Molecular Characterization of Aquaporins Genes from the Razor Clam Sinonovacula constricta and Their Potential Role in Salinity Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Cultivation
2.2. Tissue and Spatiotemporal Expression Analysis
2.3. Low- and High-Salinity Challenges
2.4. RNA Isolation and Reverse Transcription
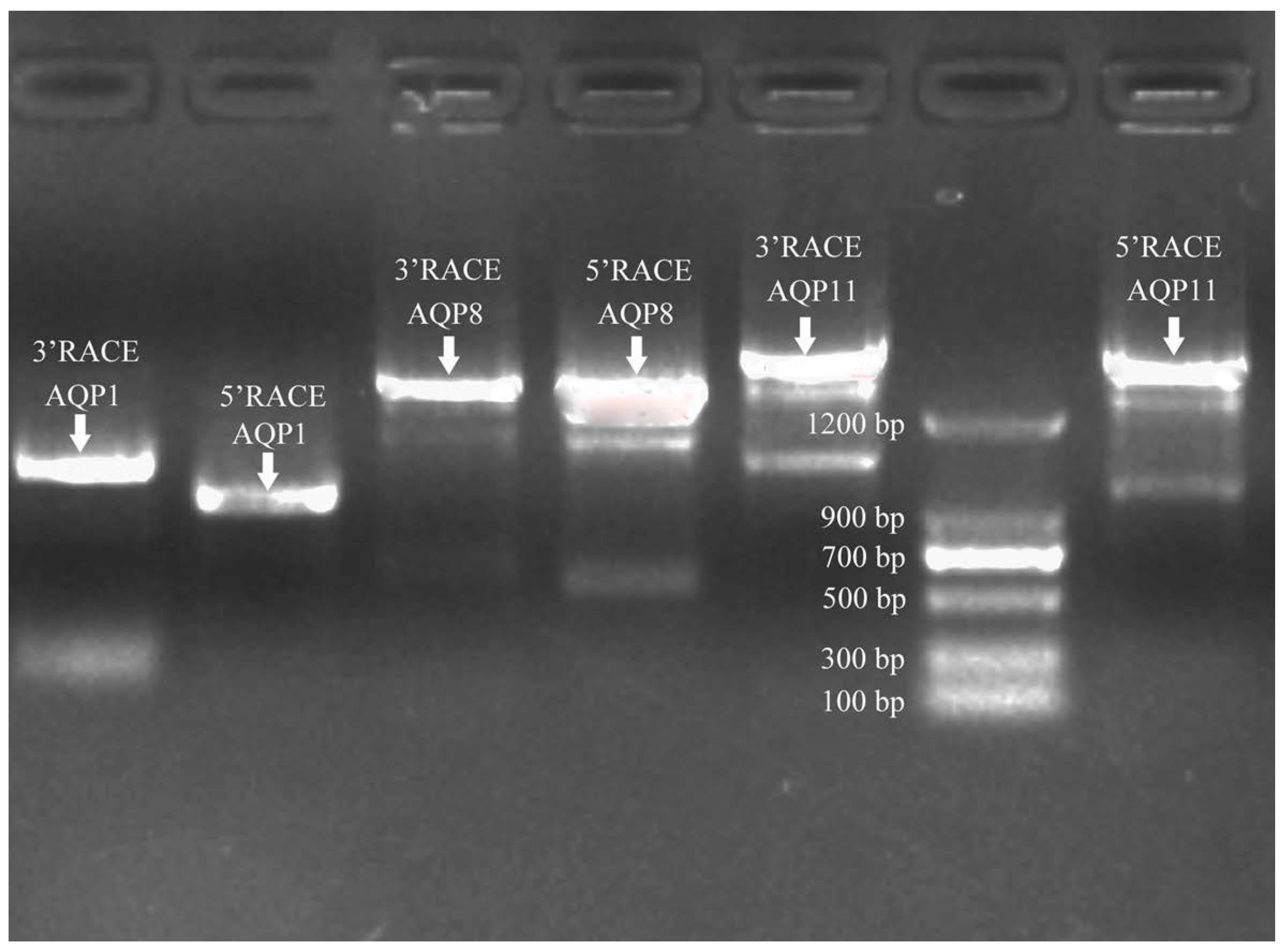
2.5. Cloning of Sc-AQP1, Sc-AQP8, and Sc-AQP11 cDNA Sequence
2.6. Bioinformatics Sequence Analysis
2.7. Multiple Sequences Alignment and Phylogenetic Analysis
2.8. qRT-PCR Analyses
2.9. RNA Interference
2.10. Fluorescence In Situ Hybridization
2.11. Statistical Analysis
3. Results
3.1. Sequence Features of Sc-AQP1, Sc-AQP8, and Sc-AQP11
3.2. Phylogenetic Tree Analysis
3.3. Spatiotemporal Expression Analysis of Sc-AQP1, Sc-AQP8, and Sc-AQP11
3.4. Quantitative Expression Analysis of Sc-AQP1, Sc-AQP8, and Sc-AQP11 mRNA after Salinity Challenge
3.5. Detection of the Osmotic Pressure of Hemolymph after Salinity Challenge
3.6. Effects of dsRNA and siRNA on Sc-AQP1, Sc-AQP8, and Sc-AQP11 Expression and Osmotic Pressure
3.7. Fluorescence In Situ Hybridization of Sc-AQP1, Sc-AQP8, and Sc-AQP11
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hachez, C.; Chaumont, F. Aquaporins: A Family of Highly Regulated Multifunctional Channels. Adv. Exp. Med. Biol. 2010, 679, 1–17. [Google Scholar] [PubMed]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol.-Renal. Physiol. 1993, 265, F463–F476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.S.; Prestont, G.M.; Smith, B.L.; Guggino, W.B.; Agre, P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994, 269, 14648–14654. [Google Scholar] [CrossRef]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef]
- Hill, A.E.; Shachar-Hill, B.; Shachar-Hill, Y. What Are Aquaporins For? J. Membr. Biol. 2004, 197, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Kosicka, E.; Grobys, D.; Kmita, H.; Lesicki, A.; Pieńkowska, J. Putative new groups of invertebrate water channels based on the snail Helix pomatia L. (Helicidae) MIP protein identification and phylogenetic analysis. Eur. J. Cell Biol. 2016, 95, 543–551. [Google Scholar] [CrossRef]
- Ren, G.; Reddy, V.S.; Cheng, A.; Melnyk, P.; Mitra, A.K. Visualization of a water-selective pore by electron crystallography in vitreous ice. Proc. Natl. Acad. Sci. USA 2001, 98, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Han, B.G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Kreida, S.; Törnroth-Horsefield, S. Trnroth-Horsefield. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, Y.; Zhang, Y.H.; Zi-Niu, Y.U. Molecular cloning, characterization, and expression of aquaprorin1 gene in Crassostrea Hongkongensis. Oceanol. Et Limnol. Sinica. 2015, 46, 1078–1087. [Google Scholar]
