Yellowstone Lake Ecosystem Restoration: A Case Study for Invasive Fish Management
Abstract
:1. Introduction
2. Study Area and Focal Species
3. Predatory Fish Invasion and Initial Management Response
4. Development of a Conservation Strategy that Embraces Uncertainty
4.1. Scientific Review Panel
- Intensify existing lake trout suppression efforts for a minimum of 6 years.
- Maintain and enhance cutthroat trout monitoring.
- Develop a statistically robust lake trout monitoring program.
- Develop a lake trout suppression plan with benchmarks for control to increase program effectiveness and ensure the conservation of the Yellowstone Lake ecosystem through the coming decades.
4.2. Planning and Environmental Compliance
- Reduction in the long-term extinction risk for native fishes;
- Restoration and maintenance of the important ecological role of native fishes; and
- Creation of sustainable native fish angling and viewing opportunities for the public.
- A direct relationship exists between lake trout gillnetting effort and the number of lake trout captured.
- Cutthroat trout will recover naturally without large-scale supplementation (stocking) once lake trout are functionally removed.
- A significant lag (in time) in the recovery of cutthroat trout will occur following lake trout suppression (cutthroat trout recovery may not be immediately perceptible).
- Large-scale supplementation (stocking) of cutthroat trout from existing hatchery broods would be detrimental because it would reduce the genetic integrity of the population.
4.3. Conceptual Ecosystem Model and Hypothesized Linkages
- regional physical/chemical forces;
- biological introductions;
- angling;
- park infrastructure and operations; and
- local physical/chemical forces.
- biogeochemical cycling;
- productivity/biomass change;
- fish functional role;
- fish life history strategy; and
- avian/terrestrial fish consumers.
