Abstract
Given the European eel population’s marked decrease since the 1980s, it has become urgent to collect information describing its regional population structure to improve management plans. The Minho River (NW-Portugal, SW-Europe) is an important basin for the eel at the southern limit of its distribution, but the species is poorly described. Thus, we aimed to study the structure of the European eel population in the Minho River using otolith shape analysis, which has proven to be effective in discriminating fish groups experiencing different environmental conditions through ontogeny. Our results showed complete discrimination between the two main types of habitats studied (tributaries and estuaries). Otoliths of eels from the estuary were rectangular and elliptic, whereas in the tributaries they presented a more round and circular form. Eels collected in both habitats were mostly yellow-stage eels with a similar age range, but the eels from the tributaries showed smaller length-at-age and lower body condition than those collected in the estuary. Additionally, the sex ratio was skewed towards males in the tributaries and females in the estuary. This study reveals that there are at least two distinct groups of eels in this basin, likely with different development characteristics.
1. Introduction
The European eel Anguilla anguilla (Linnaeus, 1758) population is currently outside of safe biological limits [1]; the number of eels reaching the European coastal areas has decreased by 90% since the 1980s [2,3,4]. Eel fishing still occurs throughout most of Europe and across most life stages [5], even though this species is considered critically endangered by the IUCN’s Red List and listed in Appendix II of the CITES and Bonn Convention [6,7,8]. The lack of basic knowledge about the status of regional stocks and its vast distribution range has precluded the development of adequate management plans [5,9,10,11,12]. Eels grow and adapt to habitats, from freshwater to saltwater, presenting a high degree of flexibility in habitat use patterns [13,14,15]. Identifying regional population structure is of outmost importance to unravel how each river basin is contributing to the spawning population.
The European eel (hereafter, eel) is a semelparous and semi-catadromous fish species with a single randomly mating population [13,15,16]. It has a remarkable life cycle which includes migration of ca. 6000 km between the spawning grounds in the Sargasso Sea on the NW-Atlantic Ocean and the nursery areas distributed along the coasts of Europe and Northern Africa [17,18,19]. Leptocephali (larvae) drift across the Atlantic Ocean and metamorphose into glass eels before entering the continental shelf [20]. They then use divergent colonization migratory tactics depending on the time of arrival and the settlement habitat [21,22]. The growth phase in coastal waters typically lasts from 2 to 20 years (i.e., the yellow eel stage), increasing with decreasing temperatures [23]. After this period, they metamorphose into silver eels and achieve sexual maturation as they swim back to their spawning grounds [24,25].
At the scale of the species distribution, eel growth rates decrease from the south to the north of Europe [23]. Additionally, as the result of the latitudinal gradients of temperature, photoperiod, hydrology, and productivity, eels achieve larger sizes with increasing latitude and distance from the spawning areas [26]. Within river basins, eel development can vary according to environmental drivers such as temperature, salinity, depth, and food availability [15,27,28]. Downstream brackish and marine environments promote higher growth rates in eels than upstream freshwater or riverine habitats [29]. Demographical factors such as sex and density are also known to influence individual development [30,31,32]. There is a predominance of males in Southern Europe and a predominance of females in Northern Europe [23]. Males generally occupy the most downstream areas in a river, grow fast, and mature earlier at a smaller size, whereas females develop slowly in upstream areas and mature later at larger sizes [23,33,34]. Eel growth is usually low at higher densities due to increased intraspecific competition for resources or habitat loss [35,36]. Males tend to predominate in such environments [28,30,37], which seems to contradict the general idea of faster growth in males [18,38]. Nonetheless, several aspects of a riverine environment are known to affect trophic interactions, fish distribution, and development, such as productivity [39], ecosystem size [40,41], and disturbance [41,42]. The interactions between these factors differ between basins, which will then reflect on the dynamic and river-specific eel biology/ecology, not following the specific expected patterns.
The observed variations in development across the eel’s distribution range could be reflected, for example, in the otolith formation [43,44,45]. Otoliths are metabolically inert structures less vulnerable to post-depositional chemical and structural modification, as they grow through uptake from the water masses through which a fish passes during its lifetime [44,46,47], being good records of its life. Otoliths are widely used for age estimation, but their microstructure can also be used to investigate habitat characteristics and regional differences or similarities between fish populations [48,49]. They have proven to successfully identify the population structure of several fish species [50,51,52]. Endogenous and exogenous factors determine both otoliths’ overall shape and growth patterns [53,54], functioning as good phenotypic markers, as their formation is influenced by feeding regime and differences in body condition and growth [55,56,57]. Otolith shape has been studied for the European eel in the Mediterranean and successfully discriminated eels that grow in different habitat types, proving to be a valuable tool to study this species ontogeny [58,59].
In this study, we aimed to investigate for the first time the population structure of the European eel in the Minho River using the otolith shape signature. The eels’ biological characteristics were recorded to explore and discuss possible differences between eel groups. This river basin is characterized by different habitats, such as upstream tributary and downstream estuary ecosystems, whose dynamics and specific characteristics will determine the ecological assemblage and its influence on eel life history. We hypothesize that eels present distinct development strategies in these different environments, which will reflect in the otolith shape characteristics. The results of this study are a step forward in understanding the population structure of the European eels in their Southern Atlantic distribution area.
2. Materials and Methods
2.1. Population Characterization
2.1.1. Study Area
Sampling took place between November 2017 and October 2020 in fixed locations considered the most relevant areas for the occurrence of this species in the Portuguese border of the International Minho river basin (unpublished results). This river is located in the NW-Iberian Peninsula (SW-Europe). The river is 343 km long, and the last 76 km serve as the north-western Portuguese–Spanish border [60]. The limit of tidal influence is about 40 km inland [61], and the uppermost 30 km are tidal freshwater wetlands (TFW) [62,63]. The estuary has 23 km2 and is partially mixed, but during periods of high floods it tends to evolve towards a salt wedge estuary [64].
The Minho River is the only area in Portugal where glass eel fishery still legally occurs, and until the ban imposed in 2011, the yellow and silver eel fisheries were also economically, socially, and culturally important. Minho has a separated eel management plan from the rest of the Portuguese rivers; therefore, the data provided here can help inform the success of its implementation. It also has a great ecological value, with several areas classified as a Natura 2000 site. In the European context, this ecosystem is of reference, as it integrates an extensive monitoring program that provides ICES with estimates for the status of the European eel in Portugal, and is of relevance as a particular example of cross-border eel management. This study was conducted under the Sudoang project, which aimed at developing common management and assessment tools, obtaining coordinated and long-term monitoring strategies, and reinforcing cooperation between stakeholders in the Southwest Europe (Sudoe) area. The Minho River was part of an eel sampling network that included 10 pilot basins in the Mediterranean and Atlantic which were representative of the different ecosystems of the Sudoe area.
In the Minho basin, the habitat available to migratory fish decreased from 17,000 km2 (at the beginning of the 20th century) to 1000 km2 [65]. The river continuum is interrupted at 76 km from the mouth of the river due to the presence of the Frieira dam, which impairs upstream fish migration. There are 30 main tributaries on the Portuguese side, with water line extensions varying from around 2000 to 46,000 m. Anthropogenic pressures, such as human or industrial pollution, water capture, water flow control, construction of small physical obstacles, or agricultural land, influence the water masses in these areas.
2.1.2. Sampling
Temperature, salinity (Aquaread aquaprobe AP2000), and river depth were determined during each sampling event. All individuals were measured (±0.1 cm), weighed (±0.1 g), and classified macroscopically as yellow or silver eels using three criteria: the color of the back and belly, presence of a well-marked lateral line, and eye diameter [66]. For eels above 30 cm, additional biometric measures were recorded for the Durif Silvering Index estimation, including the pectoral fin length and the vertical/horizontal eye diameter [67,68]. Fulton’s condition factor (FU) was calculated as a fitness indicator based on the weight and length of each eel following Fulton [69]. As normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) assumptions were met, One-Way Analysis of Variance (One-Way ANOVA) was used to test for regional differences in FU (0.05 level of significance).
