; Yu, X.; Wu, Z. Effects of Bacillus methylotrophicus WM-1 on water quality dynamics and cultural fish in grass carp culture system. Aquac. Res. 2021, 52, 3371–3380. [Google Scholar] [CrossRef]
- Li, X.Y.; Kong, J.; Zhao, F.; Yang, M.J. Application and research progress of probiotics in sturgeon feed additive. Guizhou Anim. Sci. Vet. Med. 2016, 40, 61–63. (In Chinese) [Google Scholar]
- Alizadeh, A.M.; Hosseini, H.; Meybodi, N.M.; Hashempour-Baltork, F.; Alizadeh-Sani, M.; Tajdar-Oranj, B.; Pirhadi, M.; Khaneghah, A.M. Mitigation of potentially toxic elements in food products by probiotic bacteria: A comprehensive review. Food Res. Int. 2022, 152, 110324. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Huang, J.H.; Han, W.L.; He, H.C. The effects of three probiotics on the growth and immune system of hybrid sturgeon (Huso dauricus ♀ × Acipenser schrenckii). Freshw. Fish. 2014, 1, 78–83. [Google Scholar]
- Zhang, C.N.; Li, X.F.; Xu, W.N.; Jiang, G.Z.; Lu, K.L.; Wang, L.N.; Liu, W.B. Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream (Megalobrama terminalis). Fish Shellfish. Immunol. 2013, 35, 1380–1386. [Google Scholar] [CrossRef]
- Raida, M.K.; Larsen, J.L.; Nielsen, M.E.; Buchmann, K. Enhanced resistance of rainbow trout, Oncorhynchus mykiss (Walbaum), against Yersinia ruckeri challenge following oral administration of Bacillus subtilis and B. licheniformis (BioPlus 2B). J. Fish Dis. 2003, 26, 495–498. [Google Scholar] [CrossRef]
- GB 5009.5-2016; National Food Safety Standard Determination of Protein in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard Determination of Fat in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.4-2016; National Food Safety Standard Determination of Ash in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.3-2016; National Food Safety Standard Determination of Moisture in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Gao, X.; Gai, L.Q.; Li, M.Y.; Li, M.Y.; Guo, X.X.; An, R.Y. Effects of Bacillus spp. on the growth performance and intestine bacteria in juvenile Acipenser baeri. J. Hebei Norm. Univ. 2009, 33, 377–382. [Google Scholar]
- Olmos, J.; Acosta, M.; Mendoza, G.; Pitones, V. Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch. Microbiol. 2020, 202, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Wu, J.P.; Chu, Z.P.; Xu, Q.Q.; He, J.Y.; Jin, J.L.; Chen, X. Effects of feeding Bacillus subtilis on growth and intestinal microflora of hybrid sturgeon. Mar. Fish. 2021, 43, 71–80. [Google Scholar] [CrossRef]
