Structures Associated with Oogenesis and Embryonic Development during Intraovarian Gestation in Viviparous Teleosts (Poeciliidae)
Abstract
:1. Ovarian Adaptations of Viviparous Teleosts
2. The Family Poeciliidae
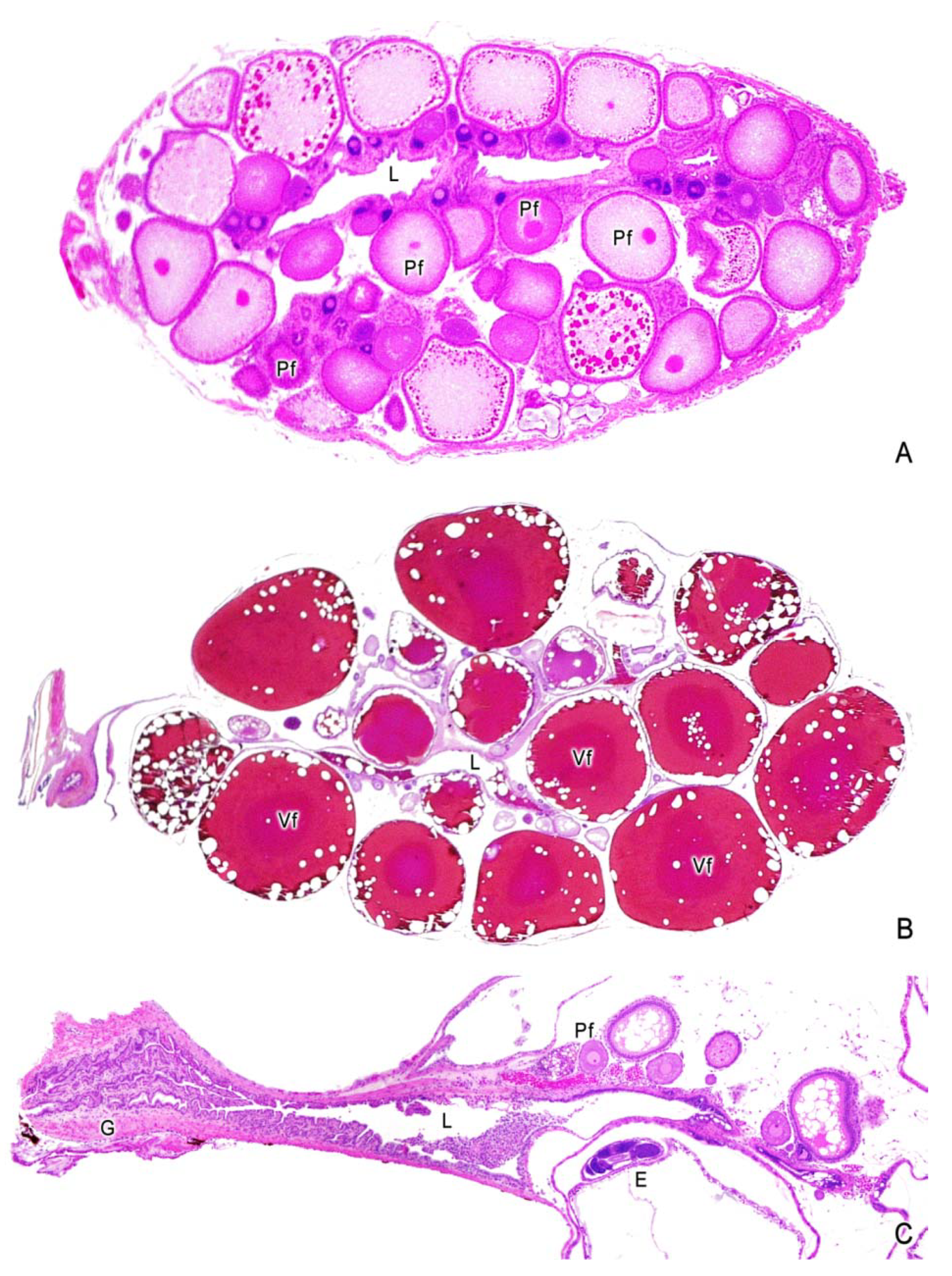
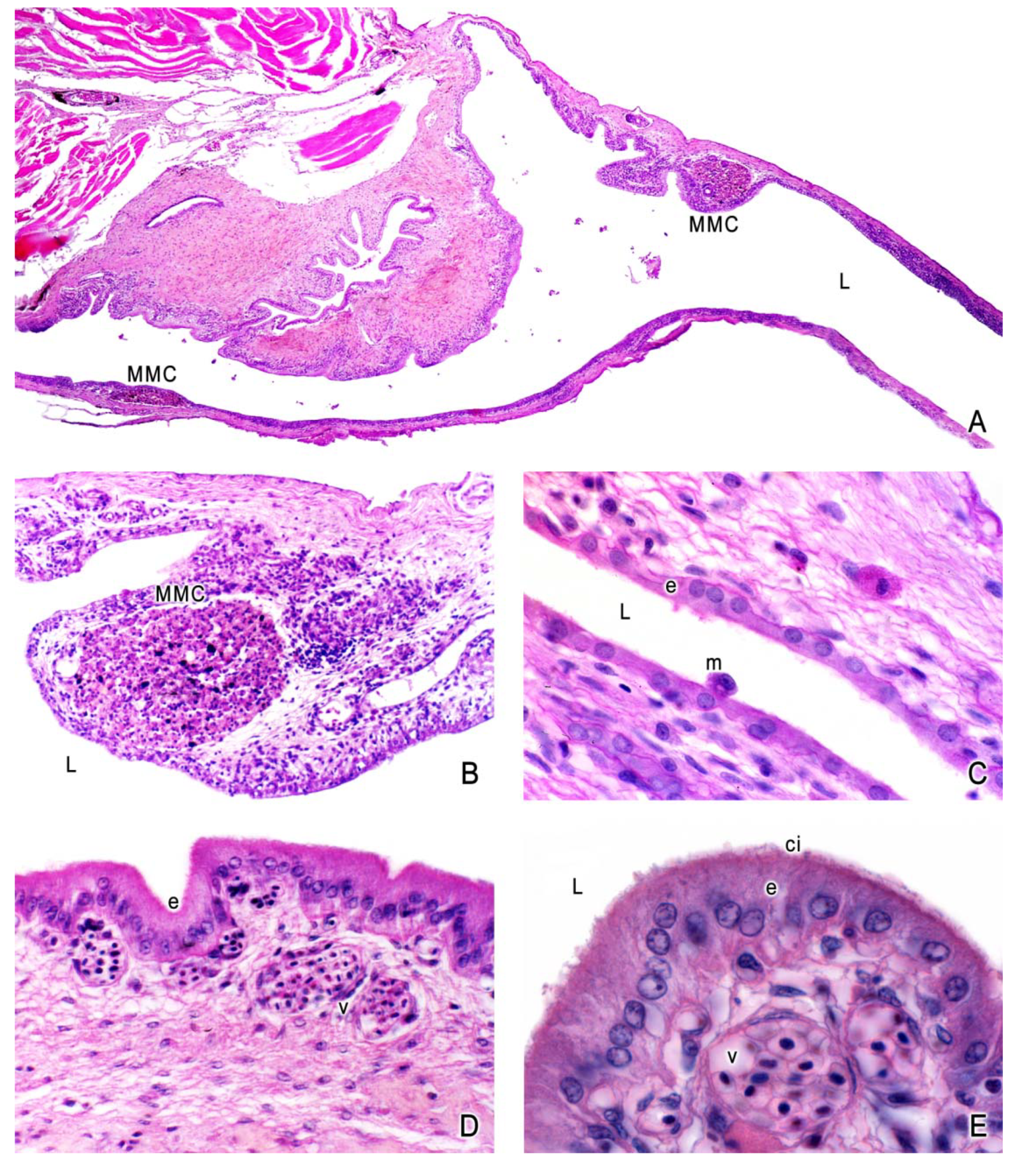
3. The Ovary of Poeciliids