- Ran, Z.; Chen, H.; Ran, Y.; Yu, S.; Li, S.; Xu, J.; Liao, K.; Yu, X.; Zhong, Y.; Ye, M.; et al. Fatty acid and sterol changes in razor clam Sinonovacula constricta (Lamarck 1818) reared at different salinities. Aquaculture 2017, 473, 493–500. [Google Scholar] [CrossRef]
- Yan, H.; Li, Q.; Liu, W.; Yu, R.; Kong, L. Seasonal changes in reproductive activity and biochemical composition of the razor clam Sinonovacula constricta (Lamarck 1818). Mar. Biol. Res. 2010, 6, 78–88. [Google Scholar] [CrossRef]
- Hu, M.Y.; Sung, P.H.; Guh, Y.J.; Lee, J.R.; Hwang, P.P.; Weihrauch, D.; Tseng, Y.C. Perfused Gills Reveal Fundamental Principles of pH Regulation and Ammonia Homeostasis in the Cephalopod Octopus vulgaris. Front. Physiol. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, S.S.; Bujak, J.; Tipsmark, C.K. Aquaporin expression in the Japanese medaka (Oryzias latipes) in freshwater and seawater: Challenging the paradigm of intestinal water transport? J. Exp. Biol. 2014, 217, 3108–3121. [Google Scholar] [PubMed] [Green Version]
- Murakami, Y.; Kinoshita, K. 3P030 A Study for the Structural and Physicochemical Properties of Sociable Ligand-Binding Sites in Proteins (01B. Protein: Structure & Function, Poster, The 52nd Annual Meeting of the Biophysical Society of Japan (BSJ2014)). Seibutsu Butsuri 2014, 54, S253. [Google Scholar]
- Liu, Y.; Yao, H.; Zhou, T.; Lin, Z.; Dong, Y. The Discovery of Circadian Rhythm of Feeding Time on Digestive Enzymes Activity and Their Gene Expression in Sinonovacula constricta within a Light/Dark Cycle. Front. Mar. Sci. 2021, 8, 744212. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhao, Q.L.; Jin, W.B.; You, S.J.; Zhang, J.N. Performance of biological phosphorus removal and characteristics of microbial community in the oxic-settling-anaerobic process by FISH analysis. J. Zhejiang Univ.-SCI. A 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Politis, S.N.; David, M.; Arianna, S.; Jose-Luis, Z.I.; Miest, J.J.; Jonna, T.; Butts, I.; Chen, T.Y. Salinity reduction benefits European eel larvae: Insights at the morphological and molecular level. PLoS ONE 2018, 13, e0198294. [Google Scholar] [CrossRef] [Green Version]
