Effects of Glutamate on Growth Performance, Gut Digestion and Antioxidant Capacity in Juvenile Little Yellow Croaker
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diet Preparation and Feeding Trial
2.2. Sample Collection
2.3. Histological Analysis
2.4. Antioxidant Enzyme Activity Assays
2.5. Digestive Enzyme Activity Assays
2.6. Plasma Biochemical Analysis
2.7. Transcriptomic Analysis
2.8. Quantitative Real-Time PCR Analysis
2.9. Calculations and Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Intestinal Morphology
3.3. Plasma Biochemistry
3.4. Antioxidant Activity

| Item | 0.0% | 0.4% | 0.8% | 1.2% | 1.6% | 2.0% |
|---|---|---|---|---|---|---|
| T-SOD (U/mL) | 53.8 ± 3.50 ab | 56.64 ± 6.24 ab | 58.20 ± 4.65 ab | 62.16 ± 4.24 a | 52.90 ± 3.90 ab | 51.46 ± 3.15 b |
| MDA (nmol/mL) | 7.56± 0.90 a | 6.64 ±1.13 a | 5.65 ± 0.95 a | 5.19 ± 0.58 b | 6.49 ± 0.43 a | 6.39 ±1.42 a |
| GSH-PX (U/mL) | 643.81 ± 7.91 b | 655.00 ± 8.17 b | 678.5 ± 8.85 a | 688.05 ± 12.13 a | 639.70 ± 12.88 b | 620.35 ± 3.83 c |
| CAT (U/mL) | 4.00 ± 0.63 c | 6.30 ± 0.71 ab | 7.40 ± 0.86 ab | 8.42 ± 0.92 a | 5.74 ± 1.64 ab | 4.52 ±0.96 b |
3.5. Digestive Enzyme
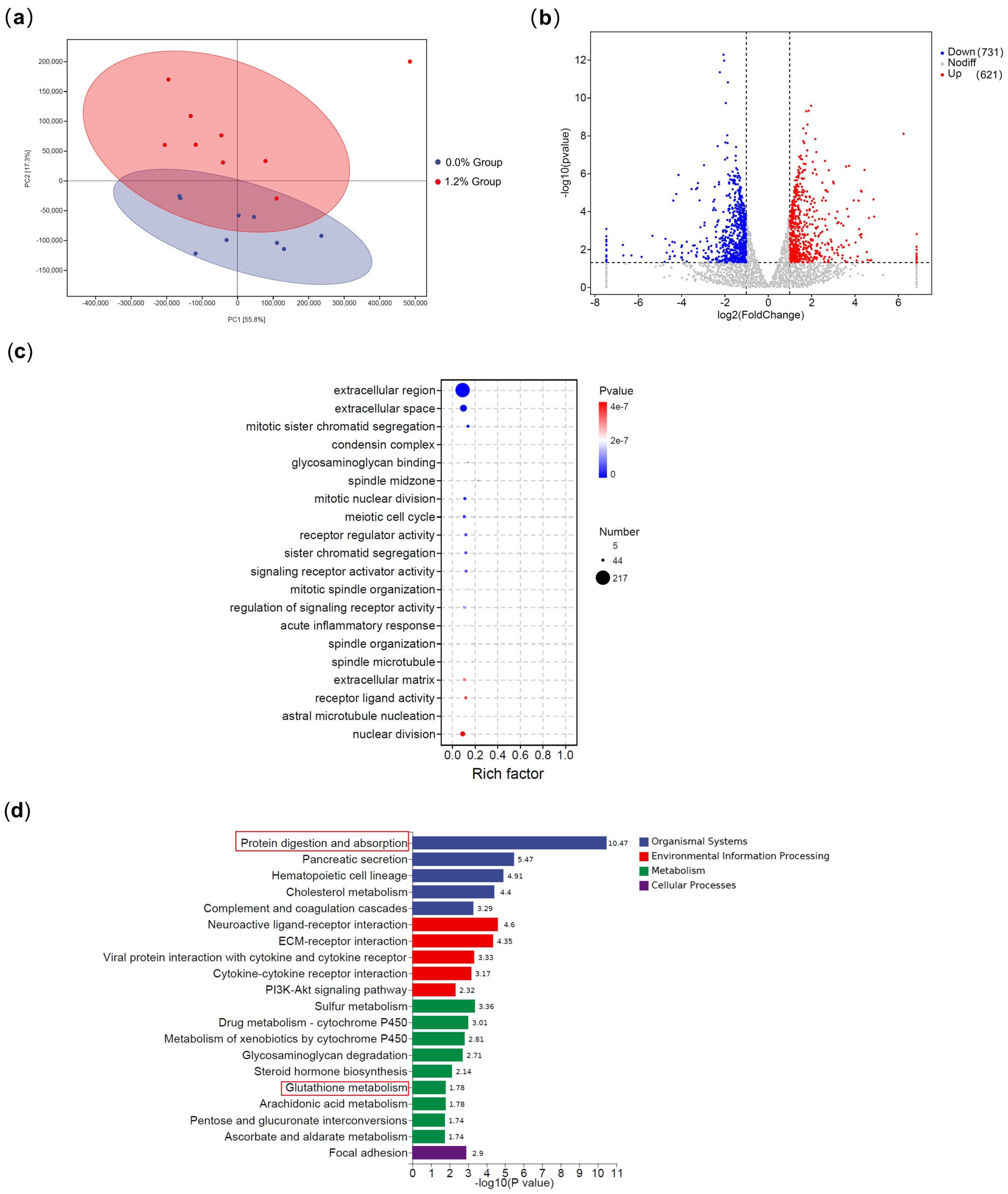
3.6. Transcriptomic Differences Between Groups Fed Diets with Varying Dietary Glutamate Levels
3.7. Relative mRNA Expression of Little Yellow Croaker
4. Discussion
4.1. Glutamate Improves Growth Performance
4.2. Glutamate Improved Intestinal Morphology
4.3. Glutamate Increases Antioxidative Capability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mangia, S.; Giove, F.; Dinuzzo, M. Metabolic Pathways and Activity-Dependent Modulation of Glutamate Concentration in the Human Brain. Neurochem. Res. 2012, 37, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lai, W.; Yao, L.; Xu, E.; Chen, X.; Zhang, Y.Y.; Li, X.G. Glutamine Regulates Gene Expression Profiles to Increase the Proliferation of Porcine Intestinal Epithelial Cells and the Expansion of Intestinal Stem Cells. Animals 2023, 13, 2917. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.L.P.F.; Xavier, W.d.S.; Guimarães, M.G.; Rodrigues, E.J.D.; Furuya, W.M.; Yamamoto, F.Y.; Pezzato, L.E.; Gatlin, D.M.; Barros, M.M. Dietary Glutamine Improves Growth and Intestinal Morphology of Juvenile Gift Tilapia (Oreochromis niloticus) but Has Limited Effects on Innate Immunity and Antioxidant Capacity. Aquaculture 2023, 563, 738976. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Gisbert, E.; Giménez, G.; Fernández, I.; Kotzamanis, Y.; Estévez, A. Development of Digestive Enzymes in Common Dentex Dentex Dentex During Early Ontogeny. Aquaculture 2008, 287, 381–387. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.-Y.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, J.; Chen, D.-F.; Zhou, X.-Q.; et al. Effects of Dietary Glutamate Supplementation on Flesh Quality, Antioxidant Defense and Gene Expression Related to Lipid Metabolism and Myogenic Regulation in Jian Carp (Cyprinus carpio var. Jian). Aquaculture 2019, 502, 212–222. [Google Scholar] [CrossRef]