A total of 1428 eels were sampled in eight tributaries (Figure 1), which are freshwater shallow areas (mean depth varying between 0.1 and 0.7 m) with temperatures ranging from 10 °C to 20 °C throughout the year. Eels were caught during spring and autumn by electrofishing using two different gears, Hans Grassl model EL62 IIG and Electrocatch International model WFC911, in deeper or shallow tributaries, respectively. This is the primary sampling method used to monitor freshwater fish abundance in rivers and streams, as it samples populations quickly, is non-lethal, and can capture the entire size range of fish present at a given location [70].
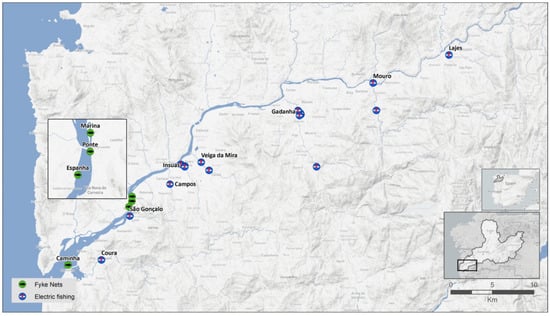
Figure 1.
Map showing the sampling locations of the Anguilla anguilla individuals collected from November 2017 to October 2020 in the Minho River basin. Green dots denote the estuary sampling locations, and blue dots show the tributaries’ sampling locations.
A total of 2676 eels were sampled in the estuary (Figure 1) in four fixed stations with similar depths (two to four meters): one located near the river mouth (mesohaline to euhaline; salinity varies with tides), with water temperatures reaching a maximum of 20 °C, and three located in the tidal freshwater (fresh to oligohaline), with water temperatures reaching a maximum of 26 °C [63,71]. Eels were collected using fyke nets of 10 mm mesh size, 7 m total length, 0.7 m mouth diameter, and 3.5 m central wing. This type of gear is selective for large eel individuals (>25 cm).
2.2. Otolith Analysis
2.2.1. Age Estimation
The sagittal otoliths from eels euthanized with an anesthetic overdosage were extracted. Macroscopic inspection of gonads was also undertaken for sex determination: eels with thin, regularly lobed organs were classified as males, and eels with broad, folded, curtain-like gonads were classified as females [28].
Eel age was assigned by counting the annual growth increments on the sagittal otoliths [72,73]. For this, all otoliths were immersed in a clearing agent (ethanol and glycerol, 1:1) to enhance their transparency during reading and examined using a stereomicroscope (Nikon SMZ800) against a dark background with polarized reflected light (Figure 2a). An age of 0 years was attributed to the glass eels to consider only the continental age. Otoliths of eels older than 5 years old (140 individuals) were treated to enhance rings’ visualization. The process consisted of embedding the otolith in epoxy resin and mounting it on a glass slide for sagittal grinding. Each otolith was grounded manually using a decreasing range in the coarseness of silicon carbide wet–dry papers (600–4000 grit) (Figure 2b). The otolith plane was etched with a drop of 5% EDTA for 3 min and rinsed with running water, stained with a drop of 1% Toluidine blue until it dried, and rinsed again (Figure 2c). Otoliths were observed with a stereomicroscope and polarized reflected light, and age was estimated. Three independent readings were undertaken, and only otoliths with consistent concordance in age were used (a total of 420 otoliths, 210 from the tributaries and 210 from the estuary). As linearity assumptions were not met, the relationship between age and length was investigated using the Spearman rank–order correlation test.
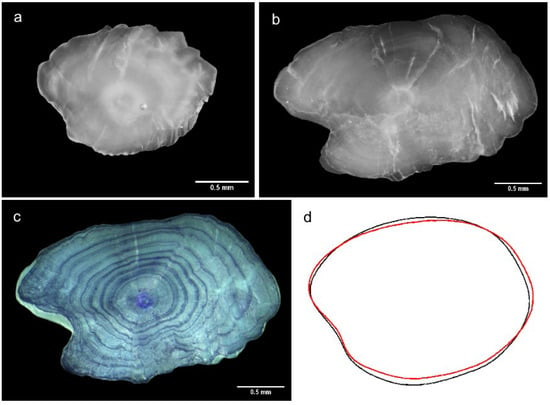
Figure 2.
Right sagittal otoliths: in the first image (a), the otolith rings are visible with the clearing agent, followed by a non-readable ground otolith (b) and the resulting rings’ visualization after coloration (c). The last image (d) is the averaged outline contour of the Elliptic Fourier analysis, in red for the estuary and black for the tributaries.
2.2.2. Shape Descriptors
Orthogonal two-dimensional digital images of the right sagittal otoliths were captured using a stereomicroscope coupled with a digital camera (Digital Sight DS-5M) at 2× magnification. Otoliths were all photographed in the same position with reflected light and dark backgrounds.
Binary otolith images were measured using the program ImageJ v. 1.50 (Bethesda, MD, USA) to assess the morphometric size parameters, otolith length (OL, mm), otolith width (OW, mm), otolith area (OA, mm2), and otolith perimeter (OP, mm) [74]. With these variables, it was possible to determine the Shape Indices (SI), Form factor (FF, (4πOA)/OP2), Roundness (RO, (4OA)/(πOL2)), Circularity (CI, OP2/OA), Ellipticity (EL, (OL − OW)/(OL + OW)), and Rectangularity (RE, OA/(OL × OW)), which describe the otolith plane [75].
The Elliptic Fourier analysis was also used. This analysis fits a closed curve to an ordered set of data points, decomposes the contour into a sum of harmonically related ellipses, and indicates the contribution of each harmonic to the total otolith shape [76,77,78]. The program Shape v.1.3 (Tsukuba, Japan) was used to capture the otolith contour and to determine the number of Elliptic Fourier Descriptors (EFD) required to describe the otolith outline adequately. A level of 95% of the accumulated variance was used to select the minimum number of harmonics [79] (Figure 2d). The first 6 harmonics reached >95% of the cumulative power, excluding coefficients c6 and d6. Moreover, after normalization to the first harmonic (EFD invariant to otolith size), the first three coefficients (a1, b1, and c1) were constant and excluded from subsequent analyses [80]. Thus, 19 Fourier coefficients (d1 to b6) could adequately explain otolith shape.
2.2.3. Statistical Analysis
Shape Indices (SI) were checked for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test). These assumptions were met after log10 (e.g., CI) or square root (e.g., FF) transformations. Differences in fish length distributions can disrupt location-specific characteristics, as the otolith shape relates to the fish development [76]. The relationship between SI and otolith size was investigated to ensure that differences in fish size among samples did not confound habitat-specific differences in otolith SI. Because all SI correlated with OL, the variables were corrected using the formula Vadj = V − (β × covariate), where Vadj is the adjusted sample value, V is the original sample value, and β is the ANCOVA slope value (sampling location as factor and OL as a covariate) [81]. Statistical analyses were performed using R studio v.3.5.1 (Boston, MA, USA) with a 0.05 level of significance.The statistical analyses described in the following paragraphs were performed for two groups, for the undifferentiated eels only, and for all the eels combined, in order to remove potential sex-related effects, which may exist due to sexual growth dimorphism [43]. The results were similar for both cases, and thus differences were presented for all eels. Comparisons were made between different habitats (estuary vs. tributary) and between locations within each habitat (sampling locations within the estuary and the tributaries).