- Yasui, M. Molecular mechanisms and drug development in aquaporin water channel diseases: Structure and function of aquaporins. J. Pharmacol. Sci. 2004, 96, 260–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Nejsum, L.N.; Zelenina, M.; Aperia, A.; Frøkiær, J.; Nielsen, S.; Nejsum, L.N.; Zelenina, M.; Aperia, A.; Frøkiær, J.; Nielsen, S. Bidirectional regulation of AQP2 trafficking and recycling: Involvement of AQP2-S256 phosphorylation. Am. J. Physiol.-Ren. Physiol. 2005, 57, F930–F938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walz, T.; Fujiyoshi, Y.; Engel, A. The AQP Structure and Functional Implications. Handb. Exp. Pharmacol. 2009, 190, 31–56. [Google Scholar]
- Törnrothhorsefield, S.; Hedfalk, K.; Fischer, G.; Lindkvistpetersson, K.; Neutze, R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010, 584, 2580–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvanese, L.; Pellegrini-Calace, M.; Oliva, R. In silico study of human aquaporin AQP11 and AQP12 channels. Protein Sci. 2013, 22, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Yakata, K.; Tani, K.; Fujiyoshi, Y. Water permeability and characterization of aquaporin-11. J. Struct. Biol. 2011, 174, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. New Members of Mammalian Aquaporins: AQP10–AQP12. Handb. Exp. Pharmacol. 2009, 190, 251. [Google Scholar]
- Giffard-Mena, I.; Boulo, V.; Aujoulat, F.; Fowden, H.; Castille, R.; Charmantier, G.; Cramb, G. Aquaporin molecular characterization in the sea-bass (Dicentrarchus labrax): The effect of salinity on AQP1 and AQP3 expression. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, H.; Xu, M.; Xu, H.; Zhang, H.; He, M. Cloning and expression analysis of aquaporin gene AQP4 cDNA from Pinctada fucata martensii. J. Trop. Oceanogr. 2020, 39, 66–75. [Google Scholar]
- Pienkowska, J.R.; Kosicka, E.; Wojtkowska, M.; Kmita, H.; Lesicki, A. Molecular identification of first putative aquaporins in snails. J. Membr. Biol. 2014, 247, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Drake, L.L.; Boudko, D.Y.; Marinotti, O.; Carpenter, V.K.; Dawe, A.L.; Hansen, I.A. The Aquaporin Gene Family of the Yellow Fever Mosquito, Aedes aegypti. PLoS ONE 2010, 5, e15578. [Google Scholar] [CrossRef] [Green Version]
- Hua, G.; Min, W.; Yang, L.; Ying, Z.; Chen, S. Molecular cloning and expression analysis of the aqp1aa gene in half-smooth tongue sole (Cynoglossus semilaevis). PLoS ONE 2017, 12, e0175033. [Google Scholar]
- Kamila, F.; Robert, T.B.; Monique, T.R.; Carolina, A.F.; Marta, M.S. Aquaporin in different moult stages of a freshwater decapod crustacean: Expression and participation in muscle hydration control. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 208, 61–69. [Google Scholar]