- Ji, M.; Yao, K.; Li, G.; Wu, X.; Chen, H.; Zhuang, Y. Control effects of Bacillus subtilis DJ-6 and pyraclostrobin alone and in combination against Fusarium oxysporum. Agric. Sci. Technol. 2014, 15, 2020–2025. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Z.; Feng, J.; Chen, Y.Q.; Wu, Z.H. Study on the digestive activity of extracellular products of Bacillus licheniformis. J. Trop. Oceanogr. 2005, 6, 6–12. [Google Scholar]
- Sangeetha, R.; Geetha, A.; Arulpandi, I. Concomitant production of protease and lipase by Bacillus licheniformis VSG1: Production, purificationand characterization. Braz. J. Microbiol. 2010, 41, 179–185. [Google Scholar] [CrossRef]
- Xu, Y. Research Progress on Application of Bacillus licheniformis in aquaculture. Fish. Res. 2018, 40, 83–88. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, X.; Liang, L.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; Ai, L.; Xia, Y. Antrodin A from Antrodia camphorate modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake. Food Funct. 2021, 12, 2925–2937. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, K.; Huang, X.Y.; Zhong, L.; Wu, L.; Huang, Q.; Tang, L. Effects of dietary Bacillus subtilis on growth performance, immunity and antioxidation function of juvenile genetic improvement of farmed tilapia (GIFT, Oreochromis niloticus). Chin. J. Anim. Nutr. 2014, 1503–1512. [Google Scholar]
- Liu, Y.; Zhao, W.X.; Yue, X.L.; Sun, X.; Guan, S.G.; Yu, C.Y. Effects of compound feeds on growth performance of Scophthalmus Maximus at different growth stages. J. Aquac. 2014, 45, 335–492. [Google Scholar]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China—A review of the past decade. Fish Shellfish. Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Huang, P.; Zhang, W.M.; He, J.H.; Kong, X.F. Effects of dietary Bacillus subtilis on growth performance and plasma biochemical parameters of nursery piglets. Chin. J. Anim. Nutr. 2020, 32, 1–8. [Google Scholar]
- Qiao, Z.G.; Jia, W.; Zhang, X.G.; Shen, F.F. Differences in growth, blood physiology and biochemical components between female and male catfish Silurus asotus. J. Dalian Ocean. Univ. 2019, 34, 376–380. [Google Scholar]
- Zhu, P.; Tang, Y.; Fan, J.; Fang, J.; Peng, X.; Cui, H. Hematological parameters and blood cell morphology of male and female Schizothorax (Racoma) davidi (Sauvage). J. World Aquac. Soc. 2017, 48, 821–830. [Google Scholar] [CrossRef]
- Shen, W.-Y.; Fu, L.-L.; Li, W.-F.; Zhu, Y.-R. Effect of dietary supplementation with Bacillus subtilis on the growth, performance, immune response and antioxidant activities of the shrimp (Litopenaeus vannamei). Aquac. Res. 2010, 41, 1691–1698. [Google Scholar] [CrossRef]