One-Way ANOVA was used to test for regional differences in individual shape variables, followed by a Tukey post-hoc test for significant differences. Multivariate Analyses of Variance (MANOVA) tested regional differences using all SI and EFD variables. For MANOVA, the approximate F-ratio statistic was reported for the most robust test of multivariate statistics (Pillai’s trace), followed by multivariate pairwise comparisons using the Hotelling’s T-squared test. Statistical analyses were performed using R studio v.3.5.1 (Boston, MA, USA) with a 0.05 level of significance.
A Linear Discriminant Analysis (LDA) was used to examine the reclassification accuracy of eels to their original location, verified through the percentage of correct reclassification accuracies of the discriminant functions using a jackknifed classification matrix. A Canonical Variates Analysis (CVA) (with Mahalanobis distances) was used to visualize differences and to identify the variables that contributed most to the discrimination. These results were presented in a two-dimensional biplot, with ellipses representing 95% confidence. Both analyses were conducted using the software PAST v.4.05 (Oslo, Norway).
3. Results
3.1. Population Characterization
The information regarding the 4104 eels caught between November 2017 and October 2020 is summarized in Table 1 and Figure 3. The size of the eels sampled in the tributaries (total = 1428 eels) varied between 6 cm and 39 cm, with an average (±SD) of 18 ± 7.32 cm, and 1.5% were macroscopically classified as silver eels. In the estuary, the size of the sampled eels (total = 2676 eels) varied between 6 cm and 88 cm (36 ± 10.64 cm), and the proportion of eels macroscopically classified as silver eels was 3.2%. Overall, size distribution was right-skewed, and there was a higher proportion of yellow-stage eels than silver eels.

Table 1.
Eels caught in the monitoring campaigns in the Minho River between November 2017 and October 2020. Data presented include: total sample size (N total), the number of silver eels classified macroscopically (N silver), minimum, maximum, and mean (±standard deviation, SD), total length (cm) of all eels, and the mean (cm ± SD) total length (cm) of the individuals characterized using the Durif Silvering Index as yellow resident (SI and SFII), female pre-migrant (SFIII), silver female (SFIV and SFV), and as silver male (SMII).
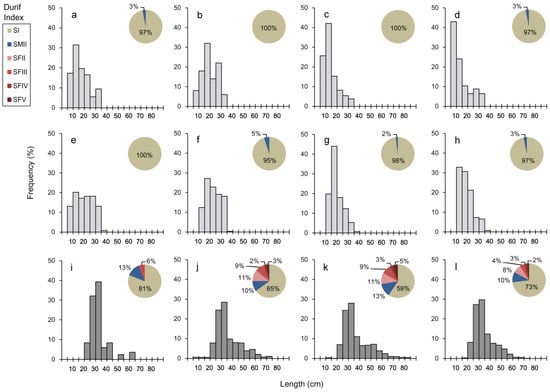
Figure 3.
Catch length composition of the eels collected in the tributaries ((a) Coura, (b) S.Gonçalo, (c) Campos, (d) Insuas, (e) V.Mira, (f) Gadanha, (g) Mouro, and (h) Lajes) and in the estuaries ((i) Caminha, (j) Espanha, (k) Ponte, and (l) Marina). Pie charts present the proportion of individuals sampled according to the life stage classification based on the Durif Silvering Index (Durif et al. 2009): yellow resident (SI and SFII), female pre-migrant (SFIII), silver female (SFIV and SFV), and silver male (SMII).
The majority of eels from the tributaries Coura (a), S.Gonçalo (b), Lajes (g), and Mouro (h) presented sizes lower than 20 cm, while those at Campos (c) and Insuas (d) were smaller than 15 cm (Figure 3). The majority of eels collected at Gadanha (f) presented sizes varying between 10 cm and 25 cm, while the sizes of the eels collected at V. Mira (e) were more evenly distributed (Figure 3). Based on the Durif Silvering Index, 97% of eels were yellow-stage eels, and silver individuals were all males (SMII) with lengths between 29 and 39 cm.
The majority of eels collected in Caminha (i), Espanha (j), Ponte (k), and Marina (l) presented sizes varying between 25 and 35 cm, and the largest eels were collected at Ponte (k), the only location with the presence of eels larger than 80 cm (Figure 3). In the estuary, 84% were yellow-stage eels, 14.5% of which were females, and the rest were classified as silver eels (4.5% females and 11.5% males). The number of females and silver eels in the estuary slightly increased with increasing distance from the river mouth (Figure 3).
3.2. Otolith Analysis
The information regarding the 420 eels used for the otolith analysis is summarized in Table 2 and Figure 4. The age of the eels varied between 0 and 10 years and sizes between 6 cm and 60 cm. Eels caught in the tributaries (Figure 4a) and estuary (Figure 4b) had similar length-at-age for the age classes 0 and 1. From the age of 2 years onwards, the eels collected in the estuary were 25% to 40% longer (mean total length) than those collected in the tributaries. In the tributaries, most of the individuals belonged to the 2, 3, and 4 age classes, whereas in the estuary most of the eels belonged to the 4, 5, and 6 age classes. The eels collected in the estuary presented higher length dispersion over an age class (Spearman correlation; rs = 0.6797, p < 0.05) than those collected in the tributaries (Spearman correlation; rs = 0.9642, p < 0.05; Figure 4). The condition of the eels collected in the tributaries only differed between Mouro and Gadanha (One-Way ANOVA, F(6, 203) = 4.55, p < 0.05; Tukey Test, p < 0.05; Table 2). The condition of the eels collected in the estuary varied between stations (One-Way ANOVA, F(3, 206) = 4.79, p < 0.05), with eels sampled in Caminha presenting the lowest mean FU value (Tukey Test, p < 0.05). Eels collected in the estuary presented higher FU values than those collected in the tributaries (One-Way ANOVA, F(1, 418) = 109.6, p < 0.05).

Table 2.
Number of eels collected in the Minho River according to habitat and sampling location (N), number of macroscopically sexed eels (males *), minimum and maximum age, weight (W), length (L), and mean (±standard error, SE), Fulton’s condition factor (FU). Data also include age, W, L, and silvering stage of undifferentiated, male, and female eels according to the habitat.
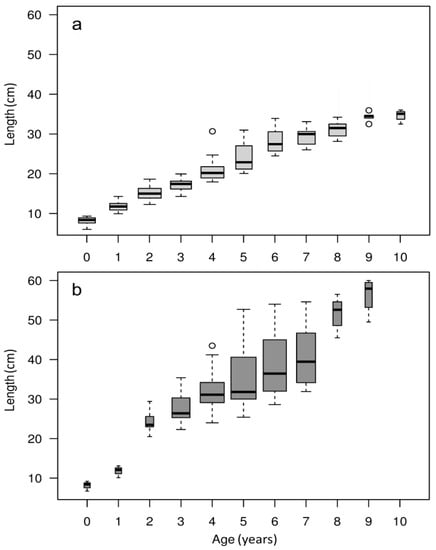
Figure 4.
Boxplot of age-at-length data for eels collected in the (a) tributaries and (b) estuary. Thicker boxes indicate a higher number of eels for the corresponding age class.