4.4. Desired Conditions
4.4.1. Primary Desired Condition
4.4.2. Secondary Desired Condition
4.4.3. Tertiary Desired Condition
5. Stakeholder Involvement and Fundraising to Support Conservation Actions
5.1. Yellowstone Fly Fishing Volunteer Program
5.2. Yellowstone Lake Workgroup
5.3. Yellowstone Forever Fund-Raising Partnership
6. Historical Development of the Gillnetting Program
7. Lake Trout Population Modeling and Gillnetting Effort Benchmarks
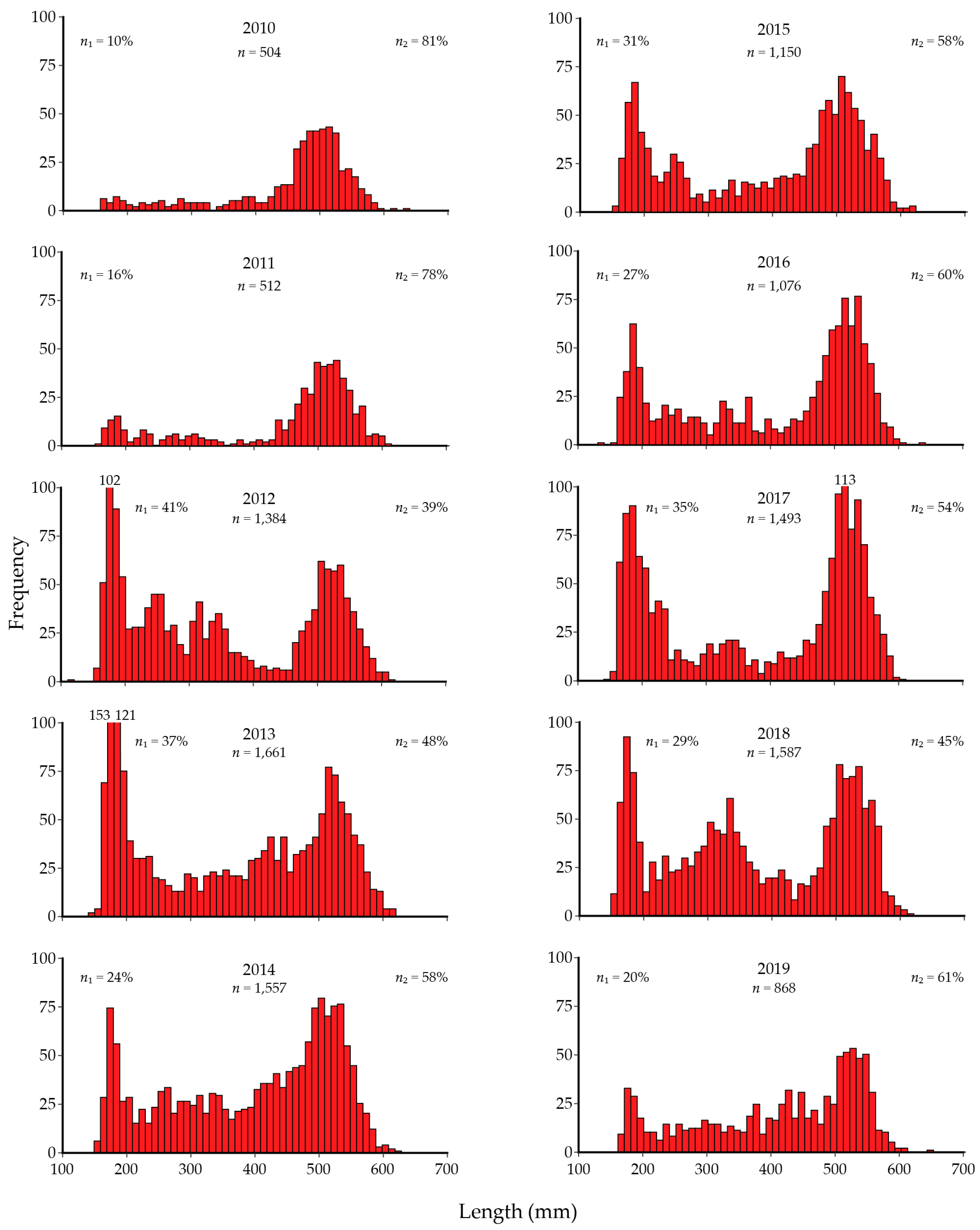
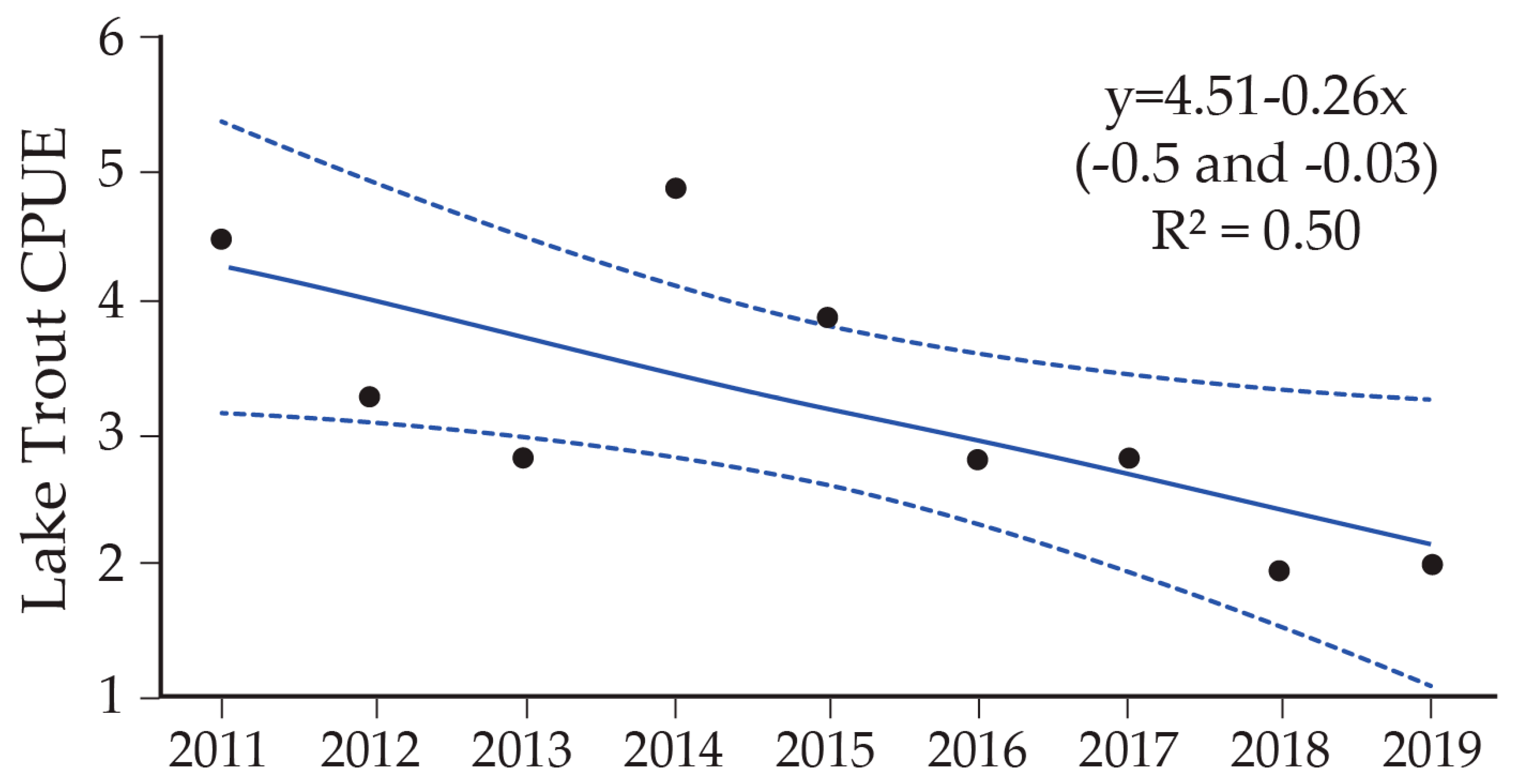
8. Lake Trout Population Response to Suppression Gillnettting
9. Cutthroat Trout Population Response to Lake Trout Suppression
10. Ecological Response to Lake Trout Suppression
11. Applied Research to Inform Decision Making
12. Need for Complementary Methods that Target Multiple Life Stages
13. Discovery of Nonnative Cisco in Yellowstone Lake
14. Discussion
14.1. Suppressing an Invasive Population Below Carrying Capacity
14.2. Why are Lake Trout Resilient to Suppression Gillnetting on Yellowstone Lake?
14.3. Transition to Suppression of Multiple Lake Trout Life Stages
15. Conclusions
16. Materials and Methods
16.1. Lake Trout Suppression Netting
16.2. Cutthroat Trout and Lake Trout Gillnet Assessment
16.3. Cutthroat Trout Tributary Spawner Assessment
16.4. Cutthroat Trout and Lake Trout Angler Catch
16.5. Lake Trout Population Modeling and Gillnetting Effort Benchmarks
16.6. Monitoring for Ecological Response
16.7. Permits and Ethical Aspects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Dedication
Appendix A
| Location | Taxon | Common Name | Scientific Name |
|---|---|---|---|
| Yellowstone | |||
| Birds | bald eagle | Haliaeetus leucocephalus (Linnaeus, 1766) | |
| common loon | Gavia immer (Brunnich, 1764) | ||
| osprey | Pandion haliaetus (Linnaeus, 1758) | ||
| trumpeter swan | Cygnus buccinator Richardson, 1832 | ||
| Fishes | cisco 1 | Coregonus artedi Lesueur, 1818 | |
| lake chub | Couesius plumbeus (Agassiz, 1850) | ||
| lake trout | Salvelinus namaycush (Walbaum, 1792) | ||
| longnose sucker | Catostomus catostomus (Forster, 1773) | ||
| redside shiner | Richardsonius balteatus (Richardson, 1836) | ||
| Yellowstone cutthroat trout 2 | Oncorhynchus clarkii bouvieri (Jordan and Gilbert, 1883) | ||
| Mammals | American bison | Bison bison (Linnaeus, 1758) | |
| American black bear | Ursus americanus Pallas, 1780 | ||
| gray wolf | Canis lupus Linnaeus, 1758 | ||
| grizzly bear | Ursus arctos (Linnaeus, 1758) | ||
| North American river otter | Lontra canadensis (Schreber, 1777) | ||
| Elsewhere | |||
| Fishes | common carp | Cyprinus carpio Linnaeus, 1758 | |
| Eurasian perch | Perca fluviatilis Linnaeus, 1758 | ||
| rock bass | Ambloplites rupestris (Rafinesque, 1817) | ||
| sea lamprey | Petromyzon marinus Linnaeus, 1758 | ||
| smallmouth bass | Micropterus dolomieu Lacepède, 1802 | ||
| yellow perch | Perca flavescens (Mitchill, 1814) |
| Year | Key Changes or Events | Public Outreach | Available URL |
|---|---|---|---|
| 1994 | Lake trout first discovered by an angler | NPS press releases | https://www.latimes.com/archives/la-xpm-1994-11-22-mn-424-story.html |
| US$10,000 reward offered for information leading to arrest | Lake trout wanted poster | http://doi.org/10.5281/zenodo.3873385 | |
| Lake trout must-kill angling regulation implemented | YNP angling regulations | https://www.nps.gov/yell/planyourvisit/upload/20FishReg_web.pdf | |
| 1995 | Scientific panel review guides management response | Report to the NPS Director | https://www.nps.gov/parkhistory/online_books/yell/trout_invasion.pdf |
| Public awareness of lake trout effect on birds and mammals | Numerous articles | https://www.nps.gov/articles/ys-25-1-shorts.htm | |
| 1996 | Lake trout telemetry locates spawning areas | Graduate research programs | https://www.researchgate.net/publication/34007273 |
| Student Conservation Association, beginning of long-term support | Internships on suppression crews | https://www.thesca.org/ | |
| Yellowstone Science, century of fisheries research and management | Special issue for the public | https://www.nps.gov/yell/learn/upload/YS_4_4_sm.pdf | |
| 1997 | Applied research documents lake trout effects on cutthroat trout | Peer-reviewed publication | https://doi.org/10.1890/1051-0761(2003)013[0023:EOILTO]2.0.CO;2 |
| 1998 | Whirling disease discovered in cutthroat trout | Peer-reviewed publication | https://doi.org/10.1577/H05-031.1 |
| Yellowstone fishes: ecology, history, and angling in the park | Book released | https://www.nps.gov/yell/learn/bookstore.htm | |
| 1999 | Angler creel survey conducted to estimate lake trout catch | Interviews with lake anglers | https://www.nps.gov/yell/learn/fishreports.htm |
| 2000 | Park visitor perceptions of lake trout evaluated | Yellowstone Science article | https://www.nps.gov/yell/learn/upload/YS_9_2_sm.pdf |
| 2001 | Gillnetting enhanced by specialized boat and dedicated crews | NPS technical report on-line | https://www.researchgate.net/publication/237614952 |
| Cutthroat trout catch-and-release only angling regulation | YNP angling regulations | https://www.nps.gov/yell/planyourvisit/upload/20FishReg_web.pdf | |
| 2002 | YNP native fish conservation reports for each year | Reports produced for the public | https://www.nps.gov/yell/learn/fishreports.htm |
| YNP fly fishing volunteer program initiated | Volunteers interact with NPS biologists | https://www.nps.gov/articles/ys-25-1-shorts.htm | |
| 2003 | Extent of whirling disease effect on cutthroat trout documented | Graduate research programs | https://doi.org/10.3354/dao071191 |
| 2004 | Montana State University, College of Engineering, Senior design | Investigate suppression alternatives | http://doi.org/10.5281/zenodo.3873394 |
| 2005 | Drought effects on cutthroat trout documented | Peer-reviewed publication | https://doi.org/10.1577/1548-8446(2005)30[10:NLTRIY]2.0.CO;2 |
| 2006 | Barbless hook only angling regulation implemented | YNP angling regulations | https://www.nps.gov/yell/planyourvisit/upload/20FishReg_web.pdf |
| 2006 | Yellowstone Science, cutthroat trout conservation | Special issue for the public | https://www.nps.gov/yell/learn/upload/YS_14_2_sm.pdf |
| 2007 | Regional drought highlights potential effects of climate change | YNP angling closures to park waters | https://www.ncdc.noaa.gov/sotc/drought/200708 |
| 2008 | Scientific panel review following cutthroat trout decline | Report to YNP Superintendent | https://www.nps.gov/yell/planyourvisit/upload/gresswell_final_updated_1_2010.pdf |
| Initiate research regarding alternate suppression techniques | Comprehensive literature review | http://files.cfc.umt.edu/cesu/NPS/MSU/2008/08Zale_YELL_trout%20embryos_lit%20review.pdf | |
| Weird but true! Researcher proposes jello to kill lake trout | Montana State University news release | https://nypost.com/2008/12/29/weird-but-true-2347/ | |
| 2009 | Contract gillnetting pilot phase begins | YNP native fish program report | https://www.nps.gov/yell/learn/fishreports.htm |
| Public scoping meetings for plan/ environmental assessment | Meetings and on-line comment period | https://parkplanning.nps.gov/projectHome.cfm?projectID=30504 | |
| 2010 | Native fish conservation plan/EA completed | Adaptive management plan | https://parkplanning.nps.gov/projectHome.cfm?projectID=30504 |
| Lake trout statistical-catch-at-age model created | Graduate research programs | https://doi.org/10.1139/cjfas-2019-0306 | |
| Aquatic trophic cascade documented | Peer-reviewed publication | https://doi.org/10.1577/T09-151.1 | |
| Public meetings at YNP gateway communities | Annually for information exchange | https://www.nps.gov/yell/learn/news/19013.htm | |
| 2011 | Scientific panel reviews implemented annually | Reports to YNP Superintendent | https://www.nps.gov/yell/planyourvisit/upload/Lake_Trout_Suppression_Workshop_LowRes_Accessible.pdf |
| Contract gillnetting fully implemented annually | YNP native fish program report | https://www.nps.gov/yell/learn/fishreports.htm | |
| Lake trout telemetry studies reinitiated | Public interpretive website | https://www.usgs.gov/centers/norock/science/yellowstone-lake-acoustic-biotelemetry-project-home-page | |
| Yellowstone Lake Workgroup formed | Memorandum of Understanding | http://doi.org/10.5281/zenodo.3873432 | |
| 2012 | Yellowstone Forever US$1,000,000 grant began annually | Save the Yellowstone cutthroat trout | https://www.yellowstone.org/what-we-do/native-fish/ |
| Trout Unlimited bloggers tour for conservation writers | Articles published on-line | http://www.sippingemergers.com/2012/08/setting-stage.html?m=1 | |
| 2013 | Native fish conservation program website | Public interpretive website | https://www.nps.gov/yell/learn/management/native-fish-conservation-program.htm |
| 2013 | Fund-raising by donating a lake trout telemetry tag | Trout Unlimited public website | https://eastyellowstonetu.org/images/savetheyellowstonecutthroat_2.html |
| Wyoming Wildlife and Natural Resource Trust grant US$771,000 | Wyoming Trout Unlimited | https://wwnrt.wyo.gov/ | |
| Grizzly bear predation links loss of native trout to migratory elk | Peer-reviewed publication | https://doi.org/10.1098/rspb.2013.0870 | |
| University science partner press releases | Articles published on-line | http://www.montana.edu/news/mountainsandminds/article.html?id=11921 | |
| 2014 | Public outreach for project support enhanced | Lake trout suppression FAQ website | https://www.usgs.gov/centers/norock/science/faq-invasive-lake-trout-yellowstone-lake |
| 2015 | Trout Unlimited document supporting science | Lake trout suppression FAQ report | http://wyomingtu.org/wp-content/uploads/2014/03/Science-Supporting-Management-of-Yellowstone-Lake-Fisheries.pdf |
| 2016 | NPS centennial celebration | Heightened public awareness | https://yellowstone.co/pdfs/centennial2016.pdf |