- Pallone, T.L.; Edwards, A.; Ma, T.; Silldorff, E.P.; Verkman, A.S. Requirement of aquaporin-1 for NaCl-driven water transport across descending vasa recta. J. Clin. Investig. 2000, 105, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Deane, E.E.; Luk, J.; Woo, N. Aquaporin 1a Expression in Gill, Intestine, and Kidney of the Euryhaline Silver Sea Bream. Front. Physiol. 2011, 2, 39. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.K.; Watanabe, S.; Kaneko, T.; Min, D.H.; Park, S.I. Expression of aquaporins 3, 8 and 10 in the intestines of freshwater- and seawater-acclimated Japanese eels Anguilla Japonica. Fish. Sci. 2010, 76, 695–702. [Google Scholar]
- Chung, J.S.; Maurer, L.; Bratcher, M.; Pitula, J.S.; Ogburn, M.B. Cloning of aquaporin-1 of the blue crab, Callinectes sapidus: Its expression during the larval development in hyposalinity. Aquat. Biosyst. 2012, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, H.; Sakamoto, J.M.; Rasgon, J.L. Functional characterization of Aquaporin-like genes in the human bed bug Cimex lectularius. Sci. Rep. 2017, 7, 3214. [Google Scholar] [CrossRef] [Green Version]
- Laforenza, U.; Cova, E.; Gastaldi, G.; Tritto, S.; Ventura, U. Aquaporin-8 Is Involved in Water Transport in Isolated Superficial Colonocytes from Rat Proximal Colon1. J. Nutr. 2005, 135, 2329–2336. [Google Scholar] [CrossRef] [Green Version]
- Ferri, D.; Mazzone, A.; Liquori, G.E.; Cassano, G.; Svelto, M.; Calamita, G. Ontogeny, distribution, and possible functional implications of an unusual aquaporin, AQP8, in mouse liver. Hepatology 2003, 38, 947–957. [Google Scholar] [CrossRef]
- Holm, L.M.; Jahn, T.P.; Mller, A.L.B.; Schjoerring, J.K.; Zeuthen, T. NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflüg. Arch. 2005, 450, 415–428. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, S.Y.; Kim, B.S.; Kim, D.S.; Nam, Y.K. Isolation and mRNA expression analysis of aquaporin isoforms in marine medaka Oryzias dancena, a euryhaline teleost. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 171, 1–8. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Calusinska, M.; Finn, R.N.; Chauvigné, F.; Lozano, J.; Cerdà, J. The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evol. Biol. 2010, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, K.; Imai, M.; Sasaki, S. Cellular Localization of Aquaporin 7 in the Rat Kidney. Exp. Nephrol. 2000, 8, 252–257. [Google Scholar] [CrossRef]