Shape Indicies (SI) and Elliptic Fourier Descriptors (EFD) values and variable individual results are in Tables S1 and S2, respectively. In the tributaries, otolith shape differed between a few locations in the tributaries: Coura from Insuas and Lajes, Mouro from Insuas and Gadanha, and S.Gonçalo from Lajes (MANOVA, Pillai´s Trace, F1.6049 = 0.999, p < 0.05). The LDA, through the Jackknife reclassification matrix, showed that Lajes was the site with the best reclassification value (40%), but the overall reclassification success was low (26%; Table 3. The lack of specific groups can be visualized in the CVA plot (Figure 5a). Vectors’ overlay shows the RO as the most prominent variable for group characterization. The otolith shape of eels collected in the brackish sampling location (Caminha) was different from those collected in the TFW (MANOVA, Pillai´s Trace, F1.799 = 0.589, p < 0.05). The LDA, through the Jackknife reclassification matrix, showed good reclassification success for Caminha (57%), but an overall poor reclassification success for the eels collected in the estuary (34%; Table 3). The discrimination of Caminha can be visualized in the CVA plot, where vector overlays show the prevalence of the otolith outline EFD variables d1, b2, and b3 (Figure 5b). Otolith shape proved to be successful in differentiating eels from tributary vs. estuary habitats (MANOVA, Pillai´s Trace, F83.883 = 0.842, p < 0.05). The LDA, through the Jackknife reclassification matrix, showed an overall reclassification success of 98%, with complete reclassification success for the tributary habitat (100%; Table 3). The discrimination of both habitats can be visualized in the CVA plot (Figure 5c). Additionally, the CVA plot of the undifferentiated eels shows similar results (Figure 5d). Vector overlays show that the SI variables RO and CI describe the otoliths of the individuals collected in the tributaries, whereas EL and RE describe the otoliths of the individuals collected in the estuary. Otolith outline EFD variables d1, b2, c2, b4, d3, d4, and b3 also contribute to the discrimination between groups.

Table 3.
Jackknife reclassification matrix of the otolith shape signatures of the eels collected in the Minho River according to (a) the tributary sampling locations, (b) the estuary sampling locations, and (c) habitat.
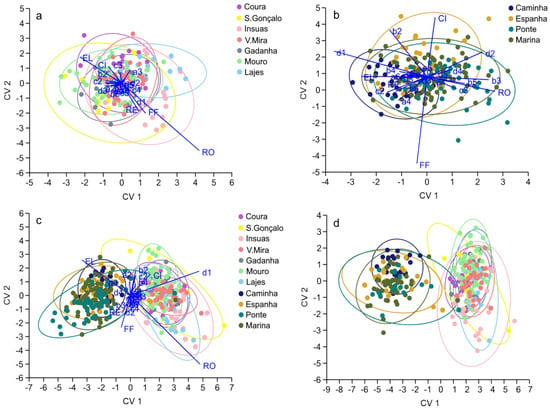
Figure 5.
Canonical Variates Analysis (CVA) plot of the otolith shape signature for eels collected in the (a) tributaries, (b) estuary, (c) tributaries and estuary combined, and (d) undifferentiated individuals only from the tributaries and estuary.
4. Discussion
This study was the first attempt to characterize the European eel population structure in the Minho River basin. Otolith shape results revealed the existence of two distinct groups of eels associated with different ecosystems: tributaries vs. estuaries. The otoliths of eels from the tributaries were round and circular, whereas the otoliths of eels from the estuary were elliptical and rectangular. Moreover, the otoliths’ outline of eels from the brackish location slightly differed from those of the tidal freshwater wetlands within the estuary. Eels collected in the tributary and estuary habitats were mostly yellow-stage eels with a similar age range, but eels from the tributaries showed lower length-at-age and lower body condition than those collected in the estuary. The sex ratio was skewed towards males in the tributaries and females in the estuary. Additionally, in the estuary, the brackish location presented a higher percentage of males and eels with a lower-body condition than those from TFW locations.
Previous studies have shown that variability in fish development can be mirrored in the shape of otoliths. Several abiotic characteristics of the environment, such as temperature fluctuations, salinity, depth, and food availability, are responsible for that variability [44,82,83,84]. Moreover, otolith shape indices have proven to be useful in discriminating eels growing in different habitats at the regional level [58,59]. For instance, Capoccioni et al. [58] found similar results to those obtained during this study, including elongated otoliths with a trimmed outline, evidence of growth effects in estuarine habitats, and maintenance of the initial glass eel circular shape throughout the eel life when growing in tributaries. The different strategies in development for the eel in a variety of river basins have been shown to reflect the complexity of the riverine habitats, which allows the understanding of habitat suitability, recruitment success, and eel productivity [85,86].
There are no studies on the effects of environmental fluctuations and differences in habitat suitability on eel development in the Minho River. Despite that, these relations were found for other basins containing similar habitat types. The smaller length-at-age and lower body condition of the eels collected in the tributaries, compared to those collected in the estuary, may indicate that tributaries are less suitable habitats for eels to grow. Previous studies have shown that growth tends to be higher in brackish environments and in freshwater marshes closer to the sea than in freshwater upstream habitats [27,87,88] due to increased productivity and food quality [29]. Although there are no estimates for the productivity in the tributaries of the Minho River, previous studies indicate that the food webs in the tributaries are mainly supported by aquatic- and terrestrial-derived detritus [89]. The detrital pathway is usually considered less efficient when compared to the phytoplankton pathway [90], which in this case, is more associated with the estuary [63]. Additionally, extreme hydrological regimes in the tributaries, characterized by droughts during the summer and torrential flooding during the winter, can impact fish responses, promoting stress conditions [91]. Moreover, eels in freshwater environments tend to present lower levels of fat accumulation and higher prevalences of the parasite Anguillicola crassus, which, in tandem with decreases in habitat quality and complexity due to human interventions, may impose further restrictions on eel growth and development with consequences for their performance, health, and survival [92,93,94]. In the Minho River, the prevalence of the parasite Anguillicola crassus has been increasing through the years [95], which could impair eel development.
The proportion of males was higher in the tributaries than in the estuary; this may indicate further habitat restrictions. One possible explanation could be related to the existence of the Frieira dam 76 km from the river mouth, which is the first main obstacle to the upstream migration of eels in the Minho River. As a result, high numbers of yellow eels concentrate in areas below the dam, a behavior already observed in other ecosystems [92,96,97]. This promotes competition for food and shelter, parasite dissemination, and long-term effects such as spawning biomass reduction due to sex ratio changes [31,97,98], specifically skewness towards males [28,30,37]. It was estimated that the habitat reduction for migratory species due to dams in this ecosystem is around 90%, and because eels cannot migrate to and from upstream areas, they are caught in the dam and distributed mainly in the downstream tributaries. The habitat reduction, both in extension and quality, and the environmental variability in tributaries may be responsible for the poorer development of eels compared to the conditions in the estuary since those in the tributaries presented lower body conditions, smaller length at a certain age, and a skewness towards males. Further studies are necessary to relate the type of habitat and environmental variability (e.g., productivity, pollution, and extreme events) with eel condition (e.g., stress, fat content, and prevalence of parasites).
Eels collected in brackish estuarine habitat (i.e., Caminha) presented a lower body condition when compared to the eels collected in the upstream tidal freshwater wetlands. This result is unexpected, as productivity tends to be higher in brackish waters. However, this could mean that eels invest more in somatic growth than in energy storage. Previous records of lighter individuals in coastal areas compared to those in inland waters were reported [28]. Although the movement patterns of eels in the Minho River are unknown, we hypothesize that this behavior could be linked to the conditions eels experienced during early life [99]. The settlement of eels in brackish habitats can be condition-dependent, with low-body-condition glass eels preferring high-salinity habitats [22]. Early migrants usually present better body condition and migrate further upstream than the late migrants, which are usually in the worst conditions and settle in the lower estuary [100,101,102]. Similar compensatory-growth behaviors were observed in other fish and invertebrate species, where they were able to increase the growth rate but maintained a low body condition when transiting to habitats with high food availability [103,104]. Another explanation, which is not mutually exclusive, could be related to movements throughout the yellow eel stage, from freshwater to the brackish estuary, as observed for this species in other ecosystems [15]. Still, additional information combining individual growth and habitat shift events is necessary to understand the influence of different environments on eels’ growth strategies and condition throughout ontogeny.