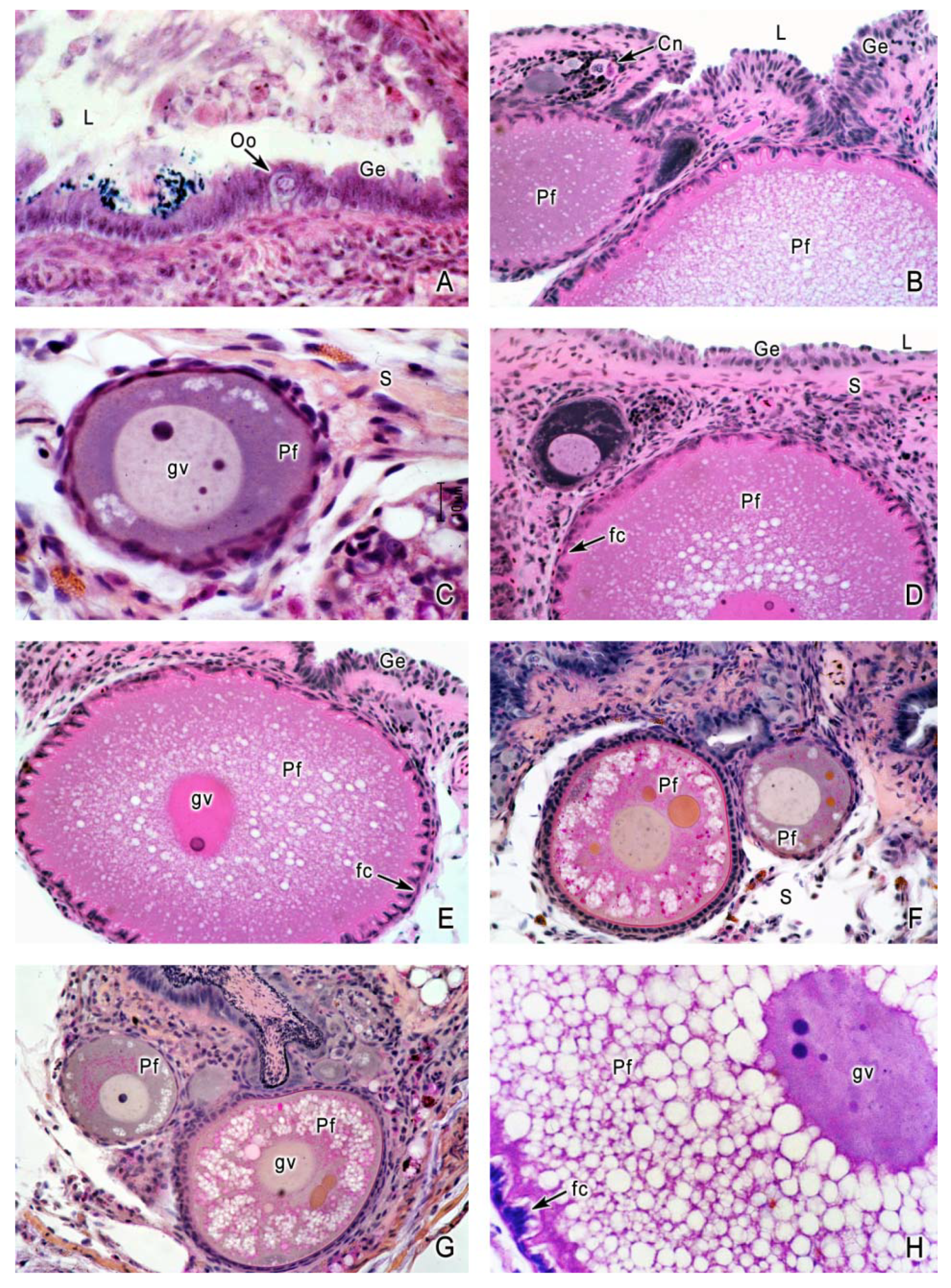
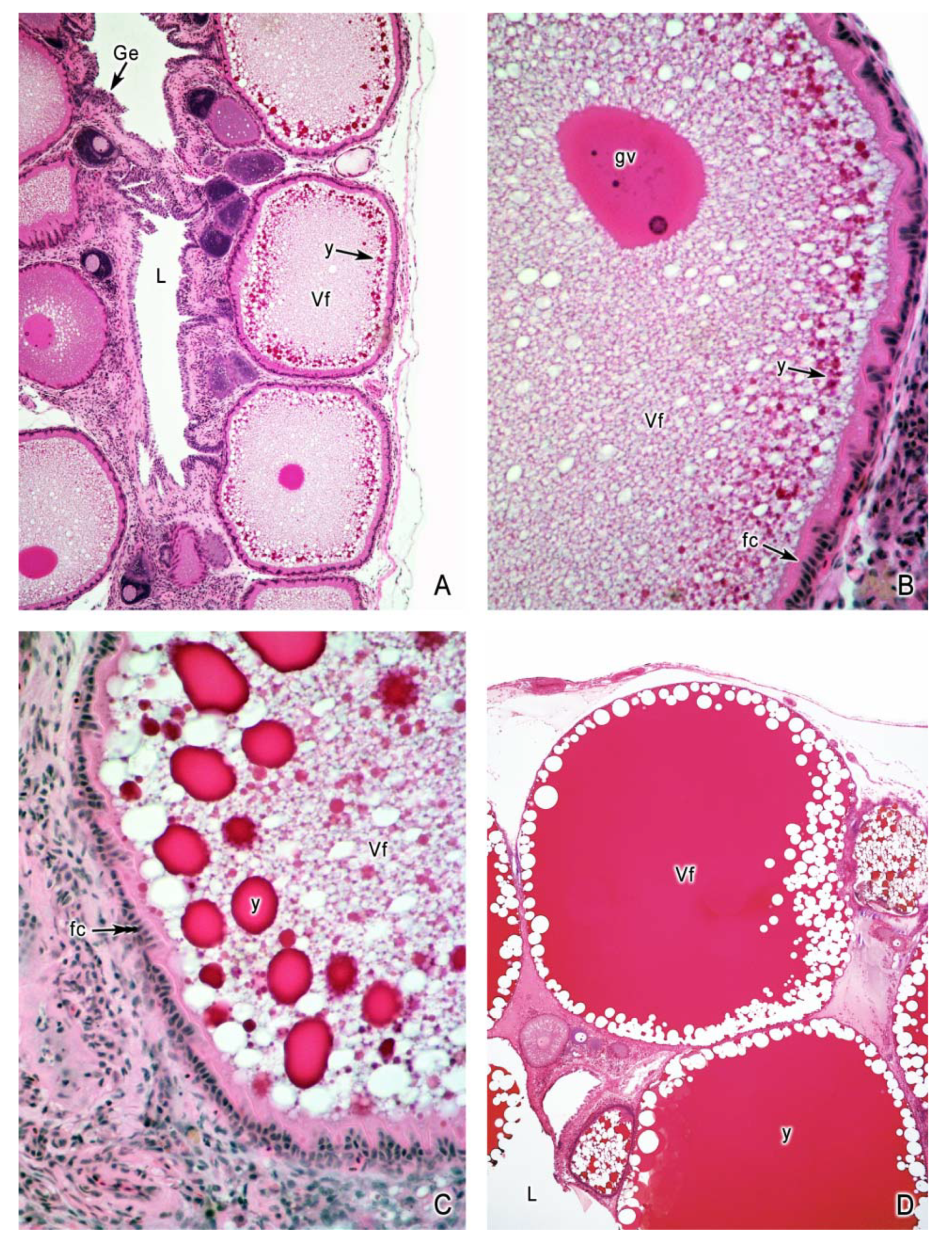
4. Oogenesis
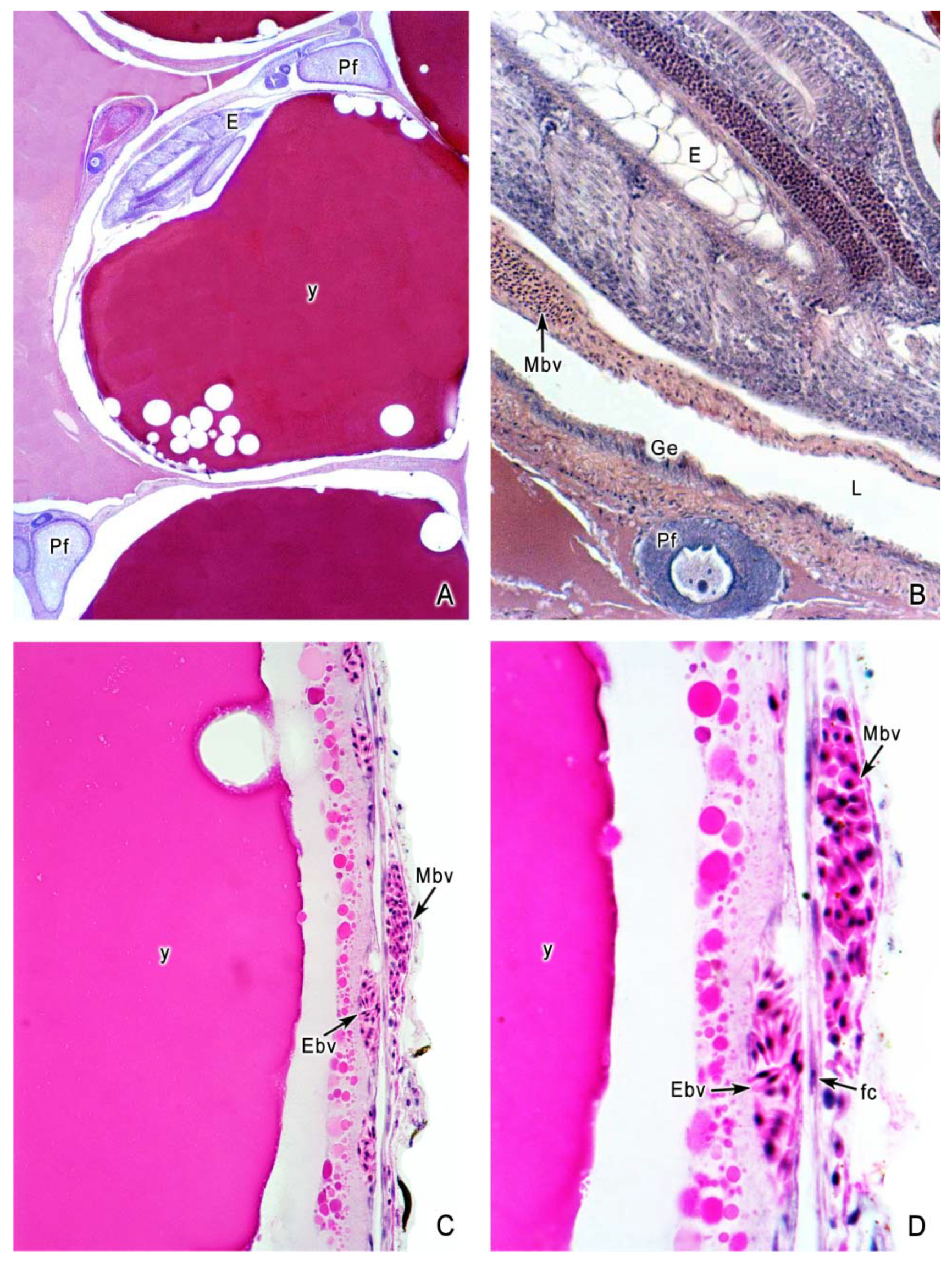
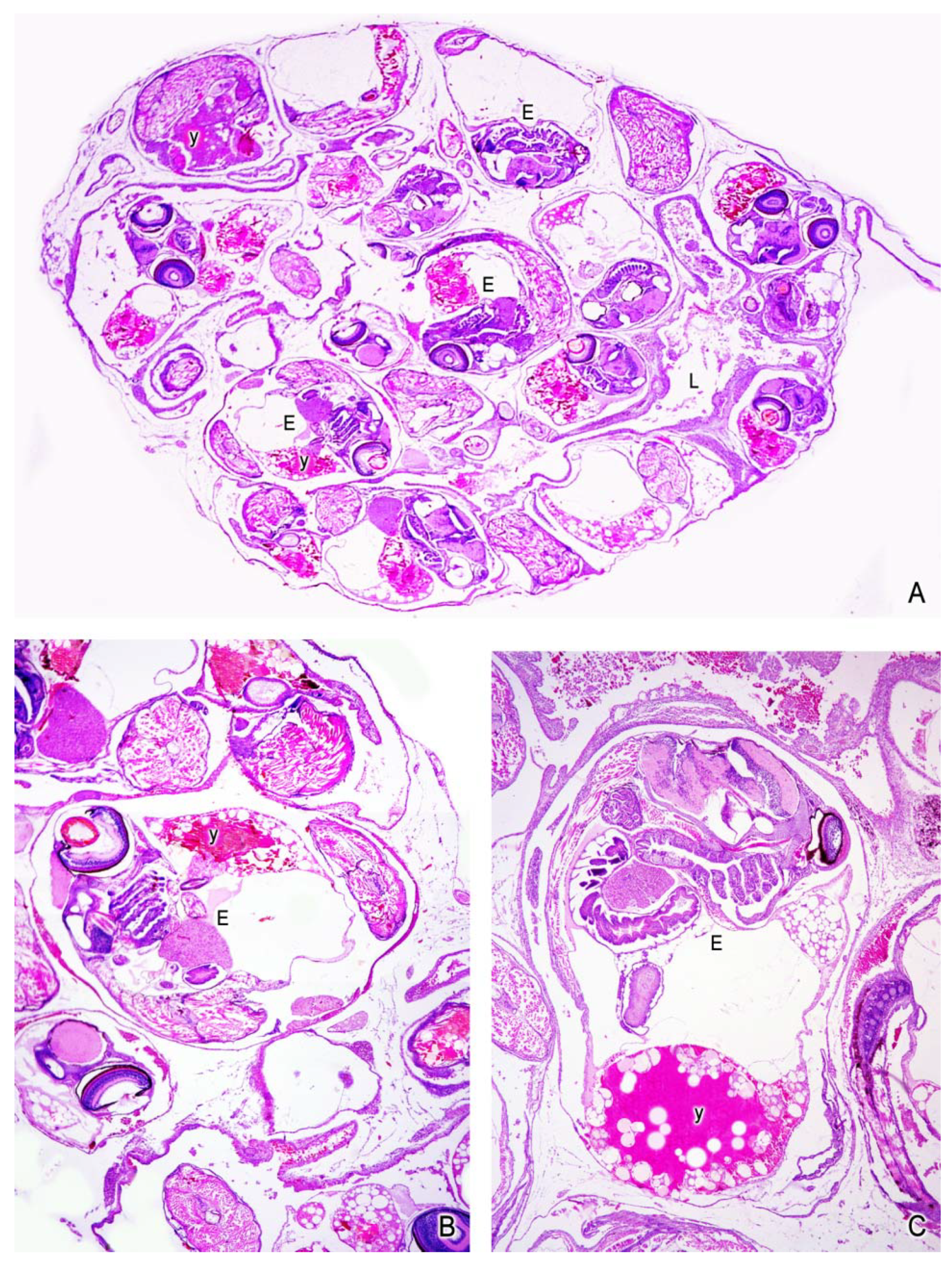
5. The Intraovarian Gestation in Poeciliids
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Froese, R.; Pauly, D. Fish Base, (10/2018) version; World Wide Web Electronic Publication, Naturalis, Leiden, the Netherlands. 2018. Available online: http://www.catalogueoflife.org/annual-checklist/2018/ (accessed on 19 June 2019).