- Zhelyazkov, G.; Stratev, D. Effect of Monosodium Glutamate on Growth Performance and Blood Biochemical Parameters of Rainbow Trout (Oncorhynchus mykiss W.). Vet. World 2019, 12, 1008–1012. [Google Scholar] [CrossRef]
- Caballero-Solares, A.; Viegas, I.; Salgado, M.C.; Siles, A.M.; Sáez, A.; Metón, I.; Baanante, I.V.; Fernández, F. Diets Supplemented with Glutamate or Glutamine Improve Protein Retention and Modulate Gene Expression of Key Enzymes of Hepatic Metabolism in Gilthead Seabream (Sparus aurata) Juveniles. Aquaculture 2015, 444, 79–87. [Google Scholar] [CrossRef]
- Oehme, M.; Grammes, F.; Takle, H.; Zambonino-Infante, J.-L.; Refstie, S.; Thomassen, M.S.; Rørvik, K.-A.; Terjesen, B.F. Dietary Supplementation of Glutamate and Arginine to Atlantic Salmon (Salmo Salar L.) Increases Growth During the First Autumn in Sea. Aquaculture 2010, 310, 156–163. [Google Scholar] [CrossRef]
- Dong, B.; Wu, L.; Wang, Y.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; Xie, S.; Liu, Z.; Jin, J. Glutamate Improves Flesh Quality and Muscle Growth of Triploid Crucian Carp. Aquac. Rep. 2023, 33, 101832. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, J.; Liu, H.; Zhang, H.; Shan, H.; Zong, J.; Cao, Q.; Jiang, J. Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass. Fishes 2025, 10, 151. [Google Scholar] [CrossRef]
- Song, X.; Hu, F.; Xu, M.; Zhang, Y.; Jin, Y.; Gao, X.; Liu, Z.; Ling, J.; Li, S.; Cheng, J. Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys polyactis) in the Southern Yellow Sea. Diversity 2024, 16, 521. [Google Scholar] [CrossRef]
- Song, D.; Xiong, Y.; Kang, Z.; Liang, L.; Wu, X.; Li, D.; Liu, D. Population Connectivity of Small Yellow Croaker (Larmichthys polyactis) in the Southern Yellow Sea: Implications from Multiple Otolith-Based Approaches. Fish. Res. 2024, 276, 107064. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Liu, Y.; Du, C.; Hou, C.; Xie, Q.; Tang, D.; Liu, F.; Lou, B.; Zhu, J. Change to the Transcriptomic Profile, Oxidative Stress, Apoptotic and Immunity in the Liver of Small Yellow Croaker (Larimichthys polyactis) under Hypoxic Stress. Aquaculture 2023, 576, 739854. [Google Scholar] [CrossRef]
- Chu, T.; Liu, F.; Qin, G.; Zhan, W.; Wang, M.; Lou, B. Transcriptome Analysis of the Larimichthys polyactis under Heat and Cold Stress. Cryobiology 2020, 96, 175–183. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Xu, J.; Zheng, Y.; Lin, J.; Zou, H. Transcriptome Analysis Reveals Dysfunction of the Endoplasmic Reticulum Protein Processing in the Sonic Muscle of Small Yellow Croaker (Larimichthys polyactis) Following Noise Exposure. Mar. Environ. Res. 2024, 194, 106299. [Google Scholar] [CrossRef]
- Sumithra, T.G.; Sharma, S.R.K.; Suresh, G.; Suja, G.; Prasad, V.; Gop, A.P.; Patil, P.K.; Gopalakrishnan, A. Gut Microbes of a High-Value Marine Fish, Snubnose Pompano (Trachinotus blochii) Are Resilient to Therapeutic Dosing of Oxytetracycline. Sci. Rep. 2024, 14, 27949. [Google Scholar] [CrossRef]
