Induced Spawning of F1 Wreckfish (Hāpuku) Polyprion oxygeneios Using a Synthetic Agonist of Gonadotropin-Releasing Hormone
Abstract
1. Introduction
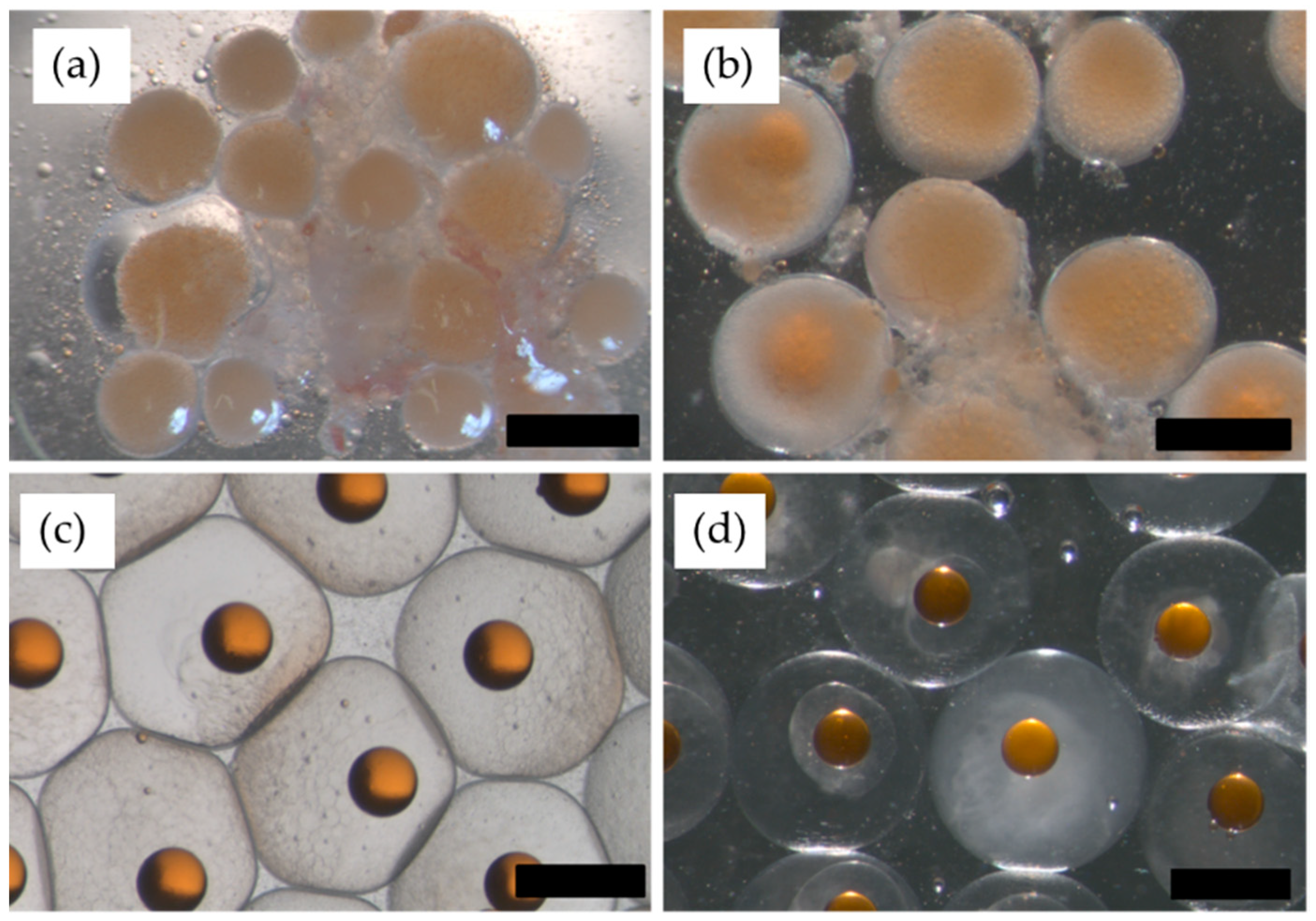
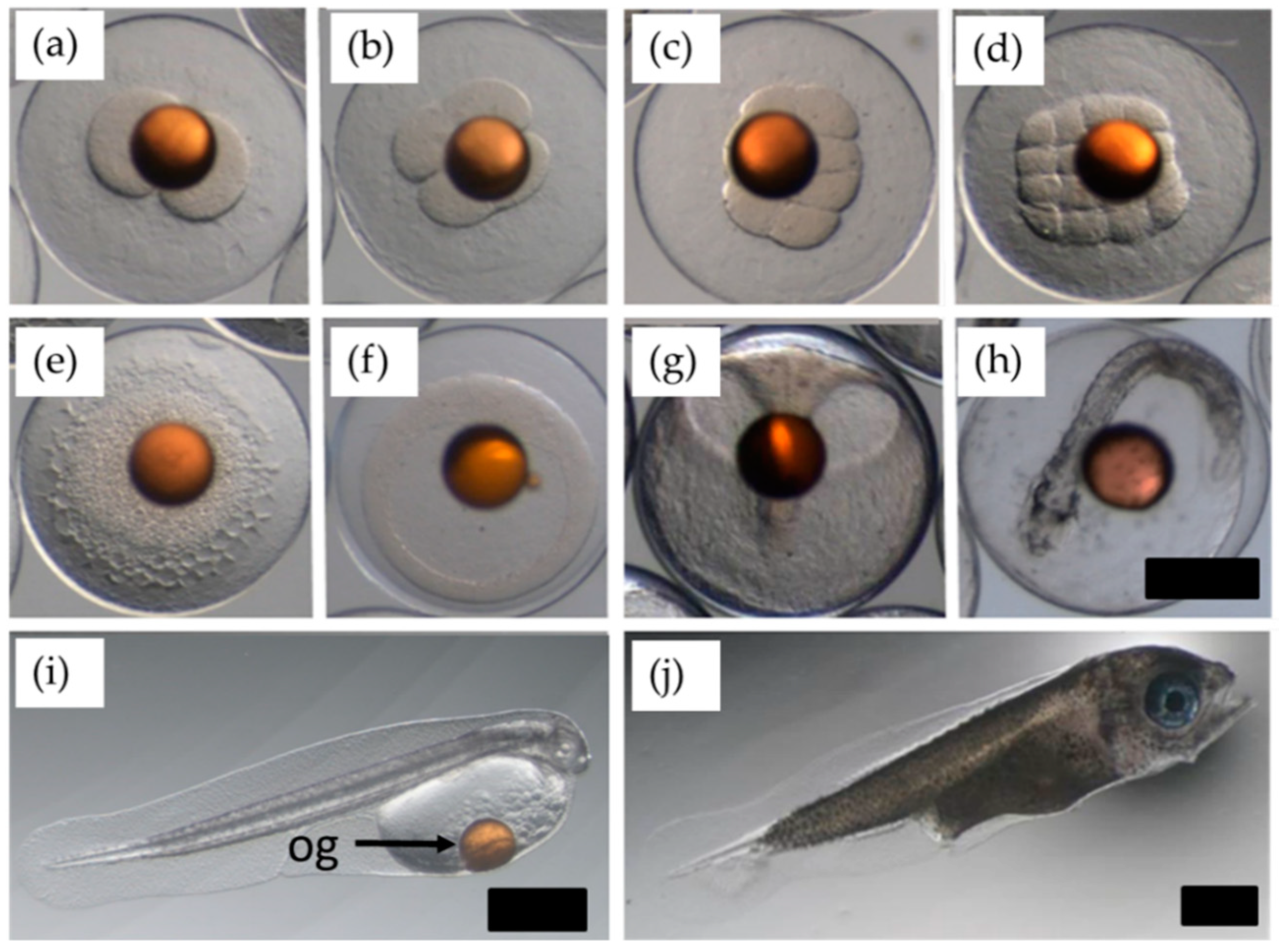
2. Results
2.1. 2013. Trial: Collection of Gametes for Strip-Spawning and In Vitro Fertilization
2.2. 2014. Trial 2A: Effect of GnRHa Administration Using EVAc Implants
2.3. 2014. Trial 2B: Effect of Different GnRHa Doses on Communal Spawning
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. 2013. Trial: Collection of Gametes for Strip-Spawning and In Vitro Fertilization
4.3. 2014. Trial 2A: Effect of GnRHa Administration Using EVAc Implants on Communal Spawning
4.4. 2014. Trial 2B: Effect of Different GnRHa Doses on Communal Spawning
4.5. Anesthesia and Assessment of Reproductive Status
4.6. Collection of Gametes for Strip-Spawning and In Vitro Fertilization
In Vitro Fertilization
4.7. Egg Sampling
4.7.1. Egg Collection and Assessment
4.7.2. Fertilization Rate and Fertilized Spawn Rate
4.7.3. Egg Incubation
4.7.4. Hatching Estimates
4.8. Genotyping (2014 Spawning Season Only)
4.9. Larval and Juvenile Rearing
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castro, L.I.; Alvarez-Lajonchère, L.; García-Aguilar, N.; de la Parra, M.I.A.; Rodríguez-lbarra, L.E. Generation cycle closure of the spotted rose snapper, Lutjanus guttatus, in captivity. Rev. Biol. Mar. Oceanog. 2012, 47, 333–337. [Google Scholar] [CrossRef]
- Symonds, J.E.; Walker, S.P.; van de Ven, I.; Marchant, A.; Irvine, G.; Pether, S.; Gublin, Y.; Bruce, M.; Anderson, R.M.; McEwan, K.M. Developing broodstock resources for farmed marine fish. Proc. N. Z. Soc. Anim. Prod. 2012, 72, 222–226. [Google Scholar]
- Camara, M.D.; Symonds, J.E. Genetic improvement of New Zealand aquaculture species: Programmes, progress and prospects. N. Z. J. Mar. Freshw. Res. 2014, 48, 466–491. [Google Scholar] [CrossRef]
- Migaud, H.; Bell, G.; Cabrita, E.; McAndrew, B.; Davie, A.; Bobe, J.; Herráez, M.P.; Carrillo, M. Gamete quality and broodstock management in temperate fish. Rev. Aquacult. 2013, 5, 194–223. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Zohar, Y. Use of GnRHa-delivery systems for the control of reproduction in fish. Rev. Fish Biol. Fish. 2001, 10, 463–491. [Google Scholar] [CrossRef]