The areas where eels were larger and had the highest body condition values were located in the tidal freshwater estuary. These are transition areas between the brackish estuary and the non-tidal freshwater streams, and tend to be less susceptible to variations in salinity or temperature and depth than the brackish estuary or the tributaries, respectively [105]. Positive effects on eel development were associated with high temperatures and depths [27,106]. In the Minho basin, the TFW area reaches maximum water temperatures of 26 °C during the summer, salinity is below 0.5 throughout most of the year, and depth is relatively constant, varying between 2 and 4 m across this area. The salinity in the brackish estuary varies daily with tides and along the year with the river flow, while in the tributaries, depths can be as low as 0.1–0.7 m during spring and autumn, and water temperature in both areas usually does not exceeds 20 °C. Thus, the relatively stable abiotic conditions and higher temperature and depth values in the TFW may promote a steady development environment, allowing eels to grow to larger sizes. However, several unknown variables could be acting together, limiting further conclusions about the influence of the environment on development [27]. Eels have sex-specific life-history strategies, with females showing long maturation periods to produce eggs, thus having higher energetic demands than males, which leads to a sexual dimorphism based on differences in length at maturity [28,68]. Males migrate at a lower length, around 35–45 cm, minimizing the duration of their yellow stage, while females migrate at sizes of 40–130 cm, optimizing their size to reach a higher fecundity [23,28,68]. This trade-off strategy in sex differentiation depends indirectly on the effects of environmental conditions on growth [107,108]. Thus, the higher proportion of females in the estuarine TFW may indicate that this habitat is more favorable for eel development.
5. Conclusions
Our study reveals the existence of at least two distinct groups of eels in the Minho River, associated with different types of habitats: tributaries vs. estuaries. Otolith shape analysis completely discriminated these two groups; the otoliths were more round and circular in the tributaries and more elliptical and rectangular in the estuary. Upstream tributary environments likely offer poorer development conditions for eels than estuarine areas, as these eels were smaller and in worse condition than those in estuaries. Moreover, eels collected from tributaries skewed towards males. The estuary was dominated by larger eels than the tributaries, skewed towards females, and presented eels with higher body condition, especially in the tidal freshwater wetlands. This information is of outmost importance to begin understanding the development strategies of the eel in the Minho River and the contribution of this river to the spawning population. Continued European eel research in this species’ wide distribution area is urgent in order to improve stock management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7030135/s1, Table S1: Shape Indices ANOVA results; Table S2: Elliptical Fourier Descriptors ANOVA results.
Author Contributions
Conceptualization, A.M. and C.A.; methodology, A.M. and C.A.; software, A.M.; validation, A.M. and C.A; formal analysis, A.M. and ED.; investigation, A.M., R.L., and C.A.; resources, C.A.; data curation, A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.M., E.D., and C.A.; visualization, A.M.; supervision, C.A.; project administration, C.A.; funding acquisition, C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by European funds, through the SUDOANG project (SOE2/P5/E0617) within the Interreg Sudoe Program and Interreg MIGRAMIÑO-MINHO (0016_MIGRA_MINHO_1_E) project, and by national funds through the FCT-Foundation for Science and Technology within the scope of UIDB/04423/2020 and UIDP/04423/2020. Additionally, Aquamuseu do Rio Minho provided most-needed resource support.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the CIIMAR’s Ethical Committee along with the CIIMAR´S Animal Welfare Body (ORBEA) in compliance with the European Directive 2010/63/EU, on the protection of animals used for scientific purposes, and its transposition to the Portuguese law. Animals were sacrificed according to the recommendations provided by the Bioterium of Aquatic Organisms (BOGA) at CIIMAR, which is certified by “Direção Geral de Alimentação e Veterinária (DGAV)” issued under Article 21°, of Decree-Law N.° 113/2013 of 7th August. The last author (C.A) has category B certification by Direção Geral de Alimentação e Veterinária (DGAV)”, which is in compliance with the Federation of European Laboratory Animal Science (FELASA) corresponding to functions (a), (c), (e), and (d) defined in national and EU Directive 2010/63/EU. Electric fishing procedures were approved by the Portuguese Nature Conservation Institute and Forestry (licenses N.° 373/2018, N.° 263/2019 and N.° 161/2020, CAPT CREDENCIAL PESCA N.° 36/2018, N.° 7/2019, and N.° 21/2020, respectively). Fyke nets sampling procedures were approved by the local Captaincy of the Port of Caminha. Otoliths used in this study came from eels sacrificed for a variety of undergoing studies, ensuring that the lethal procedure would result in gathering all the available biological information possible. The number of otoliths chosen was believed to be necessary to fairly represent the riverine systems in this study.
Data Availability Statement
Data from this study are available from the corresponding author upon request (AM.: anacatarinamoura@hotmail.com).
Acknowledgments
The authors would like to thank Eduardo Martins, Patrício Bouça, Mafalda Fernandes, and Ana Lages from Aquamuseu do Rio Minho for their assistance in the field.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ICES. Report of the Joint EIFAAC/ICES/GFCM Working Group on Eel (WGEEL); ICES Document CM 2015/ACOM: 18; ICES: Antalya, Turkey, 2015. [Google Scholar]
- Moriarty, C.; Dekker, W. Management of European eel fisheries. Irish Fish. Bull. 1997, 15, 108. [Google Scholar]
- Dekker, W. Status of the European eel stock and fisheries. In Eel Biology; Springer: Tokyo, Japan, 2003. [Google Scholar]
- Bornarel, V.; Lambert, P.; Briand, C.; Antunes, C.; Belpaire, C.; Ciccotti, E.; Diaz, E.; Diserud, O.; Doherty, D.; Domingos, I.; et al. Modelling the recruitment of European eel (Anguilla anguilla) throughout its European range. ICES J. Mar. Sci. 2018, 75, 541–552. [Google Scholar] [CrossRef]
- Dekker, W. The history of commercial fisheries for European eel commenced only a century ago. Fish. Manag. Ecol. 2019, 26, 6–19. [Google Scholar] [CrossRef]
- Jacoby, D.; Gollock, M. The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2014. [Google Scholar]