- Alfonso, S.; Mente, E.; Fiocchi, E.; Manfrin, A.; Dimitroglou, A.; Papaharisis, L.; Barkas, D.; Toomey, L.; Boscarato, M.; Losasso, C.; et al. Growth Performance, Gut Microbiota Composition, Health and Welfare of European Sea Bass (Dicentrarchus labrax) Fed an Environmentally and Economically Sustainable Low Marine Protein Diet in Sea Cages. Sci. Rep. 2023, 13, 21269. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.P.; Shen, Y.Q. A Systematic Review of Advances in Intestinal Microflora of Fish. Fish Physiol. Biochem. 2021, 47, 2041–2053. [Google Scholar] [CrossRef]
- Luise, D.; Correa, F.; Chalvon-Demersay, T.; Galosi, L.; Rossi, G.; Lambert, W.; Bosi, P.; Trevisi, P. Supplementation of Mixed Doses of Glutamate and Glutamine Can Improve the Growth and Gut Health of Piglets During the First 2 Weeks Post-Weaning. Sci. Rep. 2022, 12, 14533. [Google Scholar] [CrossRef]
- Yvon, S.; Beaumont, M.; Dayonnet, A.; Eutamène, H.; Lambert, W.; Tondereau, V.; Chalvon-Demersay, T.; Belloir, P.; Paës, C. Effect of Diet Supplemented with Functional Amino Acids and Polyphenols on Gut Health in Broilers Subjected to a Corticosterone-Induced Stress. Sci. Rep. 2024, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
- Fantatto, R.R.; Mota, J.; Ligeiro, C.; Vieira, I.; Guilgur, L.G.; Santos, M.; Murta, D. Exploring Sustainable Alternatives in Aquaculture Feeding: The Role of Insects. Aquac. Rep. 2024, 37, 102228. [Google Scholar] [CrossRef]
- Vodounnou, J.V.; Iko, R.; Okou, G.; Kpogue, D.; Ahouansou Montcho, S.; Micha, J.-C. Complete Substitution of Fish Meal with Black Soldier Flies Hermetia illucens (L. 1758) Larvae Meal at Varying Incorporation Rates for Feeding Oreochromis niloticus Raised in Captivity. Aquac. Sci. Manag. 2025, 2, 1. [Google Scholar] [CrossRef]
- Ma, B.; Wang, L.; Lou, B.; Tan, P.; Xu, D.; Chen, R. Dietary Protein and Lipid Levels affect The Growth Performance, Intestinal Digestive Enzyme Activities and Related Genes Expression of Juvenile Small Yellow Croaker (Larimichthys polyactis). Aquac. Rep. 2020, 17, 100403. [Google Scholar] [CrossRef]
- Yu, Z.; Xiao, L.; Wei, Z.; Mingming, H.; Feng, L.; Qingping, X.; Dandan, G.; Lin, C.; Bao, L. Effects of Dietary Protein Levels on Growth Performance, Muscle Composition and Fiber Recruitment of Juvenile Small Yellow Croaker (Larimichthys polyactis). Aquac. Rep. 2022, 27, 101335. [Google Scholar]
- Li, X.; Zheng, S.; Cheng, K.; Ma, X.; Wu, G. Use of Alternative Protein Sources for Fishmeal Replacement in the Diet of Largemouth Bass (Micropterus salmoides). Part II: Effects of Supplementation with Methionine or Taurine on Growth, Feed Utilization, and Health. Amino Acids 2021, 53, 49–62. [Google Scholar] [CrossRef]
- Young, T.; Walker, S.P.; Alfaro, A.C.; Fletcher, L.M.; Murray, J.S.; Lulijwa, R.; Symonds, J. Impact of Acute Handling Stress, Anaesthesia, and Euthanasia on Fish Plasma Biochemistry: Implications for Veterinary Screening and Metabolomic Sampling. Fish Physiol. Biochem. 2019, 45, 1485–1494. [Google Scholar] [CrossRef]
- Flores, C.F.Y.; de Las Mercedes Hurtado Pineda, Á.; Bonilla, V.M.C.; Sáenz-Flor, K. Sample Management: Stability of Plasma and Serum on Different Storage Conditions. Ejifcc 2020, 31, 46–55. [Google Scholar]
- Q764M9 CXCR4_PIG. Available online: https://www.uniprot.org/uniprotkb/Q764M9/entry (accessed on 9 March 2025).