- Rosen, D.E.; Bailey, R.M. The poeciliid fishes (Cyprinodontiformes), their structure, zoogeography, and systematics. Bull. Am. Museum Nat. Hist. 1963, 126, 11–76. [Google Scholar]
- Parenti, L.R. A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha). Bull. Am. Museum Nat. Hist. 1981, 168, 335–557. [Google Scholar]
- Parenti, L.R.; Grier, H.J. Evolution and phylogeny of gonad morphology in bony fishes. Integr. Comp. Biol. 2004, 44, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.G. Convergent evolution of viviparity, matrotrophy and specializations for fetal nutrition in reptiles and other vertebrates. Am. Zool. 1992, 32, 313–321. [Google Scholar] [CrossRef]
- Blackburn, D.G. Evolution of vertebrate viviparity and specializations for fetal nutrition: A quantitative and qualitative analysis. J. Morphol. 2015, 276, 961–990. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.C.; Grier, H.J.; De la Rosa Cruz, G.; García Alarcón, A. Modifications in ovarian and testicular morphology associated with viviparity in teleosts. In Reproductive Biology and Phylogeny of Fish (Agnatha and Bony Fishes), 1st ed.; Jamieson, B., Ed.; Science Publishers, Inc.: Enfield, NH, USA; Plymouth, UK, 2009; pp. 85–117. [Google Scholar]
- Wourms, J.P. Viviparity: The maternal-fetal relationship in fishes. Am. Zool. 1981, 21, 473–515. [Google Scholar] [CrossRef]
- Uribe, M.C.; Aguilar-Morales, M.; De la Rosa-Cruz, G.; García-Alarcón, A.; Campuzano-Caballero, J.C.; Guerrero-Estévez, S.M. Ovarian structure and embryonic traits associated with viviparity in poeciliids and goodeids. In Viviparous Fishes II, 1st ed.; Uribe, M.C., Grier, H.J., Eds.; New Life Publications: Homestead, FL, USA, 2010; pp. 211–229. [Google Scholar]
- Grier, H.J.; Uribe, M.C.; Parenti, L.R.; Lo Nostro, F.L.; Mims, S.D. Constancy of the germinal epithelium through 500 million years of vertebrate evolution. J. Morphol. 2016, 277, 1014–1044. [Google Scholar] [CrossRef]
- Dodd, J.M. The structure of the ovary of non-mammalian vertebrates. In The Ovary, 1st ed.; Zuckerman, S., Weir, B.J., Eds.; Ac. Press: New York, NY, USA, 1977; Volume 1, pp. 219–263. [Google Scholar]
- Hoar, W.S. Reproduction. In Fish Physiology, 1st ed.; Hoar, W.S., Randall, D.J., Eds.; Ac. Press: New York, NY, USA; London, UK, 1969; Volume 3, pp. 1–72. [Google Scholar]
- Turner, C.L. Viviparity in teleost fishes. Sci. Mon. 1947, 65, 508–518. [Google Scholar]
- Wourms, P.J.; Grove, B.D.; Lombardi, J. The maternal-embryonic relationship in viviparous fishes. In Fish Physiology, 1st ed.; Hoar, W.S., Randal, D.J., Eds.; Ac Press, Inc.: New York, NY, USA, 1988; pp. 1–134. [Google Scholar]
- Campuzano-Caballero, J.C.; Uribe, M.C. Structure of the female gonoduct of the viviparous teleost Poecilia reticulata (Poeciliidae) during non-gestation and gestation stages. J. Morphol. 2014, 275, 247–257. [Google Scholar] [CrossRef]
- Uribe, M.C.; Grier, H.J. Insemination, intrafollicular fertilization and development of the fertilization plug during gestation in Heterandria formosa (Poeciliidae). J. Morphol. 2018, 279, 970–980. [Google Scholar] [CrossRef]
- Potter, H.; Kramer, C.R. Ultrastructural observations on sperm storage in the ovary of the platyfish, Xiphophorus maculatus (Teleostei: Poeciliidae): The role of the duct epithelium. J. Morphol. 2000, 245, 110–129. [Google Scholar] [CrossRef]
- Wourms, J.P. Functional morphology, development and evolution of trophotaeniae. In Viviparous Fishes, 1st ed.; Uribe, M.C., Grier, H.J., Eds.; New Life Publications: Homestead, FL, USA, 2005; pp. 217–242. [Google Scholar]
- Marsh-Matthews, E.; Deaton, R.; Brooks, M. Survey of matrotrophy in lecithotrophic poeciliids. In Viviparous Fishes II, 1st ed.; Uribe, M.C., Grier, H.J., Eds.; New Life Publications: Homestead, FL, USA, 2010; pp. 255–258. [Google Scholar]