| Outdoor Writers Association of America Conference | Tour and articles about the program | https://owaa.org/2014/02/owaa-announces-location-2016-annual-conference/ | |
| Lake trout carcasses used to kill embryos on spawning sites | Graduate research programs | https://doi.org/10.1002/nafm.10259 | |
| 2017 | Yellowstone Science, native fish conservation issue | Special issue for the public | https://www.nps.gov/articles/series.htm?id=87AF9117-08B4-46BB-97B9C28B37A85BB2 |
| Sound of science in Yellowstone audio series | Podcast available online | https://www.nps.gov/yell/learn/photosmultimedia/onefishtwofish.htm | |
| 2018 | Journey through Yellowstone’s aquatic ecosystems | Interpretive video available online | https://www.nps.gov/yell/learn/nature/fishaquaticspecies.htm |
| Realization that lake trout may have invaded naturally | Several articles by the media | https://www.jhnewsandguide.com/news/environmental/could-lake-trout-swim-to-y-stone/article_0625cb12-8782-59bd-a942-93653e5328e5.html | |
| Effects of actions to suppress lake trout on lake ecology | Graduate research programs | http://www.mtcfru.org/ | |
| Organic pellets used to kill embryos on spawning sites | Peer-reviewed publication | https://doi.org/10.1002/tafs.10208 | |
| 2019 | Fly Fishing Film Tour features cutthroat trout return | Spring public film series | https://www.tu.org/blog/fly-fishing-film-tour-features-the-return/ |
| Partnership support of the restoration program continues | Trout Unlimited on-line articles | https://www.tu.org/blog/lake-trout-on-the-decline-in-yellowstone-lake/ | |
| 2019 | Magazine articles highlighting suppression efforts | National Geographic and others | https://www.nationalgeographic.com/animals/2019/06/how-to-eradicate-yellowstone-lake-trout/ |
| Public radio programming | Montana Public Radio | https://www.mtpr.org/post/non-native-lake-trout-numbers-declining-yellowstone-officials-say | |
| Regional and national print media | Cody Enterprise and others | https://www.codyenterprise.com/news/local/article_bd6ba2a0-93b9-11ea-b91b-2f3aadaca730.html | |
| Lake trout-induced indirect ecological effects documented | Heightened international awareness | https://advances.sciencemag.org/content/5/3/eaav1139 | |
| 2020 | Full length documentary film on the lake restoration effort | Video segments with this article | https://zenodo.org/record/3829613#.XtVlU2hKiUk |
| Program updates made available to the public | NPS press releases continue | https://www.nps.gov/yell/learn/news/19053.htm | |
| Lake trout suppression on-line information updated | Public interpretive website | https://www.nps.gov/yell/learn/nature/lake-trout.htm | |
| Yellowstone Forever fund-raising partnership continues | A race against time donor website | https://www.yellowstone.org/trout/ | |
| Scientific panel review results highlighted | Numerous articles on-line | https://www.tu.org/blog/science-panel-excited-about-numbers-on-yellowstone-lake/ | |
| Two Ocean Pass: Alternative hypothesis for lake trout invasion | Peer-reviewed publication | https://doi.org/10.3390/w12061629 | |
| Environmental assessment to expand spawning site treatments | Public scoping on-line anticipated | ||
| Global Covid-19 pandemic | Restoration program continues |
| Increased water temperatures | Altered hydrologic events (timing of max. & min. flows | Altered hydrologic events (flow volume) | Sedimentation | *Lake level declines/tributary disconnect | Physiological stress & reduced fitness | *Fewer produced or recruited to spawning population | *Direct native fish mortality | *Predation by non-native fish | *Competition/displacement | *Loss of genetic integrity (hybridization) | ||
| AGENTS OF CHANGE | ||||||||||||
| Regional Physical/Chemical Forces | ||||||||||||
| Increasing temperature (air) | X | X | X | X | X | X | ||||||
| *Changing precipitation patterns (snowpack, runoff) | X | X | X | X | X | X | ||||||
| Wildland Fire Frequency Increased | X | X | X | X | X | |||||||
| Biological Introductions | ||||||||||||
| *Historical fish stocking by management | X | X | X | X | X | |||||||
| *Stocking of fish illegally (lake trout) | X | X | X | X | ||||||||
| Aquatic nuisance species (New Zealand mudsnails) | X | X | ||||||||||
| Disease dissemination (whirling disease) | X | X | ||||||||||
| Angling | ||||||||||||
| Intentional illegal harvest | X | X | ||||||||||
| Mis-identification resulting in harvest | X | X | ||||||||||
| Catch & release mortality | X | X | ||||||||||
| Park Infrastructure/Operations | ||||||||||||
| Fire suppression | X | |||||||||||
| Backcountry trails & campsites | X | X | ||||||||||
| Land management (stock use, herbicide treatments) | X | X | ||||||||||
| Road improvements | X | X | ||||||||||
| Water treatment facilities | X | X | X | |||||||||
| Local Physical/Chemical Forces | ||||||||||||
| Natural geothermal inputs | X | X | ||||||||||
| Increased water temperatures | Altered hydrologic events (timing of max. & min. flows | Altered hydrologic events (flow volume) | Sedimentation | *Lake level declines/tributary disconnect | Physiological stress & reduced fitness | *Fewer produced or recruited to spawning population | *Direct native fish mortality | *Predation by non-native fish | *Competition/displacement | *Loss of genetic integrity (hybridization) | ||
| ECOSYSTEM RESPONSE | ||||||||||||
| Biogeochemical Cycling | ||||||||||||
| *Nutrient flux/transport altered | X | X | X | X | X | |||||||
| Productivity/Biomass Change | ||||||||||||
| *Primary production (algae) availability reduced | X | X | X | X | X | |||||||
| *Secondary production (inverts/zooplankton) availability altered/reduced | X | X | X | X | X | X | X | X | ||||
| *Secondary production (fish) availability altered/reduced | X | X | X | X | X | X | X | X | ||||
| Fish Functional Role as Secondary Consumers | ||||||||||||
| *Shift from invertivore (invert consumer) to piscivore (fish consumer/predator) | X | X | X | |||||||||
| Fish Life History Strategy | ||||||||||||
| *Shift in spawning timing | X | X | X | |||||||||
| *Disrupt migration and/or shift in spawning location | X | X | X | X | X | |||||||
| *Habitat volume (niche) available reduced | X | X | X | X | X | |||||||
| Avian/Terrestrial Tertiary Consumers | ||||||||||||
| *Displacement of grizzly bears from spawning streams | X | X | X | X | ||||||||
| *Decline in native trout use by ospreys and eagles | X | X | X | X | ||||||||
| *Increased physiological stress on river otters | X | X | X | X | ||||||||
| Cutthroat Trout | Lake Trout | |||||
|---|---|---|---|---|---|---|
| Year | YCT CPUE | Lwr 95% CL | Upr 95% CL | LKT CPUE | Lwr 95% CL | Upr 95% CL |
| 2011 | 12.46 | 8.10 | 16.82 | 4.44 | 2.91 | 5.97 |
| 2012 | 20.53 | 16.38 | 24.68 | 3.28 | 2.20 | 4.36 |
| 2013 | 24.82 | 19.03 | 30.61 | 2.80 | 2.00 | 3.60 |
| 2014 | 27.30 | 22.19 | 32.41 | 4.86 | 3.39 | 6.33 |
| 2015 | 19.42 | 14.66 | 24.18 | 3.89 | 3.00 | 4.78 |
| 2016 | 18.28 | 14.18 | 22.38 | 2.79 | 1.94 | 3.64 |
| 2017 | 20.38 | 15.99 | 24.77 | 2.80 | 2.08 | 3.52 |
| 2018 | 26.44 | 20.50 | 32.38 | 1.96 | 1.44 | 2.48 |
| 2019 | 20.96 | 16.82 | 25.10 | 2.00 | 1.11 | 2.89 |
| Year | Lake Trout | Mean | Lwr 95% CL | Upr 95% CL |
|---|---|---|---|---|
| 2012 | Abundance Total | 925,208 | 771,271 | 1,125,280 |
| 2013 | 798,996 | 652,429 | 965,909 | |
| 2014 | 774,569 | 633,884 | 943,216 | |
| 2015 | 872,537 | 700,626 | 1,071,490 | |
| 2016 | 877,724 | 706,840 | 1,089,630 | |
| 2017 | 764,868 | 594,113 | 936,550 | |
| 2018 | 572,550 | 449,699 | 741,077 | |
| 2019 | 673,983 | 493,012 | 976,925 | |
| 2012 | Abundance Age-2 | 450,672 | 364,999 | 560,782 |
| 2013 | 318,640 | 249,647 | 397,507 | |
| 2014 | 404,864 | 315,551 | 496,958 | |
| 2015 | 480,961 | 372,384 | 597,922 | |
| 2016 | 457,865 | 353,324 | 571,199 | |
| 2017 | 379,099 | 289,469 | 476,558 | |
| 2018 | 274,512 | 207,561 | 373,254 | |
| 2019 | 463,958 | 313,814 | 688,536 | |
| 2012 | Abundance Age-3 to 5 | 416,814 | 336,620 | 510,974 |
| 2013 | 433,844 | 353,892 | 538,683 | |
| 2014 | 333,291 | 268,885 | 417,800 | |
| 2015 | 355,516 | 280,185 | 448,171 | |
| 2016 | 386,503 | 301,593 | 490,933 | |
| 2017 | 363,205 | 271,289 | 454,582 | |
| 2018 | 281,511 | 213,664 | 363,813 | |
| 2019 | 197,681 | 146,741 | 282,689 | |
| 2012 | Abundance Age-6+ | 57,722 | 41,025 | 72,130 |
| 2013 | 46,512 | 31,696 | 60,086 | |
| 2014 | 36,414 | 24,458 | 48,976 | |
| 2015 | 36,060 | 25,506 | 50,249 | |
| 2016 | 33,356 | 23,762 | 48,202 | |
| 2017 | 22,563 | 14,961 | 32,736 | |
| 2018 | 16,527 | 11,088 | 25,040 | |
| 2019 | 12,345 | 7991 | 20,163 | |
| 2012 | Biomass Total | 432,017 | 343,977 | 521,873 |
| 2013 | 398,020 | 314,683 | 488,371 | |
| 2014 | 382,138 | 298,369 | 469,323 | |
| 2015 | 342,273 | 269,421 | 428,070 | |
| 2016 | 321,580 | 257,333 | 414,412 | |
| 2017 | 290,201 | 223,262 | 369,658 | |
| 2018 | 229,509 | 179,299 | 304,616 | |
| 2019 | 196,675 | 147,708 | 282,073 |
| Length Class (mm) | Time Period | Mean Kn | Lwr 95% CL | Upr 95% CL |
|---|---|---|---|---|
| 200–280 | 1995–1999 | 120.31 | 117.00 | 123.61 |
| 2000–2004 | 120.03 | 110.22 | 129.83 | |
| 2005–2009 | 121.36 | 119.22 | 123.50 | |
| 2010–2014 | 115.74 | 114.32 | 117.16 | |
| 2015–2019 | 116.26 | 114.96 | 117.56 | |
| 290–390 | 1995–1999 | 112.06 | 111.52 | 112.60 |
| 2000–2004 | 112.86 | 111.83 | 113.89 | |
| 2005–2009 | 116.59 | 114.10 | 119.08 | |
| 2010–2014 | 111.88 | 111.02 | 112.75 | |
| 2015–2019 | 113.93 | 112.90 | 114.95 | |
| 400+ | 1995–1999 | 102.78 | 102.23 | 103.33 |
| 2000–2004 | 102.04 | 100.23 | 103.84 | |
| 2005–2009 | 106.21 | 105.11 | 107.32 | |
| 2010–2014 | 107.38 | 106.45 | 108.31 | |
| 2015–2019 | 111.78 | 110.20 | 113.35 |
| Cutthroat Trout | Length Class (mm) | Decade | Mean | Lwr 95% CL | Upr 95% CL |
|---|---|---|---|---|---|
| Catch-Per-Unit-Effort | 100–280 | 1980s | 18.63 | 14.27 | 22.99 |
| 1990s | 11.95 | 8.25 | 15.64 | ||
| 2000s | 9.43 | 6.77 | 12.09 | ||
| 2010s | 6.88 | 4.34 | 9.42 | ||
| 290–390 | 1980s | 15.09 | 13.94 | 16.24 | |