- Wang, M.; Li, E.; Huang, Y.; Liu, W.; Wang, S.; Li, W.; Chen, L.; Wang, X. Dietary Supplementation with Glutamate Enhanced Antioxidant Capacity, Ammonia Detoxification and Ion Regulation Ability in Nile Tilapia (Oreochromis niloticus) Exposed to Acute Alkalinity Stress. Aquaculture 2025, 594, 741360. [Google Scholar] [CrossRef]
- Ingebrigtsen, I.A.; Berge, G.M.; Ruyter, B.; Kjær, M.A.; Mørkøre, T.; Sørensen, M.; Gjøen, T. Growth and Quality of Atlantic Cod (Gadus morhua) Fed with High and Low Fat Diets Supplemented with Glutamate. Aquaculture 2014, 433, 367–376. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and Metabolism of Glutamate and Glutamine in Fish. Amino Acids 2020, 52, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, T.-R.; Li, Q.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, J.; Zhou, X.-Q.; Jiang, J. Effect of Dietary L-Glutamate Levels on Growth, Digestive and Absorptive Capability, and Intestinal Physical Barrier Function in Jian Carp (Cyprinus carpio var. Jian). Anim. Nutr. 2020, 6, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Cahu, C.; Zambonino Infante, J. Substitution of Live Food by Formulated Diets in Marine Fish Larvae. Aquaculture 2001, 200, 161–180. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Valentín, J.M.; Fontanillas, R.; Gallardo, Á. High Dietary Carbohydrate Inclusion by Both Protein and Lipid Replacement in Gilthead Sea Bream. Changes in Digestive and Absorptive Processes. Aquaculture 2020, 520, 734977. [Google Scholar] [CrossRef]
- Ullah, S.; Feng, F.; Zhao, M.; Zhang, J.; Shao, Q. Effect of Dietary Supplementation of Lauric Acid on Growth Performance, Digestive Enzymes, Serum Immune and Antioxidant Parameters, and Intestinal Morphology in Black Sea Bream. Fish Physiol. Biochem. 2025, 51, 43. [Google Scholar] [CrossRef]
- Sichao, J.; Xinyu, L.; Shixuan, Z.; Guoyao, W. Amino Acids Are Major Energy Substrates for Tissues of Hybrid Striped Bass and Zebrafish. Amino Acids 2017, 49, 2053–2063. [Google Scholar]
- Xinyu, L.; Zheng, S.; Sichao, J.; Fei, S.; Chuanpeng, Z.; Guoyao, W. Oxidation of Energy Substrates in Tissues of Largemouth Bass (Micropterus salmoides). Amino Acids 2020, 52, 1017–1032. [Google Scholar]
- Xuan, D.T.M.; Wu, C.C.; Wang, W.J.; Hsu, H.P.; Ta, H.D.K.; Anuraga, G.; Chiao, C.C.; Wang, C.Y. Glutamine Synthetase Regulates the Immune Microenvironment and Cancer Development through the Inflammatory Pathway. Int. J. Med. Sci. 2023, 20, 35–49. [Google Scholar] [CrossRef]
- Hu, T.; Wu, J.; Lin, Z.; Lin, Y.; Lin, L.; Wei, W.; Wei, D. The Impact of Glutamine Synthetase Pbgsa on the Growth, Conidiation and Mycophenolic Acid Production of Penicillium Brevicompactum. Fungal Genet. Biol. 2024, 175, 103941. [Google Scholar] [CrossRef]
- Simon, B.R.; Learman, B.S.; Parlee, S.D.; Scheller, E.L.; Mori, H.; Cawthorn, W.P.; Ning, X.; Krishnan, V.; Ma, Y.L.; Tyrberg, B.; et al. Sweet Taste Receptor Deficient Mice Have Decreased Adiposity and Increased Bone Mass. PLoS ONE 2014, 9, e86454. [Google Scholar] [CrossRef]
- Xie, S.Z.; Yang, G.; Jiang, X.M.; Qin, D.Y.; Li, Q.M.; Zha, X.Q.; Pan, L.H.; Jin, C.S.; Luo, J.P. Polygonatum Cyrtonema Hua Polysaccharide Promotes Glp-1 Secretion from Enteroendocrine L-Cells through Sweet Taste Receptor-Mediated Camp Signaling. J. Agric. Food Chem. 2020, 68, 6864–6872. [Google Scholar] [CrossRef] [PubMed]