- Blackburn, D.G.; Starck, J.M. Morphological specializations for fetal maintenance in viviparous vertebrates: An introduction and historical retrospective. J. Morphol. 2015, 276, E1–E16. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.N.; Reznick, D.N. Life-history evolution in the fish genus Poecilia (Poeciliidae: Cyprinodontiformes: Subgenus Pamphorichthys): An evolutionary origin of extensive matrotrophy decoupled from superfetation. Biol. J. Linnean Soc. 2018, 125, 547–560. [Google Scholar] [CrossRef]
- Parenti, L.R.; Lo Nostro, F.L.; Grier, H.J. Reproductive histology of Tomeurus gracilis Eigenmann, 1909 (Teleostei: Atherinomorpha: Poeciliidae) with comments on evolution of viviparity in Atherinomorph fishes. J. Morphol. 2010, 271, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Lorier, E.; Berois, N. Reproducción y nutrición embrionaria en Cnesterodon decimmaculatus (Teleostei: Poeciliidae). Rev. Brasil. Biol. 1993, 55, 27–44. [Google Scholar]
- Stockwell, C.A.; Henkanaththegedara, S. Evolutionary conservation biology, Chapter 12. In The Evolutionary Ecology of the Livebearing Fishes, 1st ed.; Evans, J.P., Pilastro, A., Schlupp, I., Eds.; University of Chicago Press: Chicago, IL, USA, 2011; pp. 128–141. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; John Wiley & Sons: New York, NY, USA, 2016; pp. 378–380. [Google Scholar]
- Jourdan, J.; Wilhelm Miesen, F.; Zimmer, C.; Gasch, K.; Herder, F.; Schleucher, E.; Plath, M.; Bierbach, D. On the natural history of an introduced population of guppies (Poecilia reticulata Peters, 1859) in Germany. BioInvasions Rec. 2014, 3, 175–184. [Google Scholar] [CrossRef]
- Grier, H.J.; Uribe, M.C.; Parenti, L.R.; De la Rosa-Cruz, G. Fecundity, the germinal epithelium, and folliculogenesis in viviparous fishes. In Viviparous Fishes, 1st ed.; Uribe, M.C., Grier, H.J., Eds.; New Life Publications: Homestead, FL, USA, 2005; pp. 126–191. [Google Scholar]
- Grier, H.J.; Uribe, M.C.; Patiño, R. Chapter 2. The ovary, folliculogenesis and oogenesis in teleosts. In Reproductive Biology and Phylogeny of Fish (Agnatha and Bony Fishes), 1st ed.; Jamieson, B., Ed.; Science Publishers, Inc.: Enfield, NH, USA; Plymouth, UK, 2009; pp. 25–84. [Google Scholar]
- Campuzano-Caballero, J.C.; Uribe, M.C. Functional morphology of the gonoduct of the viviparous teleost Poeciliopsis gracilis (Heckel, 1848) (Poeciliidae). J. Morphol. 2017, 278, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.J. The ovarian cycle in the viviparous teleost Xiphophorus helleri. Biol. Bull. 1933, 64, 206–225. [Google Scholar] [CrossRef]
- Koya, Y.; Inoue, M.; Naruse, T.; Sawaguchi, S. Dynamics of oocyte and embryonic development during ovarian cycle of the viviparous mosquitofish Gambusia affinis. Fish. Sci. 2000, 66, 63–70. [Google Scholar] [CrossRef]
- Arcanjo, R.B.; de Souza, L.P.; Rezende, C.F.; Silva, J.R.F. Embryonic development and nourishment in the viviparous fish Poecilia vivipara (Cyprinodontiformes: Poeciliidae). Acta Zool. (Stockh.) 2014, 95, 493–500. [Google Scholar] [CrossRef]
- Ponce de León, J.L.P.; Rodríguez, R.; Acosta, M.; Uribe, M.C. Egg size and its relationship with fecundity, newborn length and female size in Cuban poeciliid fishes (Teleostei: Cyprinodontiformes). Ecol. Freshw. Fish. 2011, 20, 243–250. [Google Scholar] [CrossRef]
- Scrimshaw, N.S. 1946. Egg size in poeciliid fishes. Copeia 1963, 1, 20–23. [Google Scholar]
- Thibault, R.E.; Schultz, R.J. Reproductive adaptations among viviparous fishes (Cyprinodontiformes: Poeciliidae). Evolution 1978, 32, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.C.; Grier, H.J. Oogenesis of microlecithal oocytes in the viviparous teleost Heterandria formosa. J. Morphol. 2011, 272, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.L. Pseudoamnion, pseudochorion and follicular pseudoplacenta in poeciliid fishes. J. Morphol. 1940, 67, 59–89. [Google Scholar] [CrossRef]