| 1990s | 12.76 | 9.81 | 15.71 | ||
| 2000s | 3.09 | 2.25 | 3.93 | ||
| 2010s | 3.89 | 2.22 | 5.57 | ||
| 400+ | 1980s | 7.52 | 6.30 | 8.75 | |
| 1990s | 7.38 | 6.13 | 8.63 | ||
| 2000s | 7.45 | 5.97 | 8.93 | ||
| 2010s | 14.61 | 12.05 | 17.17 | ||
| Mean Individual Weight (g) | 100–280 | 1980s | 114.28 | 112.76 | 115.80 |
| 1990s | 113.61 | 111.46 | 115.75 | ||
| 2000s | 111.20 | 108.60 | 113.80 | ||
| 2010s | 103.90 | 100.63 | 107.18 | ||
| 290–390 | 1980s | 408.05 | 405.07 | 411.03 | |
| 1990s | 411.01 | 407.02 | 414.99 | ||
| 2000s | 425.95 | 414.21 | 437.70 | ||
| 2010s | 463.37 | 453.82 | 472.92 | ||
| 400+ | 1980s | 682.80 | 674.98 | 690.63 | |
| 1990s | 710.92 | 701.58 | 720.26 | ||
| 2000s | 1004.89 | 987.98 | 1021.81 | ||
| 2010s | 1418.60 | 1405.55 | 1431.64 | ||
| Relative Weight | 130–280 | 1980s | 58.84 | 58.46 | 59.22 |
| 1990s | 58.71 | 58.37 | 59.05 | ||
| 2000s | 62.37 | 61.47 | 63.28 | ||
| 2010s | 68.38 | 67.48 | 69.28 | ||
| 290–390 | 1980s | 56.53 | 56.35 | 56.71 | |
| 1990s | 56.71 | 56.51 | 56.91 | ||
| 2000s | 61.56 | 60.77 | 62.36 | ||
| 2010s | 70.35 | 69.70 | 71.00 | ||
| 400+ | 1980s | 55.78 | 55.43 | 56.13 | |
| 1990s | 56.36 | 55.97 | 56.74 | ||
| 2000s | 63.50 | 63.00 | 64.00 | ||
| 2010s | 67.74 | 67.44 | 68.04 |
Appendix B








References
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current knowledge on non-native freshwater fish introductions. J. Fish Biol. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Rahel, F.J.; Smith, M.A. Pathways of unauthorized fish introductions and types of management responses. Hydrobiologia 2018, 817, 41–56. [Google Scholar] [CrossRef]
- Clarkson, R.W.; Marsh, P.C.; Stefferud, S.E.; Stefferud, J.A. Conflicts between native fish and nonnative sport fish management in the southwestern United States. Fisheries 2005, 30, 20–27. [Google Scholar] [CrossRef]
- McMahon, T.E.; Bennett, D.H. Walleye and northern pike: Boost or bane to northwest fisheries? Fisheries 1996, 21, 6–13. [Google Scholar] [CrossRef]
- Moyle, P.B.; Light, T. Biological invasions of fresh water: Empirical rules and assembly theory. Biol. Conserv. 1996, 78, 149–161. [Google Scholar] [CrossRef]
- Vander Zanden, J.M.; Chandra, S.; Allen, B.C.; Reuter, J.E.; Goldman, C.R. Historical food web structure and restoration of native aquatic communities in the Lake Tahoe (California-Nevada) basin. Ecosystems 2003, 6, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Martinez, P.J.; Bigelow, P.E.; Deleray, M.A.; Fredenberg, W.A.; Hansen, B.S.; Horner, N.J.; Lehr, S.K.; Schneidervin, R.W.; Tolentino, S.A.; Viola, A.E. Western lake trout woes. Fisheries 2009, 34, 424–442. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Long–term trends of native and non–native fish faunas in the American Southwest. Anim. Biodivers. Conserv. 2005, 28, 75–89. Available online: https://www.raco.cat/index.php/ABC/article/view/56742 (accessed on 10 June 2020).
- Sanderson, B.L.; Barnas, K.A.; Rub, A.M.W. Nonindigenous species of the Pacific Northwest: An overlooked risk to endangered salmon? Bioscience 2009, 59, 245–256. [Google Scholar] [CrossRef]
- Coggins, L.G.; Yard, M.D.; Pine, W.E. Nonnative fish control in the Colorado River in Grand Canyon, Arizona: An effective program or serendipitous timing? Trans. Am. Fish. Soc. 2011, 140, 456–470. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. Bioscience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Nico, L.G.; Fuller, P.L. Spatial and temporal patterns of nonindigenous fish introductions in the United States. Fisheries 1999, 24, 16–27. [Google Scholar] [CrossRef]
- Rahel, F.J. Homogenization of fish faunas across the United States. Science 2000, 288, 854–856. [Google Scholar] [CrossRef]
- Schade, C.B.; Bonar, S.A. Distribution and abundance of nonnative fishes in streams of the western United States. N. Am. J. Fish. Manag. 2005, 25, 1386–1394. [Google Scholar] [CrossRef]
- Britton, J.R.; Gozlan, R.E.; Copp, G.H. Managing non-native fish in the environment. Fish Fish. 2011, 12, 256–274. [Google Scholar] [CrossRef]
- Rytwinski, T.; Taylor, J.J.; Donaldson, L.A.; Britton, J.R.; Browne, D.R.; Gresswell, R.E.; Lintermans, M.; Prior, K.A.; Pellatt, M.G.; Vis, C.; et al. The effectiveness of non-native fish removal techniques in freshwater ecosystems: A systematic review. Environ. Rev. 2018, 27, 71–94. [Google Scholar] [CrossRef]
- Mueller, G.A. Predatory fish removal and native fish recovery in the Colorado River mainstem. Fisheries 2005, 30, 10–19. [Google Scholar] [CrossRef]
- Fredenberg, C.R.; Muhlfeld, C.C.; Guy, C.S.; D’Angelo, V.S.; Downs, C.C.; Syslo, J.M. Suppression of invasive lake trout in an isolated backcountry lake in Glacier National Park. Fish. Manag. Ecol. 2017, 24, 33–48. [Google Scholar] [CrossRef]
- Dux, A.M.; Hansen, M.J.; Corsi, M.P.; Wahl, N.C.; Fredericks, J.P.; Corsi, C.E.; Schill, D.J.; Horner, N.J. Effectiveness of lake trout (Salvelinus namaycush) suppression in Lake Pend Oreille, Idaho: 2006–2016. Hydrobiologia 2019, 840, 319–333. [Google Scholar] [CrossRef]
- Dux, A.M.; Guy, C.S.; Fredenberg, W.A. Spatiotemporal distribution and population characteristics of a nonnative lake trout population, with implications for suppression. N. Am. J. Fish. Manag. 2011, 31, 187–196. [Google Scholar] [CrossRef]
- Klein, Z.B.; Quist, M.C.; Rhea, D.T.; Senecal, A.C. Population characteristics and the suppression of nonnative burbot. N. Am. J. Fish. Manag. 2016, 36, 1006–1017. [Google Scholar] [CrossRef]
- Kaus, D.J. Feasibility of Walleye Population Suppression in Buffalo Bill Reservoir, Wyoming. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2019. [Google Scholar]
- Quist, M.C.; Hubert, W.A. Bioinvasive species and the preservation of cutthroat trout in the western United States: Ecological, social, and economic issues. Environ. Sci. Policy 2004, 7, 303–313. [Google Scholar] [CrossRef]
- Shollenberger, H.; Dressler, E.; Mallinson, D.J. Invasive snakehead and introduced sport fish illustrate an environmental health paradox of invasive species and angler demand. Case Stud. Environ. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Syslo, J.M.; Guy, C.S.; Cox, B.S. Comparison of harvest scenarios for the cost-effective suppression of lake trout in Swan Lake, Montana. N. Am. J. Fish. Manag. 2013, 33, 1079–1090. [Google Scholar] [CrossRef]
- Hansen, M.J.; Guy, C.S.; Budy, P.; McMahon, T.E. Trout as native and nonnative species: A management paradox. In Trouts and Char of the World; Kershner, J.L., Williams, J.E., Gresswell, R.E., Lobón-Cerviá, J., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 645–684. [Google Scholar]
- Walters, C.J.; Holling, C.S. Large-scale management experiments and learning by doing. Ecology 1990, 71, 2060–2068. [Google Scholar] [CrossRef]
- McCarthy, M.A.; Possingham, H.P. Active adaptive management for conservation. Conserv. Biol. 2007, 21, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Adaptive Environmental Assessment and Management; Wiley: London, UK, 1978. [Google Scholar]
- Walters, C.J. Is adaptive management helping to solve fisheries problems? AMBIO J. Hum. Environ. 2007, 36, 304–307. [Google Scholar] [CrossRef]
- Walters, C.J. Adaptive Management of Renewable Resources; Macmillan: New York, NY, USA, 1986. [Google Scholar]
- Runge, M.C. An introduction to adaptive management for threatened and endangered species. J. Fish Wildl. Manag. 2011, 2, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301. [Google Scholar] [CrossRef] [Green Version]
- Thom, R.; St Clair, T.; Burns, R.; Anderson, M. Adaptive management of large aquatic ecosystem recovery programs in the United States. J. Environ. Manag. 2016, 183, 424–430. [Google Scholar] [CrossRef]
- Zavaleta, E.S.; Hobbs, R.J.; Mooney, H.A. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol. Evol. 2001, 16, 454–459. [Google Scholar] [CrossRef]
- Prior, K.M.; Adams, D.C.; Klepzig, K.D.; Hulcr, J. When does invasive species removal lead to ecological recovery? Implications for management success. Biol. Invasions 2018, 20, 267–283. [Google Scholar] [CrossRef]
- Parker, I.M.; Simberloff, D.; Lonsdale, W.M.; Goodell, K.; Wonham, M.; Kareiva, P.M.; Williamson, M.H.; Von Holle, B.; Moyle, P.B.; Byers, J.E.; et al. Impact: Toward a framework for understanding the ecological effects of invaders. Biol. Invasions 1999, 1, 3–19. [Google Scholar] [CrossRef]
- Kaplinski, M.A. Geomorphology and Geology of Yellowstone Lake, Yellowstone National Park, Wyoming. Master’s Thesis, Northern Arizona University, Flagstaff, AZ, USA, 1991. [Google Scholar]
- Remsen, C.C.; Maki, J.S.; Klump, J.V.; Aguilar, C.; Anderson, P.D.; Buchholz, L.; Cuhel, R.L.; Lovalvo, D.; Paddock, R.W.; Waples, J.; et al. Sublacustrine geothermal activity in Yellowstone Lake: Studies past and present. In Yellowstone Lake: Hotbed of Chaos or Reservoir of Resilience, Proceedings of the 6th Biennial Scientific Conference on the Greater Yellowstone Ecosystem; Anderson, R.J., Harmon, D., Eds.; Yellowstone Center for Resources and The George Wright Society: Yellowstone National Park, WY, USA, 2002. [Google Scholar]
- Gresswell, R.E.; Varley, J.D. Effects of a century of human influence on the cutthroat trout of Yellowstone Lake. Am. Fish. Soc. Symp. 1988, 4, 45–52. [Google Scholar]
- Koel, T.M.; Tronstad, L.M.; Arnold, J.L.; Gunther, K.A.; Smith, D.W.; Syslo, J.M.; White, P.J. Predatory fish invasion induces within and across ecosystem effects in Yellowstone National Park. Sci. Adv. 2019, 5, eaav1139. [Google Scholar] [CrossRef] [Green Version]
- Koel, T.M.; Arnold, J.L.; Bigelow, P.E.; Detjens, C.R.; Doepke, P.D.; Ertel, B.D.; Ruhl, M.E. Native Fish Conservation Program, Yellowstone Fisheries and Aquatic Sciences 2012–2014; YCR-2015-01; National Park Service, Yellowstone Center for Resources: Yellowstone National Park, WY, USA, 2015. Available online: https://www.nps.gov/yell/learn/nature/upload/2012-2014_yellowstone_fisheries.pdf (accessed on 10 June 2020).