- Toda, Y.; Hayakawa, T.; Itoigawa, A.; Kurihara, Y.; Nakagita, T.; Hayashi, M.; Ashino, R.; Melin, A.D.; Ishimaru, Y.; Kawamura, S.; et al. Evolution of the Primate Glutamate Taste Sensor from a Nucleotide Sensor. Curr. Biol. 2021, 31, 4641–4649.e5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, D.; Zhou, X.Q.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.D.; Zhao, Y. Effects of Glutamate on Growth, Antioxidant Capacity, and Antioxidant-Related Signaling Molecule Expression in Primary Cultures of Fish Enterocytes. Fish Physiol. Biochem. 2015, 41, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Knabe, D.A.; Tekwe, C.D.; Dahanayaka, S.; Ficken, M.D.; Fielder, S.E.; Eide, S.J.; Lovering, S.L.; Wu, G. Dietary Supplementation with Monosodium Glutamate Is Safe and Improves Growth Performance in Postweaning Pigs. Amino Acids 2013, 44, 911–923. [Google Scholar] [CrossRef]
- Maruyama, K.; Sunde, M.L.; Harper, A.E. Is L-Glutamic Acid Nutritionally a Dispensable Amino Acid for the Young Chick? Poult. Sci. 1976, 55, 45–60. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef]
- Acikel-Elmas, M.; Algilani, S.A.; Sahin, B.; Bingol Ozakpinar, O.; Gecim, M.; Koroglu, K.; Arbak, S. Apocynin Ameliorates Monosodium Glutamate Induced Testis Damage by Impaired Blood-Testis Barrier and Oxidative Stress Parameters. Life 2023, 13, 822. [Google Scholar] [CrossRef]
- Kayode, O.T.; Bello, J.A.; Oguntola, J.A.; Kayode, A.A.A.; Olukoya, D.K. The Interplay between Monosodium Glutamate (Msg) Consumption and Metabolic Disorders. Heliyon 2023, 9, e19675. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, L.; Limbu, S.M.; Yin, H.; Xie, Y.; Yang, Z.; Shang, Z.; Kong, L.; Rong, H. A Comparison of Digestive Strategies for Fishes with Different Feeding Habits: Digestive Enzyme Activities, Intestinal Morphology, and Gut Microbiota. Ecol. Evol. 2023, 13, e10499. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Gil-Solsona, R.; Estensoro, I.; Piazzon, M.C.; Martos-Sitcha, J.A.; Picard-Sánchez, A.; Fuentes, J.; Sancho, J.V.; Calduch-Giner, J.A.; Hernández, F.; et al. Disruption of Gut Integrity and Permeability Contributes to Enteritis in a Fish-Parasite Model: A Story Told from Serum Metabolomics. Parasites Vectors 2019, 12, 486. [Google Scholar] [CrossRef]
- Painefilú, J.C.; Bianchi, V.A.; Krock, B.; De Anna, J.S.; Kristoff, G.; Luquet, C.M. Effects of Paralytic Shellfish Toxins on the Middle Intestine of Oncorhynchus mykiss: Glutathione Metabolism, Oxidative Status, Lysosomal Function and Atp-Binding Cassette Class C (Abcc) Proteins Activity. Ecotoxicol. Environ. Saf. 2020, 204, 111069. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, B.; Wang, N.; Yang, J.; Zhou, Q.; Sun, C.; Zhao, Y. Low Fish Meal Diet Supplemented with Probiotics Ameliorates Intestinal Barrier and Immunological Function of Macrobrachium rosenbergii Via the Targeted Modulation of Gut Microbes and Derived Secondary Metabolites. Front. Immunol. 2022, 13, 1074399. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Pastorelli, G.; Tedesco, D.E.A.; Turin, L.; Guerrini, A. Alternative Protein Sources in Aquafeed: Current Scenario and Future Perspectives. Vet. Anim. Sci. 2024, 25, 100381. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, Y.; Zhao, Z.; Chu, Y.; Yang, W. The Role of Amino Acid Metabolism in Inflammatory Bowel Disease and Other Inflammatory Diseases. Front. Immunol. 2023, 14, 1284133. [Google Scholar] [CrossRef]
- Jiang, J.; Yin, L.; Li, J.-Y.; Li, Q.; Shi, D.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, Y.; et al. Glutamate Attenuates Lipopolysaccharide-Induced Oxidative Damage and Mrna Expression Changes of Tight Junction and Defensin Proteins, Inflammatory and Apoptosis Response Signaling Molecules in the Intestine of Fish. Fish Shellfish Immunol. 2017, 70, 473–484. [Google Scholar] [CrossRef]