- Constanz, J. Reproductive biology of the poeciliid fishes. In Ecology and Evolution of Live Bearing Fishes (Poeciliidae), 1st ed.; Meffe, G.K., Snelson, F.F., Eds.; Prentice Hall: New York, NY, USA, 1989; pp. 33–50. [Google Scholar]
- Marsh-Matthews, E.; Skierkowski, P.; DeMarais, A. Direct evidence for mother-to-embryo transfer of nutrients in the livebearing fish Gambusia geiseri. Copeia 2001, 1, 1–6. [Google Scholar] [CrossRef]
- Jollie, W.P.; Jollie, L.G. The fine structure of the ovarian follicle of the ovoviviparous poeciliid fish Lebistes reticulatus. I. Maturation of follicular epithelium. J. Morphol. 1964, 114, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Tlahuel, C.; Moreno-Mendoza, N.A.; Villagrán-Santa Cruz, M.; Zúñiga-Vega, J.J. Placental structures and their association with matrotrophy and superfetation in poeciliid fishes. Acta Zool. 2019, 100, 167–181. [Google Scholar] [CrossRef]
- Pollux, B.J.A.; Reznick, D.N. Matrotrophy limits a female’s ability to adaptively adjust offspring size and fecundity in fluctuating environments. Funct. Ecol. 2011, 25, 747–756. [Google Scholar] [CrossRef]
- Pollux, B.J.A.; Pires, M.N.; Banet, A.I.; Reznick, D.N. Evolution of placentas in the fish family Poeciliidae: An empirical study of macroevolution. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 271–289. [Google Scholar] [CrossRef]
- Reznick, D.N.; Callahan, H.; Llauredo, R. Maternal effects on offspring quality in poeciliid fishes. Am. Zool. 1996, 36, 147–156. [Google Scholar] [CrossRef]
- Riesch, R.; Plath, M.; Schlupp, I.; Marsh-Matthews, E. Matrotrophy in the cave molly: An unexpected provisioning strategy in an extreme environment. Evol. Ecol. 2010, 24, 789–801. [Google Scholar] [CrossRef]
- Kwan, L.; Fris, M.; Rodd, F.H.; Rowe, L.; Tuhela, L.; Panhuis, T.M. An examination of the variation in maternal placentae across the genus Poeciliopsis (Poeciliidae). J. Morphol. 2015, 276, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.N.; Arendt, J.; Reznick, D.N. The evolution of placentas and superfetation in the fish genus Poecilia (Cyprinodontiformes: Poeciliidae: Subgenera Micropoeciliia and Acanthophacelus). Biol. J. Linnean Soc. 2010, 99, 784–796. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, M.C.; De la Rosa Cruz, G.; García Alarcón, A.; Campuzano Caballero, J.C.; Guzmán Bárcenas, M.G. Structures Associated with Oogenesis and Embryonic Development during Intraovarian Gestation in Viviparous Teleosts (Poeciliidae). Fishes 2019, 4, 35. https://doi.org/10.3390/fishes4020035
Uribe MC, De la Rosa Cruz G, García Alarcón A, Campuzano Caballero JC, Guzmán Bárcenas MG. Structures Associated with Oogenesis and Embryonic Development during Intraovarian Gestation in Viviparous Teleosts (Poeciliidae). Fishes. 2019; 4(2):35. https://doi.org/10.3390/fishes4020035
Chicago/Turabian StyleUribe, Mari Carmen, Gabino De la Rosa Cruz, Adriana García Alarcón, Juan Carlos Campuzano Caballero, and María Guadalupe Guzmán Bárcenas. 2019. "Structures Associated with Oogenesis and Embryonic Development during Intraovarian Gestation in Viviparous Teleosts (Poeciliidae)" Fishes 4, no. 2: 35. https://doi.org/10.3390/fishes4020035
APA StyleUribe, M. C., De la Rosa Cruz, G., García Alarcón, A., Campuzano Caballero, J. C., & Guzmán Bárcenas, M. G. (2019). Structures Associated with Oogenesis and Embryonic Development during Intraovarian Gestation in Viviparous Teleosts (Poeciliidae). Fishes, 4(2), 35. https://doi.org/10.3390/fishes4020035