- Castro-Claros, J.D.; Checa, A.; Lucena, C.; Pearson, J.R.; Salas, C. Shell-adductor muscle attachment and Ca2+ transport in the bivalves Ostrea stentina and Anomia ephippium. Acta Biomater. 2020, 120, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of Aquaporin-11 Produces Polycystic Kidneys following Vacuolization of the Proximal Tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosling, E. Bivalve Molluscs: Biology, Ecology and Culture; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 433–435. [Google Scholar]
- Tipsmark, C.K.; Sorensen, K.J.; Madsen, S.S. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J. Exp. Biol. 2010, 213, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Shin, H.S.; Kim, N.N.; Cho, S.H.; Yamamoto, Y.; Ueda, H.; Lee, J.; Choi, C.Y. Expression of aquaporin-3 and −8 mRNAs in the parr and smolt stages of sockeye salmon, Oncorhynchus nerka: Effects of cortisol treatment and seawater acclimation. Comp. Biochem. Phys. A 2013, 165, 228–236. [Google Scholar] [CrossRef]
- Chino, N.; Arai, T. Migratory history of the giant mottled eel (Anguilla marmorata) in the Bonin Islands of Japan. Ecol. Freshw. Fish 2010, 19, 19–25. [Google Scholar] [CrossRef]
- Strathmann, R. Selection for retention or export of larvae in estuaries. In Estuarine Comparisons; Elsevier Inc.: Amsterdam, The Netherlands, 1982; pp. 521–536. [Google Scholar]
- Yeste, M.; Morató, R.; Rodríguez-Gil, J.; Bonet, S.; Prieto-Martínez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Domest. Anim. 2017, 52, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerdà, J. Molecular pathways during marine fish egg hydration: The role of aquaporins. J. Fish Biol. 2010, 75, 2175–2196. [Google Scholar] [CrossRef] [PubMed]








| Primer | Primer Sequence (5′-3′) | Purpose | Amplicon Size (bp) |
|---|---|---|---|
| 5′RACE-AQP1 | CCAGCAGTCCACCCACAATAGGC | 5RACE | 1126 |
| 5′RACE-AQP8 | CCAGGCTCCACAGTCGTGTCACCAAT | 5′RACE | 761 |
| 5′RACE-AQP11 | TCCCCCCACCAAGACTGCCGAAT | 5′RACE | 879 |
| 3′RACE-AQP1 | ACACCAGCAACTCCAGCCACCCT | 3′RACE | 1167 |
| 3′RACE-AQP8 | TGGTGACACGACTGTGGAGCCTGG | 3′RACE | 1498 |
| 3′RACE-AQP11 | CGGCTTTTGGCTCTTTATCGCTGTT | 3′RACE | 1381 |
| RT-AQP1F | CACCAGCAACTCCAGCCA | qRT-PCR | 142 |
| RT-AQP1R | CAGGACCGCCCTCCATAA | qRT-PCR | |
| RT-AQP8F | CATCTGTCCCCGATTATTGGT | qRT-PCR | 90 |
| RT-AQP8R | GGTGAAGACGAGAACCAGTGT | qRT-PCR | |
| RT-AQP11F | TGCCTGAACCAATCAAAAC | qRT-PCR | 120 |
| RT-AQP11R | CAGCGATAAAGAGCCAAAA | qRT-PCR | |
| 18SF | TCGGTTCTATTGCGTTGGTTTT | qRT-PCR | 121 |
| 18SR | CAGTTGGCATCGTTTATGGTCA | qRT-PCR | |
| dsRNAi- AQP1F1 | TAATACGACTCACTATAGGGGTCACCCCTAGCCGTCTACA | RNAi | 320 |
| dsRNAi- AQP1R1 | TAATACGACTCACTATAGGGAGCAGTCCACCCACAATAGG | RNAi | |