- Ma, Y.-M.; Ibeanu, G.; Wang, L.-Y.; Zhang, J.-Z.; Chang, Y.; Dong, J.-D.; Li, P.A.; Jing, L. Selenium Suppresses Glutamate-Induced Cell Death and Prevents Mitochondrial Morphological Dynamic Alterations in Hippocampal Ht22 Neuronal Cells. BMC Neurosci. 2017, 18, 15. [Google Scholar] [CrossRef]
- Wang, L.; Tang, X.; Liang, P.; Zhou, C.; Sun, Y.; Liang, Y. Correlation between Variants of the Creb1 and Grm7 Genes and Risk of Depression. BMC Psychiatry 2023, 23, 3. [Google Scholar] [CrossRef]
- Taysı, M.R. Assessing the Effects of Cadmium on Antioxidant Enzymes and Histological Structures in Rainbow Trout Liver and Kidney. Sci. Rep. 2024, 14, 27453. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Zhou, X.-Q.; Zeng, X.-Y.; Feng, L.; Liu, Y.; Jiang, W.-D.; Li, S.-H.; Li, D.-B.; Wu, X.-Q.; et al. Effects of Dietary Glutamate Supplementation on Growth Performance, Digestive Enzyme Activities and Antioxidant Capacity in Intestine of Grass Carp (Tenopharyngodon idella). Aquac. Nutr. 2015, 21, 935–941. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New Insights in Intestinal Oxidative Stress Damage and the Health Intervention Effects of Nutrients: A Review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Banerjee, P.; Wang, Y.; Carnevale, L.N.; Patel, P.; Raspur, C.K.; Tran, N.; Zhang, X.; Natarajan, R.; Roberts, A.J.; Baran, P.S.; et al. diAcCA, a Pro-Drug for Carnosic Acid That Activates the Nrf2 Transcriptional Pathway, Shows Efficacy in the 5xFAD Transgenic Mouse Model of Alzheimer’s Disease. Antioxidants 2025, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Stabile, A.M.; Migni, A.; Gurrado, F.; Lioci, G.; De Franco, F.; Mandarano, M.; Svegliati-Baroni, G.; Di Cristina, M.; Bellezza, G.; et al. Subcellular Distribution and Nrf2/Keap1-Interacting Properties of Glutathione S-Transferase P in Hepatocellular Carcinoma. Arch. Biochem. Biophys. 2024, 757, 110043. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.A.; Green, J.A.; Warenius, H.M. Cellular Glutathione (Gsh) and Glutathione S-Transferase (Gst) Activity in Human Ovarian Tumor Biopsies Following Exposure to Alkylating Agents. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 527–531. [Google Scholar] [CrossRef]



| Item | 0.0% | 0.4% | 0.8% | 1.2% | 1.6% | 2.0% |
|---|---|---|---|---|---|---|
| Ingredients (%) | ||||||
| Fish meal | 34.65 | 34.65 | 34.65 | 34.65 | 34.65 | 34.65 |
| Krill meal | 6 | 6 | 6 | 6 | 6 | 6 |
| Squid meal | 5 | 5 | 5 | 5 | 5 | 5 |
| Wheat gluten | 13.6 | 13.6 | 13.6 | 13.6 | 13.6 | 13.6 |
| SPC | 11 | 11 | 11 | 11 | 11 | 11 |
| Fish oil | 4.02 | 4.02 | 4.02 | 4.02 | 4.02 | 4.02 |
| Soybean oil | 4.02 | 4.02 | 4.02 | 4.02 | 4.02 | 4.02 |
| Soybean lecithin | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin premix 1 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Mineral premix 2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline chloride (60%) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Bile acid | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Ca(H2PO4)2 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Glutamate | 0 | 0.4 | 0.8 | 1.2 | 1.6 | 2 |
| α-starch | 13.1 | 13.1 | 13.1 | 13.1 | 13.1 | 13.1 |
| Microcrystalline cellulose | 5.51 | 5.11 | 4.71 | 4.31 | 3.91 | 3.51 |
| Proximate analysis (% air dry matter) | ||||||
| Crude protein | 44.26 | 44.51 | 44.36 | 44.20 | 44.93 | 44.04 |
| Crude lipid | 12.64 | 12.22 | 12.82 | 12.21 | 12.41 | 12.60 |
| Moisture | 10.45 | 10.85 | 11.12 | 9.81 | 9.68 | 11.01 |
| Crude ash | 10.28 | 10.13 | 9.85 | 9.67 | 10.56 | 9.83 |
| Gross energy (kJ g−1) | 18.02 | 18.1 | 18.11 | 18.08 | 18.06 | 18.03 |
| Glutamate | 8.17 | 8.50 | 8.82 | 9.15 | 9.48 | 9.80 |
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Tm (°C) | PCR Products (bp) |