- Zohar, Y.; Mylonas, C.C. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture 2001, 197, 99–136. [Google Scholar] [CrossRef]
- Mañanós, E.; Carrillo, M.; Sorbera, L.A.; Mylonas, C.C.; Asturiano, J.F.; Bayarri, M.J.; Zohar, Y. Luteinizing hormone and sexual steroid plasma levels after treatment of European sea bass with sustained-release delivery systems for gonadotropin-releasing hormone analogue. J. Fish Biol. 2002, 60, 328–339. [Google Scholar]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 2001, 197, 3–24. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef]
- Levavi-Sivan, B.; Bogerd, J.; Mañanós, E.L.; Gómez, A.; Lareyre, J.J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdá, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Bridges, C.; Gordin, H.; Ríos, A.B.; García, A.; De La Gándara, F.; Fauvel, C.; Suquet, M.; Medina, A.; Papadaki, M.; et al. Preparation and administration of gonadotropin-releasing hormone agonist (GnRHa) implants for the artificial control of reproductive maturation in captive-reared Atlantic bluefin tuna (Thunnus thynnus thynnus). Rev. Fish Sci. 2007, 15, 183–210. [Google Scholar] [CrossRef]
- Fernández-Palacios, H.; Schuchardt, D.; Roo, J.; Hernádez-Cruz, C.; Izquierdo, M. Spawn quality and GnRHa induction efficiency in longfin yellowtail (Seriola rivoliana) broodstock kept in captivity. Aquaculture 2015, 435, 167–172. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Salone, S.; Biglino, T.; de Mello, P.H.; Fakriadis, I.; Sigelaki, I.; Duncan, N. Enhancement of oogenesis/spermatogenesis in meagre Argyrosomus regius using a combination of temperature control and GnRHa treatments. Aquaculture 2016, 464, 323–330. [Google Scholar] [CrossRef]
- Amezawa, K.; Yazawa, R.; Takeuchi, Y.; Yoshizaki, G. Spawning induction of blue mackerel Scomber australasicus and Eastern little tuna Euthynnus affinis by oral administration of a crude gonadotropin-releasing hormone analogue. Fish. Sci. 2018, 84, 495–504. [Google Scholar] [CrossRef]