| dsRNAi- AQP8F1 | TAATACGACTCACTATAGGGTCGGGGTGACATTGTTTGTA | RNAi | 316 |
| dsRNAi- AQP8R1 | TAATACGACTCACTATAGGGCCAATAATCGGGGACAGATG | RNAi | |
| dsRNAi- AQP11F1 | TAATACGACTCACTATAGGGTGATGCCTGAACCAATCAAA | RNAi | 379 |
| dsRNAi- AQP11R1 | TAATACGACTCACTATAGGGCCGTAAAAGAACGCCACATT | RNAi | |
| siRNAi- AQP1F1 | GATCACTAATACGACTCACTATAGGGTTCAGATGTTCGGACACATTTCATT | RNAi | 22 |
| siRNAi- AQP1R1 | AATGAAATGTGTCCGAACATCTGAAGTGATC | RNAi | |
| siRNAi- AQP1F2 | AATTCAGATGTTCGGACACATTTCAGTGATC | RNAi | 22 |
| siRNAi- AQP1R2 | GATCACTAATACGACTCACTATAGGGGCTCCGAAAAATGTTCTGTTGGCTT | RNAi | |
| siRNAi- AQP8F1 | GATCACTAATACGACTCACTATAGGGCTGCTGAATGAAGAACATCGAACTT | RNAi | 22 |
| siRNAi- AQP8R1 | GCTCCGAAAAATGTTCTGTTGGCGTGATC | RNAi | |
| siRNAi- AQP8F2 | AACTGCTGAATGAAGAACATCGAACGTGATC | RNAi | 22 |
| siRNAi- AQP8R2 | GATCACTAATACGACTCACTATAGGGGTTCGATGTTCTTCATTCAGCAGTT | RNAi | |
| siRNAi- AQP11F1 | GATCACTAATACGACTCACTATAGGGGCCAACAGAACATTTTTCGGAGCTT | RNAi | 22 |
| siRNAi- AQP11R1 | AAGCTCCGAAAAATGTTCTGTTGGCGTGATC | RNAi | |
| siRNAi- AQP11F2 | AAGCCAACAGAACATTTTTCGGAGCGTGATC | RNAi | 22 |
| siRNAi- AQP11R2 | GATCACTAATACGACTCACTATAGGGGCTCCGAAAAATGTTCTGTTGGCTT | RNAi |
| Sequence Features | Sc-AQP1 | Sc-AQP8 | Sc-AQP11 |
|---|---|---|---|
| Gen Bank ID | MN186579 | MN186580 | MN186581 |
| cDNA length (bp) | 1546 | 2235 | 1713 |
| ORF (bp) | 900 | 771 | 843 |
| Length of amino acids (aa) | 299 | 256 | 280 |
| Molecular weight (kDa) | 32.34 | 26.80 | 31.52 |
| Theoretical pI | 6.19 | 6.37 | 5.89 |
| GRAVY | 0.464 | 0.738 | 0.415 |
| Asp + Glu | 21 | 17 | 25 |
| Arg + Lys | 19 | 15 | 20 |
| Instability index | 35.97 | 31.13 | 31.35 |
| Aliphatic index | 97.53 | 126.87 | 99.61 |
| 5′-UTR (bp) | 405 | 336 | 93 |
| 3′-UTR (bp) | 240 | 1128 | 777 |
| ATTTA motif | 6 | 8 | 4 |
| AATAA motif | 3 | 3 | 6 |
| NPA motifs | 2 | 2 | 1 |
| NPC motifs | 0 | 0 | 1 |
| MIP motifs | 1 | 1 | 1 |
| Transmembrane helix | 6 | 6 | 6 |
| loops | 5 | 5 | 5 |
| AQP * | Sc-AQP1 | Sc-AQP8 | Sc-AQP11 | |||
|---|---|---|---|---|---|---|
| Identity (%) | Similarity (%) | Identity (%) | Similarity (%) | Identity (%) | Similarity (%) | |
| Homo sapiens—AQP1 | 35.9 | 49.3 | 30.6 | 43.5 | 12.3 | 22.5 |
| Mus musculus—AQP1 | 35.3 | 49.7 | 31 | 44.2 | 12 | 22.8 |
| Gallus gallus—AQP1 | 25.7 | 38.3 | 26.4 | 36.4 | 10.8 | 19 |
| Xenopus tropicalis—AQP1 | 34.6 | 49.7 | 29.8 | 42.8 | 10.3 | 22.1 |
| Danio rerio—AQP1 | 32.8 | 47.7 | 30.7 | 41.4 | 9.8 | 19 |
| Sc-AQP1 | 100 | 100 | 27.5 | 39.6 | 11.3 | 21.6 |
| Aplysia californica—AQP1 | 47 | 66.2 | 23.9 | 41.1 | 10.6 | 23.9 |