- Wang, W.J.; Sun, D.Y.; Pan, B.H.; Sun, X.F. Effects of Bacillus subtilis added in feed on growth, immunity and specific intestinal microflora of grass carp. Feed. Res. 2014, 19, 43–44. [Google Scholar]
- Qin, L.; Xiang, J.; Xiong, F.; Wang, G.; Zou, H.; Li, W.; Li, M.; Wu, S. Effects of Bacillus licheniformis on the growth, antioxidant capacity, intestinal barrier and disease resistance of grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2020, 97, 344–350. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Han, H.; Zhang, M.; Wang, F.; Jiang, H. Isolation, identification and characteristics of two Bacillus associated from intestine of marine fishes. Mar. Fish. 2019, 41, 606–615. [Google Scholar]
- Lutfi, E.; Basili, D.; Falcinelli, S.; Morillas, L.; Carnevali, O.; Capilla, E.; Navarro, I. The probiotic Lactobacillus rhamnosus mimics the dark-driven regulation of appetite markers and melatonin receptors’ expression in zebrafish (Danio rerio) larvae: Understanding the role of the gut microbiome. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110634. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Q.; Liu, Y.; Gao, S.; He, Y.; Yao, C.; Ai, Q. Early life intervention using probiotic Clostridium butyricum improves intestinal development, immune response, and gut microbiota in large yellow croaker (Larimichthys crocea) larvae. Front. Immunol. 2021, 12, 640767. [Google Scholar] [CrossRef]
- Biedermann, L.; Rogler, G. The intestinal microbiota: Its role in health and disease. Eur. J. Pediatr. 2015, 174, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, C.; Dai, M.Z.; Wang, Q.; Li, C.; Hung, W. Bifidobacterium lactis Bacillus licheniformis-99 modulates intestinal inflammation and functions in zebrafish models. PLoS ONE 2022, 17, e0262942. [Google Scholar]
- Xiao, F.; Zhu, W.; Yu, Y.; Huang, J.; Li, J.; He, Z.; Yan, Q. Interactions and stability of gut microbiota in zebrafish increase with host development. Microbiol. Spectr. 2022, 10, e0169621. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Huang, Z.; Sun, B.; Liu, M.; Tang, L.; Chen, L. Metabolomic profiles in zebrafish larvae following probiotic and perfluorobutanesulfonate coexposure. Environ. Res. 2022, 204, 112380. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; Tan, B.; Dong, X.; Yang, Q.; Liu, H.; Chi, S. Replacing fish meal with fermented rice protein in diets for hybrid groupers (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂): Effects on growth, digestive and absorption capacities, inflammatory related gene expression, and intestinal microbiota. Aquac. Rep. 2021, 19, 100603. [Google Scholar] [CrossRef]