- Crim, L.W.; Sherwood, N.M.; Wilson, C.E. Sustained hormone release. II. Effectiveness of LHRH analog (LHRHa) administration by either single time injection or cholesterol pellet implantation on plasma gonadotropin levels in a bioassay model fish, the juvenile rainbow trout. Aquaculture 1988, 74, 87–95. [Google Scholar] [CrossRef]
- Lokman, P.M.; Wylie, M.J.; Downes, M.; Di Biase, A.; Damsteegt, E.L. Artificial induction of maturation in female silver eels, Anguilla australis: The benefits of androgen pre-treatment. Aquaculture 2015, 437, 111–119. [Google Scholar] [CrossRef]
- Forniés, M.A.; Mañanós, E.; Carrillo, M.; Rocha, A.; Laureau, S.; Mylonas, C.C.; Zohar, Y.; Zanuy, S. Spawning induction of individual European sea bass females (Dicentrarchus labrax) using different GnRHa-delivery systems. Aquaculture 2001, 202, 221–234. [Google Scholar] [CrossRef]
- Lee, C.S.; Tamaru, C.S.; Kelley, C.D. Technique for making chronic-release LHRH-A and 17α-methyltestosterone pellets for intramuscular implantation in fishes. Aquaculture 1986, 59, 161–168. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Woods, L.C.; Thomas, P.; Zohar, Y. Endocrine profiles of female striped bass (Morone saxatilis) in captivity, during post-vitellogenesis and induction of final oocyte maturation via controlled release GnRHa-delivery systems. Gen. Comp. Endocrinol. 1998, 110, 276–289. [Google Scholar] [CrossRef]
- Symonds, J.E.; Walker, S.P.; Pether, S.; Gublin, Y.; McQueen, D.; King, A.; Irvine, G.W.; Setiawan, A.N.; Forsythe, J.A.; Bruce, M. Developing yellowtail kingfish (Seriola lalandi) and hāpuku (Polyprion oxygeneios) for New Zealand aquaculture. N. Z. J. Mar. Freshw. Res. 2014, 48, 371–384. [Google Scholar] [CrossRef]
- Wylie, M.J.; Setiawan, A.N.; Irvine, G.W.; Symonds, J.E.; Elizur, A.; Dos Santos, M.; Lokman, P.M. Ovarian development of captive F1 wreckfish (hāpuku) Polyprion oxygeneios under constant and varying temperature regimes—Implications for broodstock management. Gen. Comp. Endocrinol. 2018, 257, 86–96. [Google Scholar] [CrossRef]
- Anderson, S.A.; Salinas, I.; Walker, S.P.; Gublin, Y.; Pether, S.; Kohn, Y.Y.; Symonds, J.E. Early development of New Zealand hapuku Polyprion oxygeneios eggs and larvae. J. Fish Biol. 2012, 80, 555–571. [Google Scholar] [CrossRef]