- Jordan, D.S. A reconnaissance of streams and lakes of Yellowstone National Park, Wyoming in the interest of the U.S. Fish Commission. Bull. U.S. Fish Comm. 1891, 9, 41–63. [Google Scholar]
- Behnke, R.J. Trout and Salmon of North America; Free Press: New York, NY, USA, 2002. [Google Scholar]
- Licciardi, J.M.; Pierce, K.L. History and dynamics of the Greater Yellowstone Glacial System during the last two glaciations. Quat. Sci. Rev. 2018, 200, 1–33. [Google Scholar] [CrossRef]
- Ertel, B.D.; McMahon, T.E.; Koel, T.M.; Gresswell, R.E.; Burckhardt, J.C. Life history migrations of adult Yellowstone cutthroat trout in the upper Yellowstone River. N. Am. J. Fish. Manag. 2017, 37, 743–755. [Google Scholar] [CrossRef] [Green Version]
- Gresswell, R.E.; Liss, W.J.; Larson, G.L. Life-history organization of Yellowstone cutthroat trout (Oncorhynchus clarkii bouvieri) in Yellowstone Lake. Can. J. Fish. Aquat. Sci. 1994, 51, 298–309. [Google Scholar] [CrossRef]
- Kaeding, L.R.; Boltz, G.D. Spatial and temporal relations between fluvial and allacustrine Yellowstone cutthroat trout, Oncorhynchus clarkii bouvieri, spawning in the Yellowstone River, outlet stream of Yellowstone Lake. Environ. Biol. Fishes 2001, 61, 395–406. [Google Scholar] [CrossRef]
- Tronstad, L.M.; Hall, R.O., Jr.; Koel, T.M. Introduced lake trout alter nitrogen cycling beyond Yellowstone Lake. Ecosphere 2015, 6, 224. [Google Scholar] [CrossRef] [Green Version]
- Felicetti, L.A.; Schwartz, C.C.; Rye, R.O.; Gunther, K.A.; Crock, J.G.; Haroldson, M.A.; Waits, L.; Robbins, C.T. Use of naturally occurring mercury to determine the importance of cutthroat trout to Yellowstone grizzly bears. Can. J. Zool. 2004, 82, 493–501. [Google Scholar] [CrossRef]
- Koel, T.M.; Bigelow, P.E.; Doepke, P.D.; Ertel, B.D.; Mahony, D.L. Nonnative lake trout result in Yellowstone cutthroat trout decline and impacts to bears and anglers. Fisheries 2005, 30, 10–19. [Google Scholar] [CrossRef]
- Baril, L.M.; Smith, D.W.; Drummer, T.; Koel, T.M. Implications of cutthroat trout declines for breeding ospreys and bald eagles at Yellowstone Lake. J. Raptor Res. 2013, 47, 234–245. [Google Scholar] [CrossRef]
- Bergum, D.J.; Gunther, K.A.; Baril, L.M. Birds and mammals that consume Yellowstone cutthroat trout in Yellowstone Lake and its tributaries. Yellowstone Sci. 2017, 25, 86–89. Available online: https://www.researchgate.net/publication/318281009 (accessed on 10 June 2020).
- Benson, N.G. Limnology of Yellowstone Lake in Relation to the Cutthroat Trout; Research Report 56; U.S. Department of the Interior, Fish and Wildlife Service, Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1961; Available online: http://doi.org/10.5281/zenodo.3890470 (accessed on 10 June 2020).
- Crait, J.R.; Ben-David, M. River otters in Yellowstone Lake depend on a declining cutthroat trout population. J. Mammal. 2006, 87, 485–494. [Google Scholar] [CrossRef]
- Swenson, J.E. Prey and foraging behavior of ospreys on Yellowstone Lake, Wyoming. J. Wildl. Manag. 1978, 42, 87–90. [Google Scholar] [CrossRef]
- Diem, K.L.; Pugesek, B.H. American white pelicans at the Molly Islands, in Yellowstone National Park: Twenty-two years of boom-and-bust breeding, 1966–1987. Colonial Waterbirds 1994, 17, 130–145. [Google Scholar] [CrossRef]
- Walker, L.E.; Smith, D.W.; Albrechtsen, M.B.; Cassidy, B.J.; Shields, E.M.; Duffy, K. Yellowstone Bird Project: Annual Report 2018; YCR-2019-01; National Park Service, Yellowstone Center for Resources: Yellowstone National Park, WY, USA, 2019. Available online: https://www.nps.gov/yell/learn/nature/upload/2018-Bird-Report_web.pdf (accessed on 10 June 2020).
- Koel, T.M.; Thomas, N.A.; Guy, C.S.; Doepke, P.D.; MacDonald, D.J.; Poole, A.S.; Sealey, W.M.; Zale, A.V. Organic pellet decomposition induces mortality of lake trout embryos in Yellowstone Lake. Trans. Am. Fish. Soc. 2020, 149, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.R. Quantifying the Spatial Structure of Invasive Lake Trout in Yellowstone Lake to Improve Suppression Efficacy. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2019. [Google Scholar]
- Flavelle, L.S.; Ridgway, M.S.; Middel, T.A.; McKinley, R.S. Integration of acoustic telemetry and GIS to identify potential spawning areas for lake trout (Salvelinus namaycush). Hydrobiologia 2002, 483, 137–146. [Google Scholar] [CrossRef]
- Bigelow, P.E. Predicting Areas of Lake Trout Spawning Habitat within Yellowstone Lake, Wyoming. Ph.D. Thesis, University of Wyoming, Laramie, WY, USA, 2009. [Google Scholar]
- Gresswell, R.E.; Liss, W.J. Values associated with management of Yellowstone cutthroat trout in Yellowstone National Park. Conserv. Biol. 1995, 9, 159–165. [Google Scholar] [CrossRef]
- Byorth, J. Trout shangri—La: Remaking the fishing in Yellowstone National Park, Montana. Mag. West. Hist. 2002, 52, 38–47. [Google Scholar]
- Varley, J.D.; Schullery, P.D. Yellowstone Fishes: Ecology, History, and Angling in the Park; Stackpole Books: Mechanicsburg, PA, USA, 1998. [Google Scholar]
- Biesinger, K.E. Studies on the Relationship of the Redside Shiner (Richardsonius balteatus) and the Longnose Sucker (Catostomus catostomus) to the Cutthroat Trout (Salmo clarki) Population in Yellowstone Lake. Master’s Thesis, Utah State University, Logan, UT, USA, 1961. [Google Scholar]
- Davenport, M. Piscivorous Avifauna on Yellowstone Lake, Yellowstone National Park; U.S. Department of the Interior, National Park Service: Yellowstone National Park, WY, USA, 1974. [Google Scholar] [CrossRef]
- Brown, C.J.D.; Graham, R.J. Observations on the longnose sucker in Yellowstone Lake. Trans. Am. Fish. Soc. 1954, 83, 38–46. [Google Scholar] [CrossRef]
- Furey, K.M.; Glassic, H.C.; Guy, C.S.; Koel, T.M.; Arnold, J.L.; Doepke, P.D.; Bigelow, P.E. Diets of longnose sucker in Yellowstone Lake, Yellowstone National Park, U.S.A. J. Freshw. Ecol. 2020. (under review). [Google Scholar]
- Madsen, D.H. Protection of native fishes in the national parks. Trans. Am. Fish. Soc. 1937, 66, 395–397. [Google Scholar] [CrossRef]
- Leopold, A.S.; Cain, S.A.; Cottam, C.M.; Gabrielson, I.N.; Kimba, T.L. Wildlife Management in the National Parks; Report to the Secretary of the Interior; Advisory Board on Wildlife Management: Washington, DC, USA, 1963; Available online: http://npshistory.com/publications/leopold_report.pdf (accessed on 10 June 2020).
- Jones, R.D.; Gresswell, R.E.; Jennings, D.E.; Rubrecht, S.M.; Varley, J.D. Fishery and Aquatic Management Program in Yellowstone National Park; Tech. Rep. 1979; U.S. Fish and Wildlife Service: Yellowstone National Park, WY, USA, 1980. [Google Scholar] [CrossRef]
- Kaeding, L.R.; Boltz, G.D.; Carty, D.G. Lake trout discovered in Yellowstone Lake threaten native cutthroat trout. Fisheries 1996, 21, 16–20. [Google Scholar] [CrossRef]
- Koel, T.M.; Detjens, C.R.; Zale, A.V. Two Ocean Pass: An alternative hypothesis for invasion of Yellowstone Lake by lake trout, and implications for future invasions. Water 2020, 12, 1629. [Google Scholar] [CrossRef]
- Scott, W.B.; Crossman, E.J. Freshwater Fishes of Canada; Bulletin 184; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1973. [Google Scholar]
- Ryder, R.A.; Kerr, S.R.; Taylor, W.W.; Larkin, P.A. Community consequences of fish stock diversity. Can. J. Fish. Aquat. Sci. 1981, 38, 1856–1866. [Google Scholar] [CrossRef]
- Healey, M.C. The dynamics of exploited lake trout populations and implications for management. J. Wildl. Manag. 1978, 42, 307–328. [Google Scholar] [CrossRef]
- Muir, A.M.; Blackie, C.T.; Marsden, J.E.; Krueger, C.C. Lake charr Salvelinus namaycush spawning behaviour: New field observations and a review of current knowledge. Rev. Fish Biol. Fish. 2012, 22, 575–593. [Google Scholar] [CrossRef]
- Ruzycki, J.R.; Beauchamp, D.A.; Yule, D.L. Effects of introduced lake trout on native cutthroat trout in Yellowstone Lake. Ecol. Appl. 2003, 13, 23–37. [Google Scholar] [CrossRef]
- Syslo, J.M.; Guy, C.S.; Bigelow, P.E.; Doepke, P.D.; Ertel, B.D.; Koel, T.M. Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Can. J. Fish. Aquat. Sci. 2011, 68, 2132–2145. [Google Scholar] [CrossRef] [Green Version]
- Syslo, J.M.; Brenden, T.O.; Guy, C.S.; Koel, T.M.; Bigelow, P.E.; Doepke, P.D.; Arnold, J.L.; Ertel, B.D. Could ecological release buffer suppression efforts for non-native lake trout (Salvelinus namaycush) in Yellowstone Lake, Yellowstone National Park? Can. J. Fish. Aquat. Sci. 2020, 77, 1010–1025. [Google Scholar] [CrossRef]
- Varley, J.D.; Schullery, P. The Yellowstone Lake Crisis: Confronting a Lake Trout Invasion; A Report to the Director of the National Park Service; Yellowstone Center for Resources, National Park Service: Yellowstone National Park, WY, USA, 1995. Available online: https://www.nps.gov/parkhistory/online_books/yell/trout_invasion.pdf (accessed on 10 June 2020).