| Mizuhopecten yessoensis—AQP1 | 44.4 | 65.1 | 26.4 | 40.4 | 10.4 | 21.1 |
| Helix pomatia—AQP1 | 46.4 | 63.5 | 24.5 | 41.5 | 11.9 | 24.7 |
| Crassostrea hongkongensis—AQP1 | 43.7 | 59.6 | 27.8 | 39.8 | 11.2 | 19.9 |
| Homo sapiens—AQP8 | 22.8 | 37 | 37 | 54.3 | 13.3 | 23.5 |
| Mus musculus—AQP8 | 22.9 | 38.1 | 38.2 | 57.1 | 13.3 | 23.5 |
| Gallus gallus—AQP8 | 21.4 | 34.4 | 37.4 | 54 | 13.7 | 26.7 |
| Alligator sinensis—AQP8 | 21.2 | 35.4 | 37.4 | 53 | 12.3 | 22.3 |
| Danio rerio—AQP8 | 19.9 | 32.8 | 33.2 | 50.6 | 14.4 | 25 |
| Sc-AQP8 | 27.5 | 39.6 | 100 | 100 | 13.2 | 23.2 |
| Crassostrea gigas—AQP8 | 23.5 | 37.8 | 54.7 | 67.6 | 12.5 | 23.1 |
| Mizuhopecten yessoensis—AQP8 | 27.7 | 42.3 | 46.1 | 60.3 | 11.5 | 20.2 |
| Pomacea canaliculata—AQP8 | 26 | 38.9 | 50.2 | 64.1 | 13.3 | 21.4 |
| Aplysia californica—AQP8 | 28.5 | 39.8 | 51 | 64.1 | 12.2 | 22.7 |
| Homo sapiens—AQP11 | 13.5 | 24.5 | 14.1 | 24.1 | 18 | 31.7 |
| Mus musculus—AQP11 | 13.8 | 26 | 14.4 | 25.1 | 18 | 32.7 |
| Gallus gallus—AQP11 | 12 | 23.7 | 16 | 30.1 | 15.5 | 28.3 |
| Xenopus laevis—AQP11 | 12 | 21.5 | 14.4 | 29.1 | 19.7 | 30 |
| Danio rerio—AQP11 | 11.4 | 20.9 | 17.2 | 30.5 | 17.7 | 28.2 |
| Sc-AQP11 | 11.3 | 21.6 | 13.2 | 23.2 | 100 | 100 |
| Crassostrea virginica—AQP11 | 11.9 | 23.5 | 11.6 | 22.5 | 57.7 | 72.2 |
| Crassostrea gigas—AQP11 | 11.4 | 22 | 11.1 | 21.3 | 54.9 | 68.9 |
| Biomphalaria glabrata—AQP11 | 9.4 | 21.1 | 12.1 | 24.3 | 49.1 | 63.6 |
| Mizuhopecten yessoensis—AQP11 | 11 | 22.8 | 15.1 | 24.4 | 51 | 65.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, W.; Dong, Y.; Lin, Z.; He, L. Molecular Characterization of Aquaporins Genes from the Razor Clam Sinonovacula constricta and Their Potential Role in Salinity Tolerance. Fishes 2022, 7, 69. https://doi.org/10.3390/fishes7020069
Ruan W, Dong Y, Lin Z, He L. Molecular Characterization of Aquaporins Genes from the Razor Clam Sinonovacula constricta and Their Potential Role in Salinity Tolerance. Fishes. 2022; 7(2):69. https://doi.org/10.3390/fishes7020069
Chicago/Turabian StyleRuan, Wenbin, Yinghui Dong, Zhihua Lin, and Lin He. 2022. "Molecular Characterization of Aquaporins Genes from the Razor Clam Sinonovacula constricta and Their Potential Role in Salinity Tolerance" Fishes 7, no. 2: 69. https://doi.org/10.3390/fishes7020069
APA StyleRuan, W., Dong, Y., Lin, Z., & He, L. (2022). Molecular Characterization of Aquaporins Genes from the Razor Clam Sinonovacula constricta and Their Potential Role in Salinity Tolerance. Fishes, 7(2), 69. https://doi.org/10.3390/fishes7020069