- Jacoby, D.M.; Casselman, J.M.; Crook, V.; DeLucia, M.B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.; Smith, K.G.; et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Miller, M.J.; Feunteun, E.; Tsukamoto, K. Did a “perfect storm” of oceanic changes and continental anthropogenic impacts cause northern hemisphere anguillid recruitment reductions? ICES J. Mar. Sci. 2016, 73, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Dekker, W.; Beaulaton, L. Climbing back up what slippery slope? Dynamics of the European eel stock and its management in historical perspective. ICES J. Mar. Sci. 2016, 73, 5–13. [Google Scholar] [CrossRef] [Green Version]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL); ICES: Antalya, Turkey, 2019. [Google Scholar]
- The European Commission. Commission Decision 2010/93/EU of 18 December 2009 adopting a multiannual Community program for the collection, management and use of data in the fisheries sector for the period 2011–2013 (notified under document C (2009) 10121). Off. J. Eur. Union L 2009, 41, 8–71. [Google Scholar]
- ICES. Report of the Workshop on Designing an Eel Data Call (WKEELDATA); ICES CM 2017/SGIEOM: 30; ICES: Rennes, France, 2017. [Google Scholar]
- Tsukamoto, K.; Nakai, I. Do all freshwater eels migrate? Nature 1998, 396, 635–636. [Google Scholar] [CrossRef]
- Limburg, K.E.; Wickstrom, H.; Svedang, H.; Elfman, M.; Kristiansson, P. Do stocked freshwater eels migrate? Evidence from the Baltic suggests “yes”. In American Fisheries Society Symposium; American Fisheries Society: New York, NY, USA, 2003; pp. 275–284. [Google Scholar]
- Daverat, F.; Limburg, K.E.; Thibault, I.; Shiao, J.C.; Dodson, J.J.; Caron, F.; Tzeng, W.N.; Iizuka, Y.; Wickström, H. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Als, T.D.; Hansen, M.M.; Maes, G.E.; Castonguay, M.; Riemann, L.; Aarestrup, K.I.M.; Munk, P.; Sparholt, H.; Hanel, R.; Bernatchez, L. All roads lead to home: Panmixia of European eel in the Sargasso Sea. Mol. Ecol. 2011, 20, 1333–1346. [Google Scholar] [CrossRef]
- Schmidt, J. On the distribution of the freshwater eels (Anguilla) throughout the world. I. Atlantic Ocean and adjacent regions. Medd. Fra Komm. Havunders. Seri Fisk. 1909, 3, 1–45. [Google Scholar]
- Tesch, F.W. Biology and Management of Anguillid Eels; CRC Press: Boco Raton, FL, USA, 1977. [Google Scholar]
- Dekker, W. On the distribution of the European eel (Anguilla anguilla) and its fisheries. Can. J. Fish. Aquat. Sci. 2003, 60, 787–799. [Google Scholar] [CrossRef]
- Antunes, C.; Tesch, F.W. A critical consideration of the metamorphosis zone when identifying daily rings in otoliths of European eel, Anguilla anguilla (L.). Ecol. Freshw. Fish 1997, 6, 102–107. [Google Scholar] [CrossRef]
- Edeline, E.; Dufour, S.; Elie, P. Role of glass eel salinity preference in the control of habitat selection and growth plasticity in Anguilla anguilla. Mar. Ecol. Prog. Ser. 2005, 304, 191–199. [Google Scholar] [CrossRef]
- Edeline, E.; Lambert, P.; Rigaud, C.; Elie, P. Effects of body condition and water temperature on Anguilla anguilla glass eel migratory behavior. J. Exp. Mar. Biol. Ecol. 2006, 331, 217–225. [Google Scholar] [CrossRef]
- Vøllestad, L.A. Geographic variation in age and length at metamorphosis of maturing European eel: Environmental effects and phenotypic plasticity. J. Anim. Ecol. 1992, 61, 41–48. [Google Scholar] [CrossRef]
- Bertin, L. Eels: A Biological Study; Cleaver-Hume Press: London, UK, 1956. [Google Scholar]
- Van Den Thillart, G.E.E.J.; Van Ginneken, V.; Körner, F.; Heijmans, R.; Van der Linden, R.; Gluvers, A. Endurance swimming of European eel. J. Fish Biol. 2004, 65, 312–318. [Google Scholar] [CrossRef]
- Helfman, G.S.; Facey, D.E.; Hales, L.S., Jr.; Bozeman, E.L., Jr. Reproductive ecology of the American eel. In American Fisheries Society Symposium; American Fisheries Society: New York, NY, USA, 1987; Volume 1, pp. 42–56. [Google Scholar]
- Daverat, F.; Beaulaton, L.; Poole, R.; Lambert, P.; Wickström, H.; Andersson, J.; Aprahamian, M.; Hizem, B.; Elie, P.; Yalçın-Özdilek, S.; et al. One century of eel growth: Changes and implications. Ecol. Freshw. Fish 2012, 21, 325–336. [Google Scholar] [CrossRef]
- Tesch, F.W. The Eel, 5th ed.; Blackwell Publishing: Oxford, UK, 2003. [Google Scholar]
- Jessop, B.M.; Shiao, J.C.; Iizuka, Y.; Tzeng, W.N. Variation in the annual growth, by sex and migration history, of silver American eels Anguilla rostrata. Mar. Ecol. Prog. Ser. 2004, 272, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Krueger, W.H.; Oliveira, K. Evidence for environmental sex determination in the American eel, Anguilla rostrata. Environ. Biol. Fishes 1999, 55, 381–389. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melia, P.; De Leo, G.A.; Gatto, M. Intra-specific scaling of natural mortality in fish: The paradigmatic case of the European eel. Oecologia 2011, 165, 333–339. [Google Scholar] [CrossRef]
- Boulenger, C.; Acou, A.; Gimenez, O.; Charrier, F.; Tremblay, J.; Feunteun, E. Factors determining survival of European eels in two unexploited sub-populations. Freshw. Biol. 2016, 61, 947–962. [Google Scholar] [CrossRef]
- Leo, G.D.; Gatto, M. A size and age-structured model of the European eel (Anguilla anguilla L.). Can. J. Fish. Aquat. Sci. 1995, 52, 1351–1367. [Google Scholar] [CrossRef]
- Poole, W.R.; Reynolds, J.D. Variability in growth rate in European eel Anguilla anguilla (L.) in a western Irish catchment. In Biology and Environment: Proceedings of the Royal Irish Academy; Royal Irish Academy: Dublin, Ireland, 1998; pp. 141–145. [Google Scholar]
- Parsons, J.; Vickers, K.U.; Warden, Y. Relationship between elver recruitment and changes in the sex ratio of silver eels Anguilla anguilla L. migrating from Lough Neagh, Northern Ireland. J. Fish Biol. 1977, 10, 211–229. [Google Scholar] [CrossRef]
- Walsh, C.T.; Pease, B.C.; Booth, D.J. Variation in the sex ratio, size and age of long finned eels within and among coastal catchments of south-eastern Australia. J. Fish Biol. 2004, 64, 1297–1312. [Google Scholar] [CrossRef]
- Kettle, A.J.; Asbjørn Vøllestad, L.; Wibig, J. Where once the eel and the elephant were together: Decline of the European eel because of changing hydrology in southwest Europe and northwest Africa? Fish Fish. 2011, 12, 380–411. [Google Scholar] [CrossRef]
- Vøllestad, L.A.; Jonsson, B. A 13-year study of the population dynamics and growth of the European eel Anguilla anguilla in a Norwegian river: Evidence for density-dependent mortality, and development of a model for predicting yield. J. Anim. Ecol. 1988, 57, 983–997. [Google Scholar] [CrossRef]
- Sakai, M.; Iwabuchi, K. Unique habitat and macroinvertebrate assemblage structures in spring-fed streams: A comparison among lowland tributaries and mainstreams in northern Japan. bioRxiv 2020, 22, 193–202. [Google Scholar] [CrossRef]
- Post, D.M.; Pace, M.L.; Hairston, N.G. Ecosystem size determines food-chain length in lakes. Nature 2000, 405, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Maceda-Veiga, A.; Mac Nally, R.; de Sostoa, A. Environmental correlates of food-chain length, mean trophic level and trophic level variance in invaded riverine fish assemblages. Sci. Total Environ. 2018, 644, 420–429. [Google Scholar] [CrossRef]
- Pimm, S.L.; Lawton, J.H. Number of trophic levels in ecological communities. Nature 1977, 268, 329–331. [Google Scholar] [CrossRef]