- McIntyre, J.D. Review and assessment of possibilities for protecting the cutthroat trout of Yellowstone Lake from introduced lake trout. In The Yellowstone Lake Crisis: Confronting a Lake Trout Invasion. A Report to the Director of the National Park Service; Varley, J.D., Schullery, P., Eds.; Yellowstone Center for Resources, National Park Service: Yellowstone National Park, WY, USA, 1995; pp. 28–33. [Google Scholar]
- Ruzycki, J.R. Impact of Lake Trout Introductions on Cutthroat Trout of Selected Western Lakes of the Continental United States. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2004. [Google Scholar]
- Koel, T.M.; Arnold, J.L.; Bigelow, P.E.; Doepke, P.D.; Ertel, B.D.; Ruhl, M.E. Yellowstone Fisheries and Aquatic Sciences: Annual Report, 2008; YCR-2010-03; National Park Service, Yellowstone Center for Resources: Yellowstone National Park, WY, USA, 2010. Available online: https://www.nps.gov/yell/planyourvisit/upload/2008_fisheries_ar_final.pdf (accessed on 10 June 2020).
- Tronstad, L.M.; Hall, R.O.; Koel, T.M.; Gerow, K.G. Introduced lake trout produced a four-level trophic cascade in Yellowstone Lake. Trans. Am. Fish. Soc. 2010, 139, 1536–1550. [Google Scholar] [CrossRef]
- Middleton, A.D.; Morrison, T.A.; Fortin, J.K.; Robbins, C.T.; Proffitt, K.M.; White, P.J.; McWhirter, D.E.; Koel, T.M.; Brimeyer, D.G.; Fairbanks, W.S.; et al. Grizzly bear predation links the loss of native trout to the demography of migratory elk in Yellowstone. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130870. [Google Scholar] [CrossRef] [Green Version]
- Gresswell, R.E. Scientific Review Panel Evaluation of the National Park Service Lake Trout Suppression Program in Yellowstone Lake, 25–29 August; Final Report, YCR–2009–05; USGS Northern Rocky Mountain Science Center: Bozeman, MT, USA, 2009. Available online: https://www.nps.gov/yell/planyourvisit/upload/gresswell_final_updated_1_2010.pdf (accessed on 10 June 2020).
- Hansen, M.J.; Horner, N.J.; Liter, M.; Peterson, M.P.; Maiolie, M.A. Dynamics of an increasing lake trout population in Lake Pend Oreille, Idaho. N. Am. J. Fish. Manag. 2008, 28, 1160–1171. [Google Scholar] [CrossRef]
- Koel, T.M.; Arnold, J.L.; Bigelow, P.E.; Ruhl, M.E. Native Fish Conservation Plan: Environmental Assessment; U.S. Department of the Interior, National Park Service: Yellowstone National Park, WY, USA, 2010. Available online: https://parkplanning.nps.gov/projectHome.cfm?projectID=30504 (accessed on 10 June 2020).
- U.S. Department of the Interior. NPS Management Policies 2006; National Park Service, U.S. Government Printing Office: Washington, DC, USA, 2006; ISBN 0-16-076874-8.
- Kaeding, L.R. New climate regime started and further shaped the historic Yellowstone Lake cutthroat trout population decline commonly attributed entirely to nonnative lake trout predation. Aquat. Ecol. 2020, 54, 641–652. [Google Scholar] [CrossRef]
- Detjens, C.R.; Voigt, W.; Voigt, J.; Koel, T.M. Fly fishing volunteers support native fish conservation in Yellowstone. Yellowstone Sci. 2017, 25, 82–84. [Google Scholar]
- Trout Unlimited. Science Supporting Management of Yellowstone Lake Fisheries: Responses to Frequently Asked Questions; Wyoming Water Project, Trout Unlimited: Lander, WY, USA, 2014; Available online: http://wyomingtu.org/wp-content/uploads/2014/03/Science-Supporting-Management-of-Yellowstone-Lake-Fisheries.pdf (accessed on 10 June 2020).
- Hansen, M.J.; Guy, C.S.; Bronte, C.R.; Nate, N.A. Life history and population dynamics. In Lake Charr Salvelinus Namaycush: Biology, Ecology, Distribution, and Management; Muir, A.M., Krueger, C.C., Hansen, M.J., Riley, S.C., Noakes, D.L.G., Eds.; Fish & Fisheries Series; Springer: New York, NY, USA. (in press)
- Morgan, L.A.; Shanks, W.C.; Lovalvo, D.A.; Johnson, S.Y.; Stephenson, W.J.; Pierce, K.L.; Harlan, S.S.; Finn, C.A.; Lee, G.; Webring, M.; et al. Exploration and discovery in Yellowstone Lake: Results from high-resolution sonar imaging, seismic reflection profiling, and submersible studies. J. Volcanol. Geotherm. Res. 2003, 122, 221–242. [Google Scholar] [CrossRef]
- Bigelow, P.E.; Doepke, P.D.; Ertel, B.E.; Guy, C.S.; Syslo, J.M.; Koel, T.M. Suppressing non-native lake trout in Yellowstone Lake. Yellowstone Sci. 2017, 25, 53–59. Available online: https://www.nps.gov/articles/suppressing-non-native-lake-trout-to-restore-native-cutthroat-trout-in-yellowstone-lake.htm (accessed on 10 June 2020).
- Gutowsky, L.F.G.; Romine, J.G.; Heredia, N.A.; Bigelow, P.E.; Parsley, M.J.; Sandstrom, P.T.; Suski, C.D.; Danylchuk, A.J.; Cooke, S.J.; Gresswell, R.E. Revealing migration and reproductive habitat of invasive fish under an active population suppression program. Conserv. Sci. Pract. 2020, 2, e119. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.S.; Hightower, J.E. Fish population dynamics: Mortality, growth, and recruitment. In Inland Fisheries Management in North America, 3rd ed.; Hubert, W.A., Quist, M.C., Eds.; American Fisheries Society: Bethesda, MD, USA, 2010; pp. 43–79. [Google Scholar]
- Quist, M.C.; Pegg, M.A.; DeVries, D.R. Age and growth. In Fisheries Techniques, 3rd ed.; Alexander, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 677–731. [Google Scholar]
- Guy, C.S.; Brown, M.L. Analysis and Interpretation of Freshwater Fisheries Data; American Fisheries Society: Bethesda, MD, USA, 2007. [Google Scholar]
- Syslo, J.M. Demography of Lake Trout in Relation to Population Suppression in Yellowstone Lake, Yellowstone National Park. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2010. [Google Scholar]
- Morris, W.F.; Doak, D.F. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Cambray, J.A. Impact on indigenous species biodiversity caused by the globalisation of alien recreational freshwater fisheries. Hydrobiologia 2003, 500, 217–230. [Google Scholar] [CrossRef]
- Reinhart, D.P. Grizzly Bear Habitat Use on Cutthroat Trout Spawning Streams in Tributaries of Yellowstone Lake. Master’s Thesis, Montana State University, Bozeman, MT, USA, 1990. [Google Scholar]
- Kaeding, L.R.; Koel, T.M. Age, growth, maturity, and fecundity of Yellowstone Lake cutthroat trout. Northwest Sci. 2011, 85, 431–444. [Google Scholar] [CrossRef]
- Syslo, J.M.; Guy, C.S.; Koel, T.M. Feeding ecology of native and nonnative salmonids during the expansion of a nonnative apex predator in Yellowstone Lake, Yellowstone National Park. Trans. Am. Fish. Soc. 2016, 145, 476–492. [Google Scholar] [CrossRef]
- Ripple, W.J.; Estes, J.A.; Schmitz, O.J.; Constant, V.; Kaylor, M.J.; Lenz, A.; Motley, J.L.; Self, K.E.; Taylor, D.S.; Wolf, C. What is a trophic cascade? Trends Ecol. Evol. 2016, 31, 842–849. [Google Scholar] [CrossRef]
- Teisberg, J.E.; Haroldson, M.A.; Schwartz, C.C.; Gunther, K.A.; Fortin, J.K.; Robbins, C.T. Contrasting past and current numbers of bears visiting Yellowstone cutthroat trout streams. J. Wildl. Manag. 2014, 78, 369–378. [Google Scholar] [CrossRef]
- Stott, W. Molecular Genetic Characterization and Comparison of Lake Trout from Yellowstone and Lewis Lakes, Wyoming; Research Completion Report for Project #1443-IA-15709-9013; National Park Service, Yellowstone National Park: Mammoth, WY, USA, 2004; Available online: https://www.researchgate.net/publication/331744804 (accessed on 10 June 2020).