- Wu, Z.X.; Feng, X.; Qu, Y.; Chen, X.X. Effects of Bacillus subtilis on the growth, digestion and intestinal flora of grass carp. Abstract Collection of Papers from the 9th World Chinese Fish and Shrimp Nutrition Academic Symposium, Xiamen, China, 12–16 November 2013; Volume 366, p. 383. [Google Scholar]
| Items | Content | |||
|---|---|---|---|---|
| Ingredients | Group A | Group B | Group C | Group D |
| Casein | 28.00 | 28.00 | 28.00 | 28.00 |
| Gelatin | 10.00 | 10.00 | 10.00 | 10.00 |
| Fish meal | 8.00 | 8.00 | 8.00 | 8.00 |
| Soybean meal | 8.00 | 8.00 | 8.00 | 8.00 |
| Fish oil | 7.00 | 7.00 | 7.00 | 7.00 |
| Soybean oil | 7.00 | 7.00 | 7.00 | 7.00 |
| Dextrin | 21.50 | 21.40 | 21.30 | 21.10 |
| Choline | 0.20 | 0.20 | 0.20 | 0.20 |
| Ca(H2PO4)2 | 1.00 | 1.00 | 1.00 | 1.00 |
| Premix a | 2.50 | 2.50 | 2.50 | 2.50 |
| Cellulose | 6.80 | 6.80 | 6.80 | 6.80 |
| probiotics | 0.00 | 0.10 | 0.20 | 0.40 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrients level b | ||||
| Crude protein | 43.61 | 43.60 | 43.60 | 43.60 |
| Crude lipid | 12.11 | 12.10 | 12.10 | 12.10 |
| (MJ·kg−1) Gross energy | 19.49 | 19.48 | 19.41 | 19.39 |
| Lysine | 3.03 | 3.03 | 3.03 | 3.03 |
| Methionine | 1.02 | 1.02 | 1.02 | 1.02 |
| Items | Group A | Group B | Group C | Group D | p Value |
|---|---|---|---|---|---|
| % SR | 92.50 ± 2.01 | 95.00 ± 2.11 | 97.50 ± 2.22 | 97.50 ± 2.04 | 0.847 |
| g IBW | 850.10 ± 12.37 | 851.31 ± 11.22 | 853.24 ± 11.35 | 853.44 ± 13.11 | 0.782 |
| g FBW | 1442.33 ± 14.77 a | 1468.45 ± 15.64 a | 1552.11 ± 15.41 b | 1502.12 ± 14.50 ab | 0.045 |
| % WG | 69.67 ± 3.34 a | 72.49 ± 2.85 a | 81.90 ± 2.88 b | 76.01± 2.93 ab | 0.032 |
| SGR (%·d−1) | 0.44 ± 0.02 a | 0.45 ± 0.02 a | 0.50 ± 0.02 b | 0.47 ± 0.01 ab | 0.022 |
| FCR | 1.88 ± 0.03 a | 1.55 ± 0.04 b | 1.46 ± 0.05 b | 1.50 ± 0.04 b | 0.018 |
| % VSI | 13.75 ± 0.43 | 13.40 ± 0.26 | 13.02 ± 0.29 | 13.11 ± 0.30 | 0.523 |
| % HSl | 8.88 ± 0.15 | 8.63 ± 0.14 | 8.22 ± 0.18 | 8.33 ± 0.16 | 0.634 |
| Items | Group A | Group B | Group C | Group D | p Value |
|---|---|---|---|---|---|
| CP | 18.44 ± 0.64 a | 19.95 ± 0.61 b | 20.21 ± 0.62 b | 20.11 ± 0.57 b | 0.032 |
| EE | 5.58 ± 0.51 a | 6.61 ± 0.52 b | 6.96 ± 0.55 b | 6.87 ± 0.47 b | 0.044 |
| Ash | 3.33 ± 0.37 | 3.45 ± 0.44 | 3.61 ± 0.41 | 3.57 ± 0.40 | 0.511 |
| MS | 79.67 ± 0.25 | 80.19 ± 0.21 | 80.44 ± 0.23 | 80.32 ± 0.25 | 0.722 |
| Items | Group A | Group B | Group C | Group D | p Value |
|---|---|---|---|---|---|
| TP (g/L) | 19.11 ± 0.22 | 19.43 ± 0.22 | 19.75 ± 0.21 | 19.61 ± 0.15 | 0.931 |
| TG (mmol/L) | 7.70 ± 0.23 a | 8.93 ± 0.25 b | 9.22 ± 0.21 b | 9.11 ± 0.24 b | 0.021 |
| TC (mmol/L) | 2.42 ± 0.11 a | 3.55 ± 0.13 b | 3.87 ± 0.14 b | 3.41 ± 0.15 b | 0.029 |