- Panfili, J.; Ximénès, M.C.; Crivelli, A.J. Sources of variation in growth of the European eel (Anguilla anguilla) estimated from otoliths. Can. J. Fish. Aquat. Sci. 1994, 51, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Lin, Y.J.; Ložys, L.; Shiao, J.C.; Iizuka, Y.; Tzeng, W.N. Growth differences between naturally recruited and stocked European eel Anguilla anguilla from different habitats in Lithuania. J. Fish Biol. 2007, 71, 1773–1787. [Google Scholar] [CrossRef]
- Thorrold, S.R.; Campana, S.E.; Jones, C.M.; Swart, P.K. Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish. Geochim. Cosmochim. Acta 1997, 61, 2909–2919. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Gillanders, B.M. Reconstructing migratory patterns of fish based on environmental influences on otolith chemistry. Rev. Fish Biol. Fish. 2003, 13, 217–235. [Google Scholar] [CrossRef]
- Begg, G.A.; Campana, S.E.; Fowler, A.J.; Suthers, I.M. Otolith research and application: Current directions in innovation and implementation. Mar. Freshw. Res. 2005, 56, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Campana, S.E. Otolith science entering the 21st century. Mar. Freshw. Res. 2005, 56, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Correia, A.T. Otolith shape analysis as a tool to infer the population structure of the blue jack mackerel, Trachurus picturatus, in the NE Atlantic. Fish. Res. 2019, 209, 40–48. [Google Scholar] [CrossRef]
- Moura, A.; Muniz, A.A.; Mullis, E.; Wilson, J.M.; Vieira, R.P.; Almeida, A.A.; Pinto, E.; Brummer, G.J.A.; Gaever, P.V.; Gonçalves, J.M.S.; et al. Population structure and dynamics of the Atlantic mackerel (Scomber scombrus) in the North Atlantic inferred from otolith chemical and shape signatures. Fish. Res. 2020, 230, 105621. [Google Scholar] [CrossRef]
- Muniz, A.A.; Moura, A.; Triay-Portella, R.; Moreira, C.; Santos, P.T.; Correia, A.T. Population structure of the chub mackerel (Scomber colias) in the North-east Atlantic inferred from otolith shape and body morphometrics. Mar. Freshw. Res. 2020, 72, 341–352. [Google Scholar] [CrossRef]
- Jones, C.M. Development and application of the otolith increment technique. In Otolith Microstructure Examination and Analysis; Canadian Special Publication of Fisheries and Aquatic Sciences 117; Publishing Supply and Services Canada: Ottawa, ON, Canada, 1992; Volume 117, pp. 1–11. [Google Scholar]
- Lombarte, A.; Torres, G.J.; Morales-Nin, B. Specific Merluccius otolith growth patterns related to phylogenetics and environmental factors. Marine Biological Association of the United Kingdom. J. Mar. Biol. Assoc. 2003, 83, 277. [Google Scholar] [CrossRef] [Green Version]
- Bacha, M.; Jemaa, S.; Hamitouche, A.; Rabhi, K.; Amara, R. Population structure of the European anchovy, Engraulis encrasicolus, in the SW Mediterranean Sea, and the Atlantic Ocean: Evidence from otolith shape analysis. ICES J. Mar. Sci. 2014, 71, 2429–2435. [Google Scholar] [CrossRef]
- Vieira, A.R.; Neves, A.; Sequeira, V.; Paiva, R.B.; Gordo, L.S. Otolith shape analysis as a tool for stock discrimination of forkbeard (Phycis phycis) in the Northeast Atlantic. Hydrobiologia 2014, 728, 103–110. [Google Scholar] [CrossRef]
- Jemaa, S.; Bacha, M.; Khalaf, G.; Dessailly, D.; Rabhi, K.; Amara, R. What can otolith shape analysis tell us about population structure of the European sardine, Sardina pilchardus, from Atlantic and Mediterranean waters? J. Sea Res. 2015, 96, 11–17. [Google Scholar] [CrossRef]
- Capoccioni, F.; Costa, C.; Aguzzi, J.; Menesatti, P.; Lombarte, A.; Ciccotti, E. Ontogenetic and environmental effects on otolith shape variability in three Mediterranean European eel (Anguilla anguilla, L.) local stocks. J. Exp. Mar. Biol. Ecol. 2011, 397, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Milošević, D.; Bigović, M.; Mrdak, D.; Milašević, I.; Piria, M. Otolith morphology and microchemistry fingerprints of European eel, Anguilla anguilla Linnaeus, 1758) stocks from the Adriatic Basin in Croatia and Montenegro. Sci. Total Environ. 2021, 786, 147478. [Google Scholar] [CrossRef]
- Antunes, C.; Araújo, M.J.; Braga, C.; Roleira, A.; Carvalho, R.; Mota, M. Valorização dos recursos naturais da bacia hidrográfica do rio Minho. In Final Report from the Project Natura Miño-Minho; Centro interdisciplinar de Investigação Marinha e Ambiental; Universidade do Porto: Porto, Portugal, 2011. [Google Scholar]
- Vilas, F.; Somoza, L. El estuario del rio Miño: Observaciones previas de su dinâmica. Thalassas 1984, 2, 87–92. [Google Scholar]
- Sousa, R.; Dias, S.C.; Guilhermino, L.; Antunes, C. Minho River tidal freshwater wetlands: Threats to faunal biodiversity. Aquat. Biol. 2008, 3, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Dias, E.; Morais, P.; Cotter, A.M.; Antunes, C.; Hoffman, J.C. Estuarine consumers utilize marine, estuarine and terrestrial organic matter and provide connectivity among these food webs. Mar. Ecol. Prog. Ser. 2016, 554, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R.; Guilhermino, L.; Antunes, C. Molluscan fauna in the freshwater tidal area of the River Minho estuary, NW of Iberian Peninsula. Ann. Limnol.-Int. J. Limnol. 2005, 41, 141–147. [Google Scholar] [CrossRef]
- Mota, M.; Rochard, E.; Antunes, C. Status of the diadromous fish of the Iberian Peninsula: Past, present and trends. Limnetica 2016, 35, 1–18. [Google Scholar]
- Feunteun, E.; Acou, A.; Laffaille, P.; Legault, A. European eel (Anguilla anguilla): Prediction of spawner escapement from continental population parameters. Can. J. Fish. Aquat. Sci. 2000, 57, 1627–1635. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The silvering process of Anguilla anguilla: A new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Durif, C.; Guibert, A.; Elie, P. Morphological discrimination of the silvering stages of the European eel. In American Fisheries Society Symposium; American Fisheries Society: New York, NY, USA, 2009; Volume 58, pp. 103–111. [Google Scholar]
- Fulton, T.W. The rate of growth of fishes. In Twenty-Second Annual Report; Fisheries Board of Scotland: Edinburgh, UK, 2009; pp. 141–241. [Google Scholar]
- Howard, S.W.; Crow, S.K.; Jellyman, P.G. Site-Specific Selectivity of Electric-Fishing Gear. New Zealand Fisheries Assessment Report. 2019. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi5zODSr5r4AhXzg2MGHff_B2cQFnoECAgQAQ&url=https%3A%2F%2Fwww.mpi.govt.nz%2Fdmsdocument%2F33166-far-201906-site-specific-selectivity-of-electric-fishing-gear&usg=AOvVaw3dPD5unARJBiQ69KpXsxdG (accessed on 22 May 2022).
- Souza, A.T.; Dias, E.; Nogueira, A.; Campos, J.; Marques, J.C.; Martins, I. Population ecology and habitat preferences of juvenile flounder Platichthys flesus (Actinopterygii: Pleuronectidae) in a temperate estuary. J. Sea Res. 2013, 79, 60–69. [Google Scholar] [CrossRef]
- ICES. Workshop on Age Reading of European and American Eel (WKAREA); ICES CM 2009\ACOM; ICES: Bordeaux, France, 2009; p. 48. [Google Scholar]
- ICES. Third Workshop on Age Reading of European and American Eel (WKAREA3); ICES: Bordeaux, France, 2020. [Google Scholar]
- Rasband, W. ImageJ v. 1.50i; National Institute of Health: Bethesda, MD, USA, 2009. Available online: http://imagej.nih.gov/ij/ (accessed on 22 May 2022).