- Munro, A.R.; McMahon, T.E.; Ruzycki, J.R. Natural chemical markers identify source and date of introduction of an exotic species: Lake trout (Salvelinus namaycush) in Yellowstone Lake. Can. J. Fish. Aquat. Sci. 2005, 62, 79–87. [Google Scholar] [CrossRef]
- Stapp, P.; Hayward, G.D. Estimates of predator consumption of Yellowstone cutthroat trout (Oncorhynchus clarkii bouvieri) in Yellowstone Lake. J. Freshw. Ecol. 2002, 17, 319–329. [Google Scholar] [CrossRef]
- Koel, T.M.; Kerans, B.L.; Barras, S.C.; Hanson, K.C.; Wood, J.S. Avian piscivores as vectors for Myxobolus cerebralis in the Greater Yellowstone Ecosystem. Trans. Am. Fish. Soc. 2010, 139, 976–988. [Google Scholar] [CrossRef]
- Koel, T.M.; Mahony, D.L.; Kinnan, K.L.; Rasmussen, C.; Hudson, C.J.; Murcia, S.; Kerans, B.L. Myxobolus cerebralis in native cutthroat trout of the Yellowstone Lake ecosystem. J. Aquat. Anim. Health 2006, 18, 157–175. [Google Scholar] [CrossRef]
- Murcia, S.; Kerans, B.L.; Koel, T.M.; MacConnell, E. Myxobolus cerebralis (Hofer) infection risk in native cutthroat trout Oncorhynchus clarkii (Richardson) and its relationships to tributary environments in the Yellowstone Lake basin. J. Fish Dis. 2014, 38, 637–652. [Google Scholar] [CrossRef]
- Murcia, S.; Kerans, B.L.; MacConnell, E.; Koel, T.M. Myxobolus cerebralis infection patterns in Yellowstone cutthroat trout after natural exposure. Dis. Aquat. Org. 2006, 71, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murcia, S.; Kerans, B.L.; MacConnell, E.; Koel, T.M. Correlation of environmental attributes with histopathology of native Yellowstone cutthroat trout naturally infected with Myxobolus cerebralis. Dis. Aquat. Org. 2011, 93, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.D.; Kerans, B.L.; Koel, T.M.; Rasmussen, C. Context-specific parasitism in Tubifex tubifex in geothermally influenced stream reaches in Yellowstone National Park. J. N. Am. Benthol. Soc. 2011, 30, 853–867. [Google Scholar] [CrossRef]
- Stewart, K.P. Use of Otolith Microchemistry to Identify Yellowstone Cutthroat Trout and Lake Trout Natal Origins and Movement Patterns in Yellowstone Lake, Wyoming. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2016. [Google Scholar]
- Syslo, J.M.; Guy, C.S.; Arnold, J.L.; Koel, T.M.; Ertel, B.D. Standard Operating Procedures for Distribution Netting in Yellowstone Lake; 2010–2012 Final Report; USGS, Montana Cooperative Fishery Research Unit: Yellowstone National Park, WY, USA, 2014; Available online: https://www.researchgate.net/publication/339697733 (accessed on 10 June 2020).
- Gresswell, R.E.; Heredia, N.A.; Romine, J.G.; Gutowsky, L.F.G.; Sandstrom, P.T.; Parsley, M.J.; Bigelow, P.E.; Suski, C.D.; Ertel, B.D. Identifying movement patterns and spawning areas of lake trout in Yellowstone Lake. Yellowstone Sci. 2017, 25, 66–69. Available online: https://www.nps.gov/articles/identifying-movement-patterns-and-spawning-areas-of-lake-trout-in-yellowstone-lake.htm (accessed on 10 June 2020).
- Williams, J.R.; Guy, C.S.; Koel, T.M.; Bigelow, P.E. Targeting aggregations of telemetered lake trout to increase gillnetting suppression efficacy. N. Am. J. Fish. Manag. 2020, 40, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Simard, L.G.; Marsden, J.E.; Gresswell, R.E.; Euclide, M. Rapid early development and feeding benefits an invasive population of lake trout. Can. J. Fish. Aquat. Sci. 2019, 77, 496–504. [Google Scholar] [CrossRef]
- Detjens, C.R.; Carim, K.J. Environmental DNA: A new approach to monitoring fish in Yellowstone National Park. Yellowstone Sci. 2017, 25, 26–27. [Google Scholar]
- Roddewig, M.R.; Churnside, J.H.; Hauer, F.R.; Williams, J.; Bigelow, P.E.; Koel, T.M.; Shaw, J.A. Airborne lidar detection and mapping of invasive lake trout in Yellowstone Lake. Appl. Opt. 2018, 57, 4111–4116. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Report on the First Session of FAO Panel of Experts on Integrated Pest Control; FAO: Rome, Italy, 1968. [Google Scholar]
- Dent, D. Integrated Pest Management; Chapman and Hall: New York, NY, USA, 1995. [Google Scholar]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef]
- Flint, M.L.; Van den Bosch, R. Introduction to Integrated Pest Management; Plenum Press: New York, NY, USA, 1981. [Google Scholar] [CrossRef]
- Peshin, R.; Bandral, R.S.; Zhang, W.; Wilson, L.; Dhawan, A.K. Integrated pest management: A global overview of history, programs, and adoption. In Integrated Pest Management: Innovation-Development Process, Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–49. [Google Scholar]
- Lechelt, J.D.; Bajer, P.G. Modeling the potential for managing invasive common carp in temperate lakes by targeting their winter aggregations. Biol. Invasions 2016, 18, 831–839. [Google Scholar] [CrossRef]
- Weber, M.J.; Hennen, M.J.; Brown, M.L.; Lucchesi, D.O.; St. Sauver, T.R. Compensatory response of invasive common carp Cyprinus carpio to harvest. Fish. Res. 2016, 179, 168–178. [Google Scholar] [CrossRef]
- Pearson, J.; Dunham, J.; Ryan Bellmore, J.; Lyons, D. Modeling control of common carp (Cyprinus carpio) in a shallow lake–wetland system. Wetlands Ecol. Manag. 2019, 27, 663–682. [Google Scholar] [CrossRef]
- Sawyer, A.J. Prospects for integrated pest management of the sea lamprey (Petromyzon marinus). Can. J. Fish. Aquat. Sci. 1980, 37, 2081–2092. [Google Scholar] [CrossRef]
- Christie, G.C.; Goddard, C.I. Sea lamprey international symposium (SLIS II): Advances in the integrated management of sea lamprey in the Great Lakes. J. Great Lakes Res. 2003, 29, 1–14. [Google Scholar] [CrossRef]
- Johnson, N.S.; Yun, S.-S.; Thompson, H.T.; Brant, C.O.; Li, W. A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc. Natl. Acad. Sci. USA 2009, 106, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, P.C.; Taylor, W.W.; Hayes, D.B. Evaluation of age-0 survival and its effect on lake trout rehabilitation in the Michigan waters of Lake Superior. J. Great Lakes Res. 1995, 21, 218–224. [Google Scholar] [CrossRef]
- Cox, B.S.; Guy, C.S.; Fredenberg, W.A.; Rosenthal, L.R. Baseline demographics of a non-native lake trout population and inferences for suppression from sensitivity-elasticity analyses. Fish. Manag. Ecol. 2013, 20, 390–400. [Google Scholar] [CrossRef]
- Bernhart, B.; Blackwood, C.; Gale, R.; Held, E. Yellowstone National Park Lake Trout Crisis: Report Prepared to Fulfill the Requirements of ME 404/ChE 411, Senior Design; College of Engineering, Montana State University: Bozeman, MT, USA, 2004. [Google Scholar] [CrossRef]
- Gross, J.A.; Farokhkish, B.; Gresswell, R.E.; Webb, M.A.H.; Guy, C.S.; Zale, A.V. Techniques for Suppressing Invasive Fishes in Lacustrine Systems: A Literature Review; Final Report for Project RM-CESU H1200040001; National Park Service, Rocky Mountains Cooperative Ecosystem Studies Unit: Yellowstone National Park, WY, USA, 2010; Available online: http://files.cfc.umt.edu/cesu/NPS/MSU/2008/08Zale_YELL_trout%20embryos_lit%20review.pdf (accessed on 10 June 2020).
- Brown, P.J.; Guy, C.S.; Meeuwig, M.H. A comparison of two mobile electrode arrays for increasing mortality of lake trout embryos. N. Am. J. Fish. Manag. 2017, 37, 363–369. [Google Scholar] [CrossRef]
- Doepke, P.D.; Koel, T.M.; Guy, C.S.; Poole, A.S.; Thomas, N.A.; Zale, A.V. Lake trout suppression alternatives to gillnetting. Yellowstone Sci. 2017, 25, 70–73. Available online: https://www.nps.gov/yell/learn/ys-25-1-lake-trout-suppression-alternatives-to-gillnetting.htm (accessed on 10 June 2020).
- Thomas, N.A. Evaluation of Suppression Methods Targeting Non-Native Lake Trout Embryos in Yellowstone Lake, Yellowstone National Park, Wyoming, USA. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2017. [Google Scholar]
- Thomas, N.A.; Guy, C.S.; Koel, T.M.; Zale, A.V. In-situ evaluation of benthic suffocation methods for suppression of invasive lake trout embryos in Yellowstone Lake. N. Am. J. Fish. Manag. 2019, 39, 104–111. [Google Scholar] [CrossRef]
- Poole, A.S.; Koel, T.M.; Thomas, N.A.; Zale, A.V. Suppression of invasive lake trout embryos by fish carcasses and sedimentation in Yellowstone Lake, Yellowstone National Park. N. Am. J. Fish. Manag. (under review).
- Koel, T.M.; Arnold, J.L.; Bigelow, P.E.; Detjens, C.R.; Doepke, P.D.; Ertel, B.D.; MacDonald, D.J. Native Fish Conservation Program, Yellowstone National Park: Report 2015–2018; YCR-2019-04; National Park Service, Yellowstone Center for Resources: Yellowstone National Park, WY, USA, 2019. Available online: https://home.nps.gov/yell/learn/upload/2015-2018-Fish-Report-_Web.pdf (accessed on 10 June 2020).
- Poole, A.S. Evaluation of Embryo Suppression Methods for Nonnative Lake Trout in Yellowstone Lake, Yellowstone National Park, Wyoming, USA. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2019. [Google Scholar]
- Dwyer, W.P. Effect of lowering water temperature on hatching time and survival of lake trout eggs. Prog. Fish-Cult. 1987, 49, 175–176. [Google Scholar] [CrossRef]
- Bronte, C.R.; Selgeby, J.H.; Saylor, J.H.; Miller, G.S.; Foster, N.R. Hatching, dispersal, and bathymetric distribution of age-0 wild lake trout at the Gull Island shoal complex, Lake Superior. J. Great Lakes Res. 1995, 21, 233–245. [Google Scholar] [CrossRef]
- Mullins, M.S. Biology and Predator Use of Cisco (Coregonus artedi) in Fort Peck Reservior, Montana. Master’s Thesis, Montana State University, Bozeman, MT, USA, 1991. [Google Scholar]
- Cherry, T.L.; Shogren, J.F. Invasive species management for the Yellowstone Lake ecosystem: What do visitors think? Yellowstone Sci. 2001, 9, 10–15. Available online: https://www.nps.gov/yell/learn/upload/YS_9_2_sm.pdf (accessed on 10 June 2020).
- National Park Service. Organic Act 16 USC 1—4. ch. 408, 39 Stat. 53511. 25 August 1916. Available online: https://www.nps.gov/parkhistory/online_books/fhpl/nps_organic_act.pdf (accessed on 10 June 2020).