|---|---|---|---|---|
| 18S | GGAGGCATGGTGGTGGAT | AAAACTGGACCTGGTTGGAAA | 60 | 142 |
| β-actin | GTTATGCCCTGCCCCATG | TGTCACGCACGATTTCCCT | 56 | 132 |
| Bcl-2 | ACCGCAGGTGGACAACATC | CAGCCATACCGAAGACCGT | 60 | 173 |
| Keap1 | GCCAAACGCATCATAACACG | GCCACTCCAGCACCCAGAC | 60 | 100 |
| Nrf2 | TGAGAAGAGCCAGAACACCAC | AATGCGAGGAACAAGGAAGAT | 60 | 169 |
| GST | GTTCTGCCTCCCTCTGTCAC | GGCGTATATCTGTCGGGCAA | 60 | 180 |
| GGT | GTGTTTGGGTGTGGTGCATC | CCTCTGAGCATACCTGGCAC | 60 | 198 |
| GS | GGGACATCGTAGAGTGCCAC | GCCTGTAACTGGTTTGGGGT | 60 | 218 |
| IL-6R | GCCTCACAAAGTGCCACATC | CCAAAGGTCACCAGAGCCTG | 60 | 207 |
| IL-6 | GCCAAGGGCCTGTTCACTTA | GTGGGTGTGTCGATGTTCCT | 60 | 198 |
| IL-10 | ACAAGTCCAGTGTGCGTCAA | AATGCTGTTGATGGCGTGAC | 60 | 195 |
| Creb1 | GGTGGTCATGGCTTCATCC | TCCTTCTTCTTCCTGCGACA | 60 | 126 |
| Item | 0.0% | 0.4% | 0.8% | 1.2% | 1.6% | 2.0% |
|---|---|---|---|---|---|---|
| SR (%) | 75.56 ± 4.16 b | 84.47 ± 3.14 ab | 85.56 ± 1.57 ab | 91.11 ± 3.14 a | 83.33 ± 2.72 ab | 82.22 ± 4.16 ab |
| IW (g) | 30.93 ± 0.04 a | 30.59 ± 0.15 a | 30.45 ± 0.27 a | 30.64 ± 0.09 a | 30.83 ± 0.06 a | 30.43 ± 0.10 a |
| FW (g) | 36.56 ± 1.14 b | 39.85 ± 1.14 b | 41.58 ± 0.77 ab | 45.43 ± 1.07 a | 41.44 ± 2.26 ab | 39.66 ± 2.00 b |
| WGR (%) | 18.24 ± 3.68 c | 30.31 ± 3.96 bc | 36.57 ± 3.49 ab | 48.26 ± 3.53 a | 34.41 ± 7.31 ab | 30.16 ± 6.83 b |
| SGR (% day −1) | 0.28 ± 0.05 b | 0.44 ± 0.05 ab | 0.52 ± 0.04 ab | 0.66 ± 0.04 a | 0.49 ± 0.10 ab | 0.44 ± 0.10 b |
| DFI (g tail −1 day −1) | 0.16 ± 0.04 b | 0.27 ± 0.04 ab | 0.31 ± 0.04 a | 0.38 ± 0.03 a | 0.30 ± 0.06 ab | 0.22 ± 0.04 b |
| PER | 1.55 ± 0.08 c | 1.69 ± 0.04 b | 1.76 ± 0.02 ab | 1.85 ± 0.03 a | 1.75 ± 0.04 ab | 1.53 ± 0.04 c |
| FCR | 1.75 ± 0.03 a | 1.72 ± 0.05 a | 1.61 ± 0.03 ab | 1.57 ± 0.03 b | 1.65 ± 0.05 ab | 1.69 ± 0.08 ab |
| Item | 0% | 0.4% | 0.8% | 1.2% | 1.6% | 2.0% |
|---|---|---|---|---|---|---|
| Muscle thickness (μm) | 53.83 ± 3.50 b | 56.64 ± 6.24 a | 58.20 ± 4.65 a | 62.16 ± 4.24 a | 52.90 ± 3.90 a | 51.46 ± 3.15 b |
| Villus height (μm) | 153.71 ± 28.96 b | 186.08 ± 14.69 b | 237.62 ± 31.81 a | 273.55 ± 27.82 a | 248.26 ± 34.20 a | 199.58 ± 18.89 ab |
| Item | 0% | 0.4% | 0.8% | 1.2% | 1.6% | 2.0% |
|---|---|---|---|---|---|---|
| Lipase (U/mg) | 6.80 ± 0.31 bc | 7.71 ± 0.69 b | 9.70 ± 0.39 a | 10.04 ± 0.55 a | 10.40 ± 1.00 a | 6.07 ± 0.40 c |
| Trypsin (U/mg) | 15.52 ± 1.96 b | 19.62 ± 0.67 a | 20.80 ± 1.38 a | 22.57 ± 2.95 a | 20.33 ± 1.30 a | 16.87 ± 1.85 b |
| Pepsin (U/mg) | 6.14 ± 0.40 d | 7.09 ± 0.40 c | 7.23 ± 0.41 c | 12.32 ± 0.40 a | 10.12 ± 0.41 b | 5.73 ± 0.40 d |
| α-Amylase (U/mg) | 0.67 ± 0.05 d | 0.74 ± 0.07 d | 0.92 ± 0.07 c | 1.05 ± 0.05 ab | 1.07 ± 0.06 a | 0.40 ± 0.07 e |
| Name | Id | p Value | Fold Change | KEGG | Length | Swiss-Prot |
|---|---|---|---|---|---|---|
| pepsin A | evm.TU.Scaffold1008.105 | 0.030 | 3.287 | K06002 | 528 | sp|P00792|GN = Pepsin A |
| trypsin | evm.TU.Scaffold47.69 | 0.000 | 13.382 | K01312 | 1307 | sp|P70059|GN = Trypsin |
| collagen type I alpha | evm.TU.Scaffold22.60 | 0.012 | 2.627 | K06236 | 4582 | sp|P02452|GN = CO1A1_HUMAN Collagen alpha-1(I) |
| neprilysin | evm.TU.Scaffold69.303 | 0.658 | 0.916 | K01389 | 5214 | sp|Q61391|GN = Neprilysin |
| carboxypeptidase B | evm.TU.Scaffold11.357 | 0.000 | 6.274 | K01291 | 1239 | sp|P19223|GN = CBPB1_RAT Carboxypeptidase B |
| potassium large conductance calcium-activated channel subfamily M alpha member 1 | evm.TU.Scaffold63.151 | 0.006 | 0.192 | K04936 | 1239 | sp|Q12791|KCMA1_HUMAN Calcium-activated potassium channel subunit alpha-1 OS = Homo sapiens OX = 9606 GN = KCNMA1 PE = 1 SV = 2 |
