Development of a Single-Piece Sperm Counting Chamber (SSCC) for Aquatic Species
Abstract
:1. Introduction
2. Materials and Methods
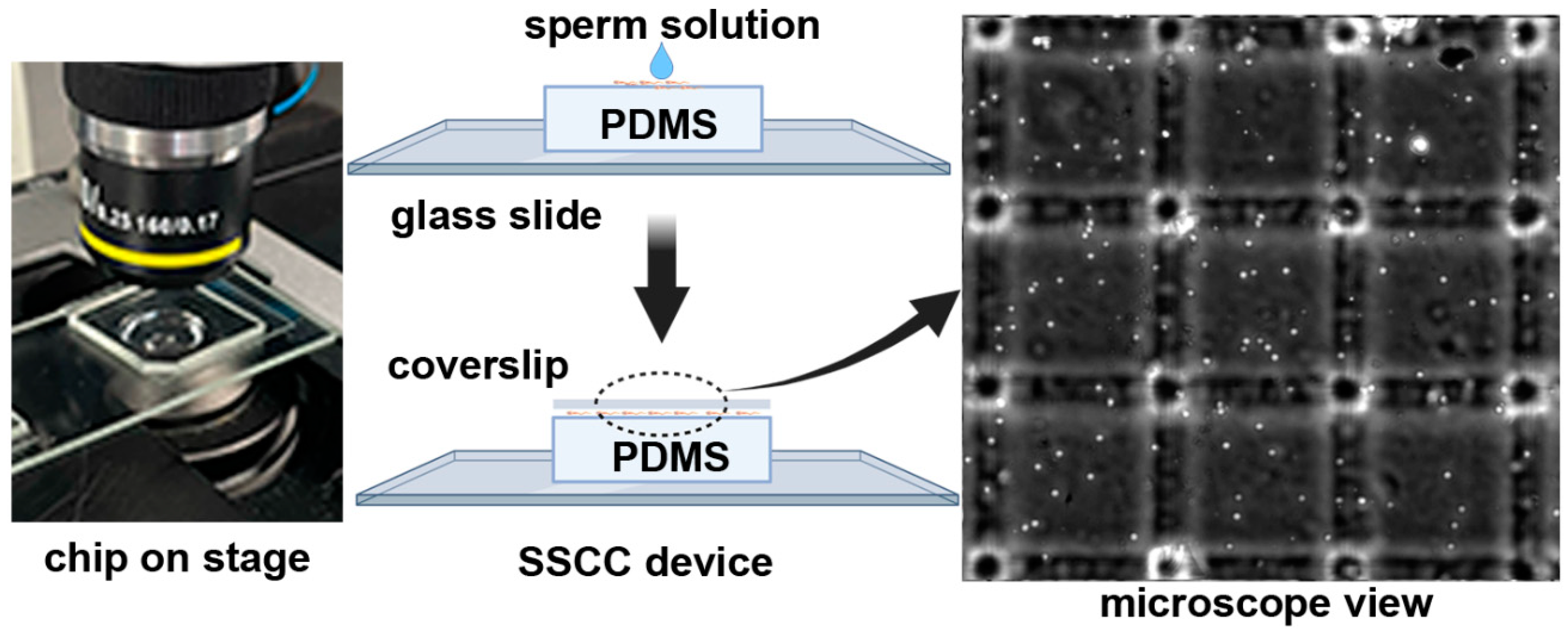
2.1. Design and Component Prototyping
2.2. Sperm Collection
2.3. Evaluation of Operational Utility and Functionality
2.3.1. Evaluation of Different Chamber Designs
2.3.2. Evaluation of the Feasibility of Repeated Use