- Tuset, V.M.; Lozano, I.J.; González, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Campana, S.E.; Casselman, J.M. Stock discrimination using otolith shape analysis. Can. J. Fish. Aquat. Sci. 1993, 50, 1062–1083. [Google Scholar] [CrossRef]
- Kuhl, F.P.; Giardina, C.R. Elliptic Fourier features of a closed contour. Comput. Graph. Image Process. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- Ponton, D. Is geometric morphometrics efficient for comparing otolith shape of different fish species? J. Morphol. 2006, 267, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.J.; Ward, T.M.; Gillanders, B.M. Otolith shape and elemental composition: Complementary tools for stock discrimination of mulloway (Argyrosomus japonicus) in southern Australia. Fish. Res. 2011, 110, 75–83. [Google Scholar] [CrossRef]
- Pothin, K.; Gonzalez-Salas, C.; Chabanet, P.; Lecomte-Finiger, R. Distinction between Mulloidichthys flavolineatus juveniles from Reunion Island and Mauritius Island (south-west Indian Ocean) based on otolith morphometrics. J. Fish Biol. 2006, 69, 38–53. [Google Scholar] [CrossRef]
- Campana, S.E.; Chouinard, G.A.; Hanson, J.M.; Frechet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Wilson, R.R., Jr. Depth-related changes in sagitta morphology in six macrourid fishes of the Pacific and Atlantic Oceans. Copeia 1985, 4, 1011–1017. [Google Scholar] [CrossRef]
- Campana, S.E.; Neilson, J.D. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 1985, 42, 1014–1032. [Google Scholar] [CrossRef]
- Morales-Nin, B. Review of the growth regulation processes of otolith daily increment formation. Fish. Res. 2000, 46, 53–67. [Google Scholar] [CrossRef]
- Schiavina, M.; Bevacqua, D.; Melia, P.; Crivelli, A.J.; Gatto, M.; De Leo, G.A. A user-friendly tool to assess management plans for European eel fishery and conservation. Environ. Model. Softw. 2015, 64, 9–17. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Schiavina, M.; Crivelli, A.J.; De Leo, G.A.; Gatto, M. A demographic model for the conservation and management of the European eel: An application to a Mediterranean coastal lagoon. ICES J. Mar. Sci. 2019, 76, 2164–2178. [Google Scholar] [CrossRef]
- Edeline, E.; Elie, P. Is salinity choice related to growth in juvenile eel Anguilla anguilla. Cybium 2004, 28, 77–82. [Google Scholar]
- Daverat, F.; Tomas, J. Tactics and demographic attributes in the European eel Anguilla anguilla in the Gironde watershed, SW France. Mar. Ecol. Prog. Ser. 2006, 307, 247–257. [Google Scholar] [CrossRef]
- Dias, E.; Miranda, M.L.; Sousa, R.; Antunes, C. Riparian vegetation subsidizes sea lamprey ammocoetes in a nursery area. Aquat. Sci. 2019, 81, 1–13. [Google Scholar] [CrossRef]
- Rooney, N.; McCann, K.S. Integrating food web diversity, structure and stability. Trends Ecol. Evol. 2011, 27, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Rytwinski, T.; Harper, M.; Taylor, J.J.; Bennett, J.R.; Donaldson, L.A.; Smokorowski, K.E.; Clarke, K.; Bradford, M.J.; Ghamry, H.; Olden, J.D.; et al. What are the effects of flow-regime changes on fish productivity in temperate regions? A systematic map. Environ. Evid. 2020, 9, 1–26. [Google Scholar]
- Domingos, I.; Costa, J.L.; Costa, M.J. Factors determining length distribution and abundance of the European eel, Anguilla anguilla, in the River Mondego (Portugal). Freshw. Biol. 2006, 51, 2265–2281. [Google Scholar] [CrossRef]
- Gravato, C.; Guimarães, L.; Santos, J.; Faria, M.; Alves, A.; Guilhermino, L. Comparative study about the effects of pollution on glass and yellow eels (Anguilla anguilla) from the estuaries of Minho, Lima and Douro Rivers (NW Portugal). Ecotoxicol. Environ. Saf. 2010, 73, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Marohn, L.; Jakob, E.; Hanel, R. Implications of facultative catadromy in Anguilla anguilla. Does individual migratory behaviour influence eel spawner quality? J. Sea Res. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Pereira, L.; Braga, A.C.; Moura, A.; Antunes, C. Prevalence of the Anguillicola crassus parasite in the International Minho River. Environ. Smoke 2021, 17, 64–74. [Google Scholar] [CrossRef]
- Feunteun, E.; Acou, A.; Guillouët, J.; Laffaille, P.; Legault, A. Spatial distribution of an eel population (Anguilla anguilla L.) in a small coastal catchment of Northern Brittany (France). Consequences of hydraulic works. Bulletin Français de la Pêche et de la Pisciculture 1998, 349, 129–139. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Monteiro, R.; Castro, N.; Quintella, B.R.; Almeida, P.R.; Domingos, I. Can a restocking event with European (glass) eels cause early changes in local biological communities and its ecological status? Glob. Ecol. Conserv. 2020, 21, e00884. [Google Scholar] [CrossRef]
- Davey, A.J.; Jellyman, D.J. Sex determination in freshwater eels and management options for manipulation of sex. Rev. Fish Biol. Fish. 2005, 15, 37–52. [Google Scholar] [CrossRef]
- Taborsky, B. The influence of juvenile and adult environments on life-history trajectories. Proc. R. Soc. B Biol. Sci. 2005, 273, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elie, P. Contribution a L’etude des Montees de Civelles Anguilla anguilla Linne (Poisson, Teleosteen, Anguilliforme), Dans L’estuaire de la Loire: Peche, Ecologie, Ecophysiologie et Elevage. Ph.D. Thesis, University of Rennes, Brittany, France, 1979. [Google Scholar]
- Charlon, N.; Blanc, J.M. Etude des civelles d´Anguilla anguilla L. dans la region du bassin de l´adour. I. Caracteristiques biometriques de longueur et poids en function de la pigmentation. Arch. Hydrobiol. 1982, 93, 238–255. [Google Scholar]
- Edeline, E.; Dufour, S.; Elie, P. Proximate and ultimate control of eel continental dispersal. In Spawning Migration of the European Eel; Springer: Dordrecht, The Netherlands, 2009; pp. 433–461. [Google Scholar]
- Johansen, S.J.S.; Ekli, M.; Stangnes, B.; Jobling, M. Weight gain and lipid deposition in Atlantic salmon, Salmo salar, during compensatory growth: Evidence for lipostatic regulation? Aquac. Res. 2002, 32, 963–974. [Google Scholar] [CrossRef]
- Zeller, M.; Koella, J.C. Effects of food variability on growth and reproduction of Aedes aegypti. Ecol. Evol. 2016, 6, 552–559. [Google Scholar] [CrossRef]
- Whigham, D.F.; Baldwin, A.H.; Barendregt, A. Tidal Freshwater Wetlands. In Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 619–640. [Google Scholar]
- Laffaille, P.; Feunteun, E.; Baisez, A.; Robinet, T.; Acou, A.; Legault, A.; Lek, S. Spatial organization of European eel (Anguilla anguilla L.) in a small catchment. Ecol. Freshw. Fish 2003, 12, 254–264. [Google Scholar] [CrossRef]
- Melià, P.A.C.O.; Bevacqua, D.; Crivelli, A.J.; Panfili, J.; De Leo, G.A.; Gatto, M. Sex differentiation of the European eel in brackish and freshwater environments: A comparative analysis. J. Fish Biol. 2006, 69, 1228–1235. [Google Scholar] [CrossRef]
- Geffroy, B.; Bardonnet, A. Sex differentiation and sex determination in eels: Consequences for management. Fish Fish. 2016, 17, 375–398. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).