- Tromp, J.J.; Jones, P.L.; Symonds, J.E.; Walker, S.P.; Pope, A.; Pether, S.M.; Afonso, L.O. Effects of commercial diets and temperature on the growth performance and stress response of hāpuku (Polyprion oxygeneios). Aquaculture 2016, 452, 128–133. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50, 195–219. [Google Scholar] [CrossRef]
- Zohar, Y.; Pagelson, G.; Tosky, M. Daily changes in reproductive hormone levels in the female gilthead seabream Sparus aurata at the spawning period. In Reproduction in Fish: Basic and Applied Aspects of Endocrinology and Genetics; Zohar, Y., Breton, B., Eds.; INRA Press: Paris, France, 1988; pp. 119–125. [Google Scholar]
- Rasines, I.; Gómez, M.; Martín, I.; Rodríguez, C.; Mañanós, E.; Chereguini, O. Artificial fertilization of Senegalese sole (Solea senegalensis): Hormone therapy administration methods, timing of ovulation and viability of eggs retained in the ovarian cavity. Aquaculture 2012, 326, 129–135. [Google Scholar] [CrossRef]
- Francis, M.P.; Mulligan, K.P.; Davies, N.M.; Beentjes, M.P. Age and growth estimates for New Zealand hāpuku, Polyprion oxygeneios. Fish. Bull. 1999, 97, 227–242. [Google Scholar]
- Steven, C.; Gothilf, Y.; Holland, M.C.H.; Stubblefield, J.; Mylonas, C.C.; Zohar, Y. Differential expression of the three GnRH genes in wild and captive striped bass, Morone saxatilis, in response to natural and hormonally induced maturation. In Reproductive Physiology of Fish; Norberg, B., Kjesbu, O.S., Taranger, G.L., Andersson, E., Eds.; University of Bergen: Bergen, Norway, 2000; p. 66. [Google Scholar]
- Newman, D.M.; Jones, P.L.; Ingram, B.A. Age-related changes in ovarian characteristics, plasma sex steroids and fertility during pubertal development in captive female Murray cod Maccullochella peelii peelii. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 150, 444–451. [Google Scholar] [CrossRef]
- Lastein, S.; Gregersen, F.; Døving, K.B. Seasonal variations in olfactory sensory neurons—Fish sensitivity to sex pheromones explained? Chem. Senses 2008, 33, 119–123. [Google Scholar]
- Munakata, A.; Kobayashi, M. Endocrine control of sexual behavior in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 456–468. [Google Scholar] [CrossRef]