| ALP (U/L) | 151.13 ± 7.12 | 154.22 ± 7.03 | 158.41 ± 6.80 | 156.10 ± 7.40 | 0.755 |
| ALT (U/L) | 7.02 ± 0.68 a | 7.85 ± 0.54 ab | 8.07 ± 0.51 b | 8.00 ± 0.54 b | 0.041 |
| AST (U/L) | 556.41 ± 23.11 a | 600.23 ± 24.13 ab | 677.81 ± 25.96 b | 660.18 ± 26.22 b | 0.038 |
| GLU (mmol/L) | 2.50 ± 0.14 a | 3.11 ± 0.15 b | 3.74 ± 0.13 ab | 2.94 ± 0.12 ab | 0.026 |
| Items | Group A | Group B | Group C | Group D | p Value |
|---|---|---|---|---|---|
| OTU | 265.11 ± 7.61 a | 211.01 ± 8.42 a | 185.44 ± 8.32 ab | 143.24 ± 7.61 b | 0.011 |
| ACE | 277.19 ± 3.15 a | 265.40 ± 2.41 a | 211.42 ± 2.11 ab | 175.10 ± 2.32 b | 0.045 |
| Chao1 | 188.11 ± 4.12 a | 205.44 ± 3.16 ab | 279.30 ± 3.02 b | 231.22 ± 3.22 b | 0.033 |
| Simpson | 0.68 ± 0.02 a | 0.81 ± 0.02 b | 0.87 ± 0.02 b | 0.84 ± 0.01 b | 0.022 |
| Shannon | 0.48 ± 0.03 a | 0.65 ± 0.03 b | 0.79 ± 0.14 b | 0.68 ± 0.05 b | 0.041 |
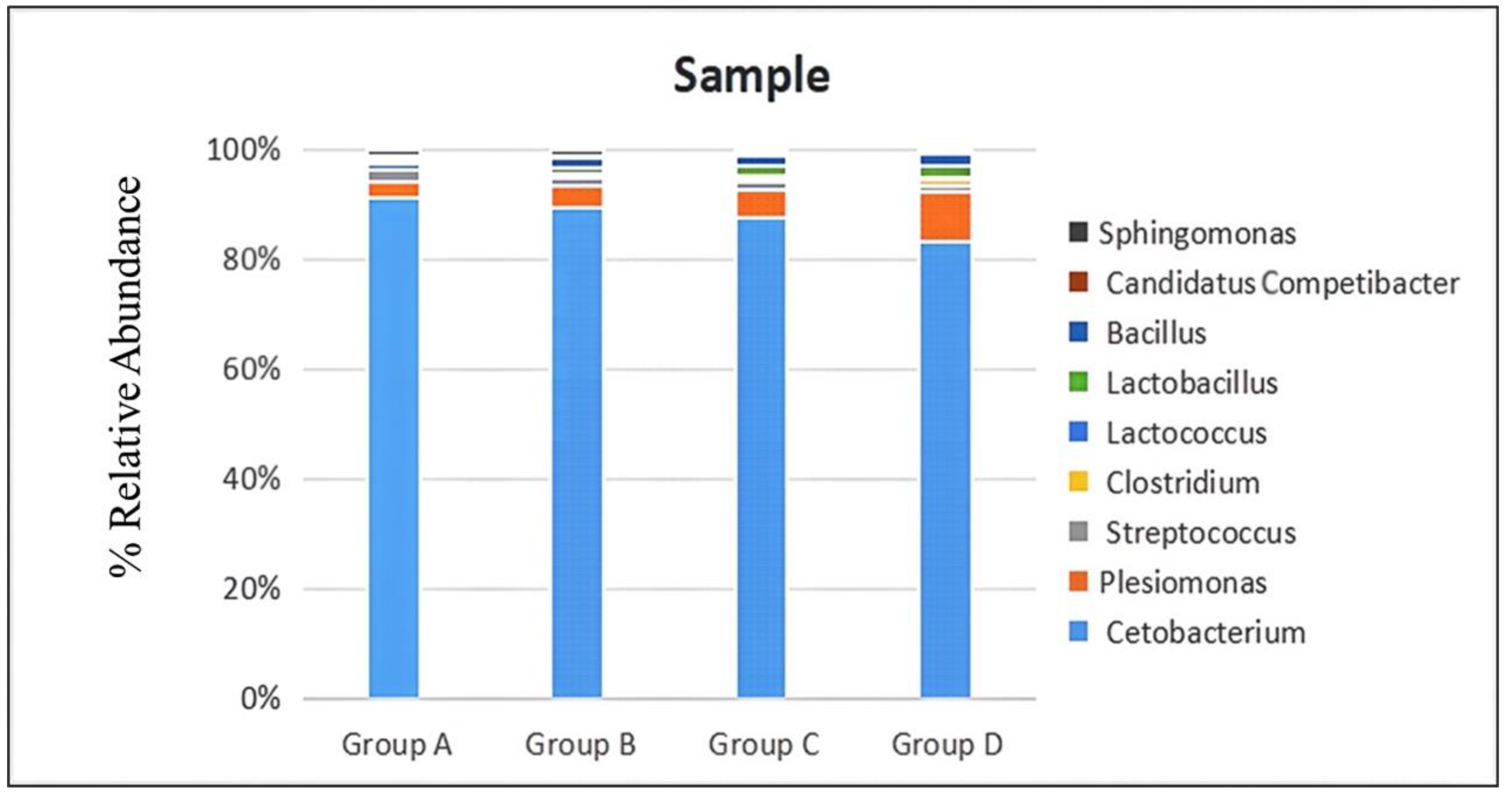
| Gut Microbe | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Cetobacterium | 91.22 | 89.23 | 87.65 | 83.22 |
| Plesiomonas | 3.01 | 4.16 | 5.02 | 9.21 |
| Streptococcus | 2.02 | 1.55 | 1.33 | 1.01 |
| Clostridium | 0.1 | 0.19 | 0.55 | 1.1 |
| Lactococcus | 0.85 | 0.57 | 0.52 | 0.42 |
| Lactobacillus | 0.89 | 1.02 | 1.78 | 1.98 |
| Bacillus | 0.01 | 1.75 | 2.00 | 2.12 |
| Candidatus Competibacter | 0.55 | 0.31 | 0.27 | 0.19 |
| Sphingomonas | 1.35 | 1.22 | 0.88 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Wang, Y.; Huang, X.; Liu, Y.; Yang, M.; Xing, H.; Yang, C.; Hu, C.; Pan, M.; Qi, Z. Effects of Bacillus licheniformis Feeding on the Growth Performance, Blood Parameters and Intestinal Microbiota of Adult Hybrid Sturgeon. Fishes 2025, 10, 189. https://doi.org/10.3390/fishes10050189
Xie Q, Wang Y, Huang X, Liu Y, Yang M, Xing H, Yang C, Hu C, Pan M, Qi Z. Effects of Bacillus licheniformis Feeding on the Growth Performance, Blood Parameters and Intestinal Microbiota of Adult Hybrid Sturgeon. Fishes. 2025; 10(5):189. https://doi.org/10.3390/fishes10050189
Chicago/Turabian StyleXie, Quansen, Yu Wang, Xinyu Huang, Yiran Liu, Mingjian Yang, Haochun Xing, Caimei Yang, Caihong Hu, Mingzhu Pan, and Zhitao Qi. 2025. "Effects of Bacillus licheniformis Feeding on the Growth Performance, Blood Parameters and Intestinal Microbiota of Adult Hybrid Sturgeon" Fishes 10, no. 5: 189. https://doi.org/10.3390/fishes10050189
APA StyleXie, Q., Wang, Y., Huang, X., Liu, Y., Yang, M., Xing, H., Yang, C., Hu, C., Pan, M., & Qi, Z. (2025). Effects of Bacillus licheniformis Feeding on the Growth Performance, Blood Parameters and Intestinal Microbiota of Adult Hybrid Sturgeon. Fishes, 10(5), 189. https://doi.org/10.3390/fishes10050189