- Sax, D.F.; Stachowicz, J.J.; Brown, J.H.; Bruno, J.F.; Dawson, M.N.; Gaines, S.D.; Grosberg, R.K.; Hastings, A.; Holt, R.D.; Mayfield, M.M.; et al. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007, 22, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Sax, D.F. An essay on some topics concerning invasive species. Austral Ecol. 2004, 29, 530–536. [Google Scholar] [CrossRef]
- Morgan, L.A.; Shanks, P.; Lovalvo, D.; Pierce, K.; Lee, G.; Webring, M.; Stephenson, W.; Johnson, S.; Finn, C.; Schulze, B.; et al. The floor of Yellowstone Lake is anything but quiet! New discoveries in lake mapping. Yellowstone Sci. 2003, 11, 15–30. Available online: https://www.nps.gov/yell/learn/upload/YS_11_2_sm.pdf (accessed on 10 June 2020).
- Pianka, E.R. On r- and K-Selection. Am. Nat. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- Reznick, D.; Bryant, M.J.; Bashey, F. r- and K-selection revisited: The role of population regulation in life-history evolution. Ecology 2002, 83, 1509–1520. [Google Scholar] [CrossRef] [Green Version]
- Sibly, R.M.; Barker, D.; Denham, M.C.; Hone, J.; Pagel, M. On the regulation of populations of mammals, birds, fish, and insects. Science 2005, 309, 607. [Google Scholar] [CrossRef] [Green Version]
- Martin, N.; Olver, C.H. The lake charr, Salvelinus namaycush. In Charrs: Salmonid Fishes of the Genus Salvelinus; Balon, E., Ed.; Kluwer Boston, Inc.: Hingham, MA, USA, 1980; pp. 205–277. [Google Scholar]
- Westley, P.A.H.; Fleming, I.A. Landscape factors that shape a slow and persistent aquatic invasion: Brown trout in Newfoundland 1883–2010. Divers. Distrib. 2011, 17, 566–579. [Google Scholar] [CrossRef]
- Gunn, J.M. Spawning behavior of lake trout: Effects on colonization ability. J. Great Lakes Res. 1995, 21, 323–329. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NY, USA, 1967. [Google Scholar]
- Eklöv, P.; Svanbäck, R. Predation risk influences adaptive morphological variation in fish populations. Am. Nat. 2006, 167, 440–452. [Google Scholar] [CrossRef]
- Suski, C.D.; Cooke, S.J. Conservation of aquatic resources through the use of freshwater protected areas: Opportunities and challenges. Biodivers. Conserv. 2007, 16, 2015–2029. [Google Scholar] [CrossRef]
- Hedges, K.J.; Koops, M.A.; Mandrak, N.E.; Johannsson, O.E. Use of aquatic protected areas in the management of large lakes. Aquat. Ecosyst. Health Manag. 2010, 13, 135–142. [Google Scholar] [CrossRef]
- Parker, S.R.; Mandrak, N.E.; Truscott, J.D.; Lawrence, P.L.; Kraus, D.; Bryan, G.; Molnar, M. Status and extent of aquatic protected areas in the Great Lakes. George Wright Forum 2017, 34, 381–393. [Google Scholar]
- Ricker, W.E. Stock and recruitment. J. Fish. Res. Board Can. 1954, 11, 559–623. [Google Scholar] [CrossRef]
- Hilborn, R.; Walters, C.J.; Ludwig, D. Sustainable exploitation of renewable resources. Ann. Rev. Ecol. Syst. 1995, 26, 45–67. [Google Scholar] [CrossRef]
- Rose, K.A.; Cowan Jr, J.H.; Winemiller, K.O.; Myers, R.A.; Hilborn, R. Compensatory density dependence in fish populations: Importance, controversy, understanding and prognosis. Fish Fish. 2001, 2, 293–327. [Google Scholar] [CrossRef] [Green Version]
- Abrams, P.A. When does greater mortality increase population size? The long history and diverse mechanisms underlying the hydra effect. Ecol. Lett. 2009, 12, 462–474. [Google Scholar] [CrossRef]
- Hansen, M.J. Lake trout in the Great Lakes: Basinwide stock collapse and binational restoration. In Great Lakes Fisheries Policy and Management; Taylor, W.W., Ferreri, C.P., Eds.; Michigan State University Press: East Lansing, MI, USA, 1999; pp. 417–454. [Google Scholar]
- Post, J.R. Resilient recreational fisheries or prone to collapse? A decade of research on the science and management of recreational fisheries. Fish. Manag. Ecol. 2013, 20, 99–110. [Google Scholar] [CrossRef]
- Embke, H.S.; Rypel, A.L.; Carpenter, S.R.; Sass, G.G.; Ogle, D.; Cichosz, T.; Hennessy, J.; Essington, T.E.; Vander Zanden, M.J. Production dynamics reveal hidden overharvest of inland recreational fisheries. Proc. Natl. Acad. Sci. USA 2019, 116, 24676. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; van Leeuwen, A.; Cameron, T.C. When less is more: Positive population-level effects of mortality. Trends Ecol. Evol. 2014, 29, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Ellrott, B.J.; Marsden, J.E. Lake trout reproduction in Lake Champlain. Trans. Am. Fish. Soc. 2004, 133, 252–264. [Google Scholar] [CrossRef]
- Jonas, J.L.; Claramunt, R.M.; Fitzsimons, J.D.; Marsden, J.E.; Ellrott, B.J. Estimates of egg deposition and effects of lake trout (Salvelinus namaycush) egg predators in three regions of the Great Lakes. Can. J. Fish. Aquat. Sci. 2005, 62, 2254–2264. [Google Scholar] [CrossRef]
- Bajer, P.G.; Chizinski, C.J.; Silbernagel, J.J.; Sorensen, P.W. Variation in native micro-predator abundance explains recruitment of a mobile invasive fish, the common carp, in a naturally unstable environment. Biol. Invasions 2012, 14, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Bajer, P.G.; Sorensen, P.W. Recruitment and abundance of an invasive fish, the common carp, is driven by its propensity to invade and reproduce in basins that experience winter-time hypoxia in interconnected lakes. Biol. Invasions 2010, 12, 1101–1112. [Google Scholar] [CrossRef]
- Claramunt, R.M.; Jonas, J.L.; Fitzsimons, J.D.; Marsden, J.E. Influences of spawning habitat characteristics and interstitial predators on lake trout egg deposition and mortality. Trans. Am. Fish. Soc. 2005, 134, 1048–1057. [Google Scholar] [CrossRef]
- Fitzsimons, J.D.; Perkins, D.L.; Krueger, C.C. Sculpins and crayfish in lake trout spawning areas in Lake Ontario: Estimates of abundance and egg predation on lake trout eggs. J. Great Lakes Res. 2002, 28, 421–436. [Google Scholar] [CrossRef]
- Riley, J.W.; Marsden, J.E. Predation on emergent lake trout fry in Lake Champlain. J. Great Lakes Res. 2009, 35, 175–181. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Ingram, T.; Stutz, W.E.; Snowberg, L.K.; Lau, O.L.; Paull, J.S. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B Biol. Sci. 2010, 277, 1789–1797. [Google Scholar] [CrossRef]
- Zipkin, E.F.; Kraft, C.E.; Cooch, E.G.; Sullivan, P.J. When can efforts to control nuisance and invasive species backfire? Ecol. Appl. 2009, 19, 1585–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zipkin, E.F.; Sullivan, P.J.; Cooch, E.G.; Kraft, C.E.; Shuter, B.J.; Weidel, B.C. Overcompensatory response of a smallmouth bass (Micropterus dolomieu) population to harvest: Release from competition? Can. J. Fish. Aquat. Sci. 2008, 65, 2279–2292. [Google Scholar] [CrossRef] [Green Version]
- Karatayev, V.A.; Kraft, C.E.; Zipkin, E.F. Racing through life: Maturation rate plasticity regulates overcompensation and increases persistence. Ecosphere 2015, 6, art203. [Google Scholar] [CrossRef]
- Ohlberger, J.; Langangen, Ø.; Edeline, E.; Claessen, D.; Winfield, I.J.; Stenseth, N.C.; Vøllestad, L.A. Stage-specific biomass overcompensation by juveniles in response to increased adult mortality in a wild fish population. Ecology 2011, 92, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.J. Personnal Communication; U.S. Geological Survey, Great Lakes Science Center, Hammond Bay Biological Station: Millersburg, MI, USA, 2020. [Google Scholar]
- Syslo, J.M. Dynamics of Yellowstone Cutthroat Trout and Lake Trout in the Yellowstone Lake Ecosystem: A Case Study for the Ecology and Management of Non-Native Fishes. Ph.D. Thesis, Montana State University, Bozeman, MT, USA, 2015. [Google Scholar]
- Neumann, R.M.; Guy, C.S.; Willis, D.W. Length, weight, and associated structural indices. In Fisheries Techniques, 3rd ed.; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 637–676. [Google Scholar]
- Wickham, H.; François, R.; Lionel, H.; Müller, K. dplyr: A Grammar of Data Manipulation, R Package Version 0.8.4; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2019. [Google Scholar]














| Desired Conditions | Conservation Actions | Quantitative Responses | Performance Metrics |
|---|---|---|---|
Primary
|
|
|
|
|
| ||
Secondary
|
|
|
|
|
| ||
Tertiary
|
|
|
|
|
| ||
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koel, T.M.; Arnold, J.L.; Bigelow, P.E.; Brenden, T.O.; Davis, J.D.; Detjens, C.R.; Doepke, P.D.; Ertel, B.D.; Glassic, H.C.; Gresswell, R.E.; et al. Yellowstone Lake Ecosystem Restoration: A Case Study for Invasive Fish Management. Fishes 2020, 5, 18. https://doi.org/10.3390/fishes5020018
Koel TM, Arnold JL, Bigelow PE, Brenden TO, Davis JD, Detjens CR, Doepke PD, Ertel BD, Glassic HC, Gresswell RE, et al. Yellowstone Lake Ecosystem Restoration: A Case Study for Invasive Fish Management. Fishes. 2020; 5(2):18. https://doi.org/10.3390/fishes5020018
Chicago/Turabian StyleKoel, Todd M., Jeffery L. Arnold, Patricia E. Bigelow, Travis O. Brenden, Jeffery D. Davis, Colleen R. Detjens, Philip D. Doepke, Brian D. Ertel, Hayley C. Glassic, Robert E. Gresswell, and et al. 2020. "Yellowstone Lake Ecosystem Restoration: A Case Study for Invasive Fish Management" Fishes 5, no. 2: 18. https://doi.org/10.3390/fishes5020018
APA StyleKoel, T. M., Arnold, J. L., Bigelow, P. E., Brenden, T. O., Davis, J. D., Detjens, C. R., Doepke, P. D., Ertel, B. D., Glassic, H. C., Gresswell, R. E., Guy, C. S., MacDonald, D. J., Ruhl, M. E., Stuth, T. J., Sweet, D. P., Syslo, J. M., Thomas, N. A., Tronstad, L. M., White, P. J., & Zale, A. V. (2020). Yellowstone Lake Ecosystem Restoration: A Case Study for Invasive Fish Management. Fishes, 5(2), 18. https://doi.org/10.3390/fishes5020018