- Rekwot, P.I.; Ogwu, D.; Oyedipe, E.O.; Sekoni, V.O. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 2001, 65, 157–170. [Google Scholar] [CrossRef]
- Carazo, I.; Martin, I.; Hubbard, P.; Chereguini, O.; Maatanas, E.; Canário, A.; Duncan, N. Reproductive behaviour, the absence of reproductive behaviour in cultured (G1 generation) and chemical communication in the Senegalese sole (Solea senegalensis). Indian J. Sci. Technol. 2011, 4, 96–97. [Google Scholar]
- Meiri, I.; Gothilf, Y.; Zohar, Y.; Elizur, A. Physiological changes in the spawning gilthead seabream Sparus aurata, succeeding the removal of males. J. Exp. Zool. 2002, 292, 555–564. [Google Scholar] [CrossRef]
- Soyano, K.; Izumida, D.; Nakachi, M.; Murata, R.; Nakamura, M. The final oocyte maturation regulated by the pheromone released from male in the honeycomb grouper. In Proceedings of the 7th International Symposium on Fish Endocrinology, Buenos Aries, Argentina, 3–6 September 2012. [Google Scholar]
- Rainwater, F.L. Courtship and reproductive behavior of the Siamese fighting fish Betta splendens Regan (Pisces, Belontiidae). Proc. OK Acad. Sci. 1967, 47, 98–114. [Google Scholar]
- Pankhurst, N.W. Hormones and reproductive behaviour in male damselfish. Bull. Mar. Sci. 1995, 57, 569–581. [Google Scholar]
- Mohagheghi Samarin, A.; Policar, T.; Lahnsteiner, F. Fish oocyte aging and its effects on egg quality. Rev. Fish. Sci. Aquac. 2015, 23, 302–314. [Google Scholar] [CrossRef]
- Papadaki, M.; Peleteiro, J.; Alvarez-Blázquez, B.; Rodríguez Villanueva, J.; Linares, F.; Vilar, A.; Pérez Rial, E.; Lluch, N.; Fakriadis, I.; Sigelaki, I.; et al. Description of the annual reproductive cycle of wreckfish Polyprion americanus in captivity. Fishes 2018, 3, 43. [Google Scholar] [CrossRef]
- Whatmore, P.; Nguyen, N.H.; Miller, A.; Lamont, R.; Powell, D.; D’Antignana, T.; Bubner, E.; Elizur, A.; Knibb, W. Genetic parameters for economically important traits in yellowtail kingfish Seriola lalandi. Aquaculture 2013, 400, 77–84. [Google Scholar] [CrossRef]