| solute carrier family 4 (anion exchanger), member 2 | evm.TU.Scaffold22.7 | 0.045 | 0.209 | K13855 | 3316 | sp|O95477|ABCA1_HUMAN Phospholipid-transporting ATPase ABCA1 OS = Homo sapiens OX = 9606 GN = ABCA1 PE = 1 SV = 3 |
| potassium large conductance calcium-activated channel subfamily M alpha member 1 | evm.TU.Scaffold63.151 | 0.006 | 0.192 | K04936 | 6627 | sp|P53760|LCAT_CHICK Phosphatidylcholine-sterol acyltransferase (Fragment) OS = Gallus gallus OX = 9031 GN = LCAT PE = 1 SV = 1 |
| ATP-binding cassette, subfamily A (ABC1), member 1 | evm.TU.Scaffold236.288 | 0.000 | 0.489 | K05641 | 2290 | sp|O14841|OPLA_HUMAN 5-oxoprolinase OS = Homo sapiens OX = 9606 GN = OPLAH PE = 1 SV = 3 |
| lecithin-cholesterol acyltransferase | evm.TU.Scaffold843.27 | 0.034 | 2.120 | K00650 | 2250 | sp|P78417|GSTO1_HUMAN Glutathione S-transferase omega-1 OS = Homo sapiens OX = 9606 GN = GSTO1 PE = 1 SV = 2 |
| 5-oxoprolinase (ATP-hydrolysing) | evm.TU.Scaffold123.35 | 0.000 | 4.749 | K01469 | 720 | sp|O12990|JAK1_DANRE Tyrosine-protein kinase JAK1 OS = Danio rerio OX = 7955 GN = jak1 PE = 1 SV = 1 |
| glutathione S-transferase | evm.TU.Scaffold63.186 | 0.000 | 3.965 | K00799 | 12,123 | sp|Q9IB11|SOMA_SCIOC Somatotropin OS = Sciaenops ocellatus OX = 76340 GN = gh PE = 2 SV = 2 |
| Janus kinase 1 | evm.TU.Scaffold81.61 | 0.008 | 0.805 | K11217 | 768 | sp|P22105|TENX_HUMAN Tenascin-X OS = Homo sapiens OX = 9606 GN = TNXB PE = 1 SV = 5 |
| growth hormone | evm.TU.scaffold66s1.4 | 0.019 | 1.630 | K05438 | 5009 | sp|Q1LVF0|LAMC1_DANRE Laminin subunit gamma-1 OS = Danio rerio OX = 7955 GN = lamc1 PE = 2 SV = 2 |
| tenascin | evm.TU.scaffold20s1.38 | 0.047 | 3.112 | K06252 | 6185 | sp|Q28178|TSP1_BOVIN Thrombospondin-1 OS = Bos taurus OX = 9913 GN = THBS1 PE = 2 SV = 2 |
| laminin, gamma 1 | evm.TU.scaffold1079s1.2_evm.TU.scaffold1079s1.3 | 0.374 | 1.313 | K05635 | 3471 | sp|Q06274|ITA5_XENLA Integrin alpha-5 OS = Xenopus laevis OX = 8355 GN = itga5 PE = 2 SV = 1 |
| thrombospondin 1 | evm.TU.Scaffold754.152 | 0.474 | 0.199 | K16857 | 2970 | sp|Q5EBF6|SDF1_XENTR Stromal cell-derived factor 1 OS = Xenopus tropicalis OX = 8364 GN = cxcl12 PE = 3 SV = 1 |
| integrin alpha 5 | evm.TU.Scaffold804.20 | 0.000 | 2.192 | K06484 | 2661 | sp|Q5EBF6|SDF1_XENTR Stromal cell-derived factor 1 OS = Xenopus tropicalis OX = 8364 GN = cxcl12 PE = 3 SV = 1 |
| C-X-C motif chemokine 12 | evm.TU.Scaffold310.328 | 0.000 | 0.357 | K10031 | 1914 | sp|Q764M9|CXCR4_PIG C-X-C chemokine receptor type 4 OS = Sus scrofa OX = 9823 GN = CXCR4 PE = 2 SV = 1 |
| B-cell lymphoma-2 | evm.TU.Scaffold38.508 | 0.849 | 0.440 | k02470 | 222 | sp|P02478|GN = bcl-2-like protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Zhang, Y.; Liang, X.; Lou, B.; Zhu, J. Effects of Glutamate on Growth Performance, Gut Digestion and Antioxidant Capacity in Juvenile Little Yellow Croaker. Fishes 2025, 10, 188. https://doi.org/10.3390/fishes10040188
Liu R, Zhang Y, Liang X, Lou B, Zhu J. Effects of Glutamate on Growth Performance, Gut Digestion and Antioxidant Capacity in Juvenile Little Yellow Croaker. Fishes. 2025; 10(4):188. https://doi.org/10.3390/fishes10040188
Chicago/Turabian StyleLiu, Ruining, Yu Zhang, Xiao Liang, Bao Lou, and Junquan Zhu. 2025. "Effects of Glutamate on Growth Performance, Gut Digestion and Antioxidant Capacity in Juvenile Little Yellow Croaker" Fishes 10, no. 4: 188. https://doi.org/10.3390/fishes10040188
APA StyleLiu, R., Zhang, Y., Liang, X., Lou, B., & Zhu, J. (2025). Effects of Glutamate on Growth Performance, Gut Digestion and Antioxidant Capacity in Juvenile Little Yellow Croaker. Fishes, 10(4), 188. https://doi.org/10.3390/fishes10040188