- Bobe, J. Egg quality in fish: Present and future challenges. Anim. Front. 2015, 5, 66–72. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Scott, A.P.; Vermeirssen, E.L.; Zohar, Y. Changes in plasma gonadotropin II and sex steroid hormones, and sperm production of striped bass after treatment with controlled-release gonadotropin-releasing hormone agonist-delivery systems. Biol. Reprod. 1997, 57, 669–675. [Google Scholar] [CrossRef][Green Version]
- Rainis, S.; Mylonas, C.C.; Kyriakou, Y.; Divanach, P. Enhancement of spermiation in European sea bass (Dicentrarchus labrax) at the end of the reproductive season using GnRHa implants. Aquaculture 2003, 219, 873–890. [Google Scholar] [CrossRef]
- Vermeirssen, E.L.; de Quero, C.M.; Shields, R.J.; Norberg, B.; Kime, D.E.; Scott, A.P. Fertility and motility of sperm from Atlantic halibut (Hippoglossus hippoglossus) in relation to dose and timing of gonadotrophin-releasing hormone agonist implant. Aquaculture 2004, 230, 547–567. [Google Scholar] [CrossRef]
- Fauvel, C.; Suquet, M.; Severe, A.; Mylonas, C.C.; Papandroulakis, N. Slow-release GnRHa treatment prevented atresia during vitellogenesis and induced ovulation of captive wreckfish (Polyprion americanus). Cybium 2008, 32, 191. [Google Scholar]
- Butts, I.A.; Trippel, E.A.; Litvak, M.K. The effect of sperm to egg ratio and gamete contact time of fertilisation success in Atlantic Cod Gadus morhuna L. Aquaculture 2009, 286, 89–94. [Google Scholar] [CrossRef]
- Kohn, Y.Y.; Symonds, J.E. Evaluation of egg quality parameters as predictors of hatching success and early larval survival in hāpuku (Polyprion oxygeneios). Aquaculture 2012, 342, 42–47. [Google Scholar] [CrossRef]


| Temp Group | ♀ | Body Weight (kg) | GnRHa Dose (µg/kg−1) × 2 | First Injection (Time Zero) Date: 11 November 2013 | Second Injection (24 h Post-First Injection) Date: 12 November 2013 | Fertilization Rate (%) | ||
|---|---|---|---|---|---|---|---|---|
| Mean OD (mm) | Ovulated Egg Volume (mL) | Mean OD (mm) | Ovulated Egg Volume (mL) | |||||
| VT | A | 9.06 | 30 | 1.24 | 0 | 1.25 | 34 | 0 |
| VT | B | 8.45 | 30 | 1.24 | 0 | 1.85 | 93 | 84 |
| VT | C | 10.12 | 30 | 1.20 | 0 | 1.20 | 0 | - |
| VT | D | 10.43 | 30 | 1.29 | 0 | 1.22 | 18 | 0 |
| CT | I | 7.86 | 30 | 1.2 | 0 | 1.13 | 0 | - |
| CT | J | 5.80 | 30 | No biopsy | 0 | 1.06 | 24 | 0 |
| CT | K | 6.76 | 30 | 1.07 | 0 | No biopsy | 0 | - |
| VT | E | 7.28 | 0 | 1.30 | 0 | 1.24 | 0 | - |
| VT | F | 9.58 | 0 | 0.94 | 0 | 1.07 | 0 | - |
| VT | G | 10.83 | 0 | 1.16 | 0 | 1.08 | 0 | - |
| VT | H | 8.35 | 0 | 1.13 | 0 | 1.09 | 0 | - |
| CT | L | 7.05 | 0 | 1.07 | 0 | 1.07 | 0 | - |
| CT | M | 9.55 | 0 | 0.94 | 0 | No biopsy | (female died) | - |
| Trial | Tank | Target GnRHa Dose (µg/kg−1) | Spawning (Yes/No) | No. Batches Genotyped | Total No. Females in Tank | No. Females Detected by Genotyping | Total No. Males in Tank | No. Males Detected by Genotyping |
|---|---|---|---|---|---|---|---|---|
| Trial 2A | Tank 1 | 100 | Yes | 1 | 7 | 1 | 5 | 1 |
| Tank 2 | 100 | Yes | 5 | 6 | 3 | 5 | 1 | |
| Tank 3 | 100 | Yes | 2 | 8 | 3 | 9 | 3 | |
| Tank 4 | 0 | No | - | 6 | - | 4 | - | |
| Tank 5 | 0 | No | - | 4 | - | 11 | - | |
| Tank 6 | 0 | No | - | 8 | - | 8 | - | |
| Trial 2B | Tank 7 | 100 | Yes | 9 | 5 | 3 | 9 | 5 |
| Tank 8 | 50 | No | - | 5 | - | 9 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wylie, M.J.; Setiawan, A.N.; Irvine, G.W.; Elizur, A.; Zohar, Y.; Symonds, J.E.; Lokman, P.M. Induced Spawning of F1 Wreckfish (Hāpuku) Polyprion oxygeneios Using a Synthetic Agonist of Gonadotropin-Releasing Hormone. Fishes 2019, 4, 41. https://doi.org/10.3390/fishes4030041
Wylie MJ, Setiawan AN, Irvine GW, Elizur A, Zohar Y, Symonds JE, Lokman PM. Induced Spawning of F1 Wreckfish (Hāpuku) Polyprion oxygeneios Using a Synthetic Agonist of Gonadotropin-Releasing Hormone. Fishes. 2019; 4(3):41. https://doi.org/10.3390/fishes4030041
Chicago/Turabian StyleWylie, Matthew J., Alvin N. Setiawan, Glen W. Irvine, Abigail Elizur, Yonathan Zohar, Jane E. Symonds, and P. Mark Lokman. 2019. "Induced Spawning of F1 Wreckfish (Hāpuku) Polyprion oxygeneios Using a Synthetic Agonist of Gonadotropin-Releasing Hormone" Fishes 4, no. 3: 41. https://doi.org/10.3390/fishes4030041
APA StyleWylie, M. J., Setiawan, A. N., Irvine, G. W., Elizur, A., Zohar, Y., Symonds, J. E., & Lokman, P. M. (2019). Induced Spawning of F1 Wreckfish (Hāpuku) Polyprion oxygeneios Using a Synthetic Agonist of Gonadotropin-Releasing Hormone. Fishes, 4(3), 41. https://doi.org/10.3390/fishes4030041