2.3.3. Evaluation of the Feasibility of Bonding to Glass Slides for Durability
2.4. Accuracy Comparison of Performance Prototypes and Commercial Products
2.5. Data Analysis
3. Results
3.1. Design and Component Prototyping
3.2. Evaluation of Chamber Prototypes for Operational Utility and Functionality
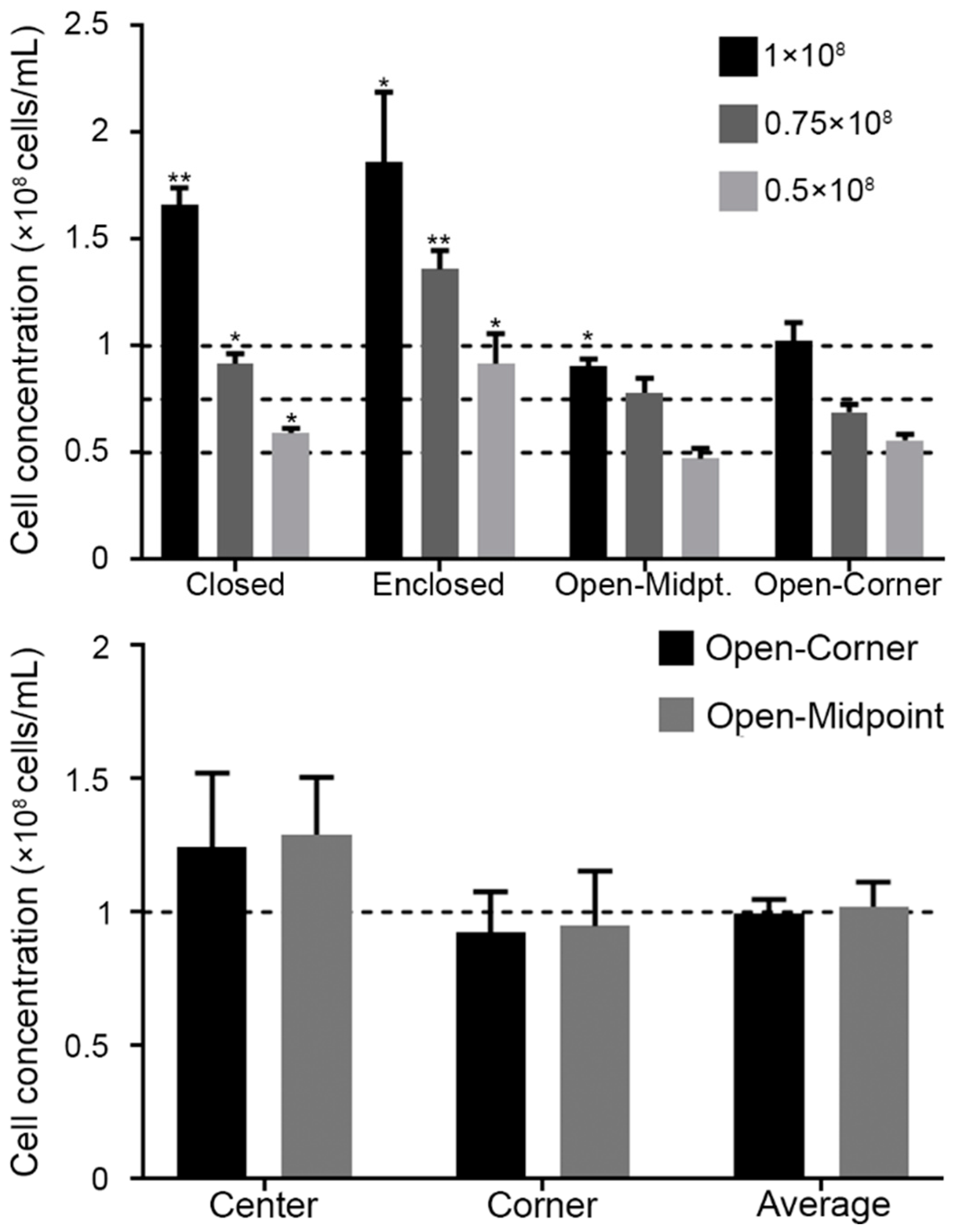
3.2.1. Comparison of Sperm Counting Accuracy for Each Design
3.2.2. Uniformity of Sperm Distribution within Chambers
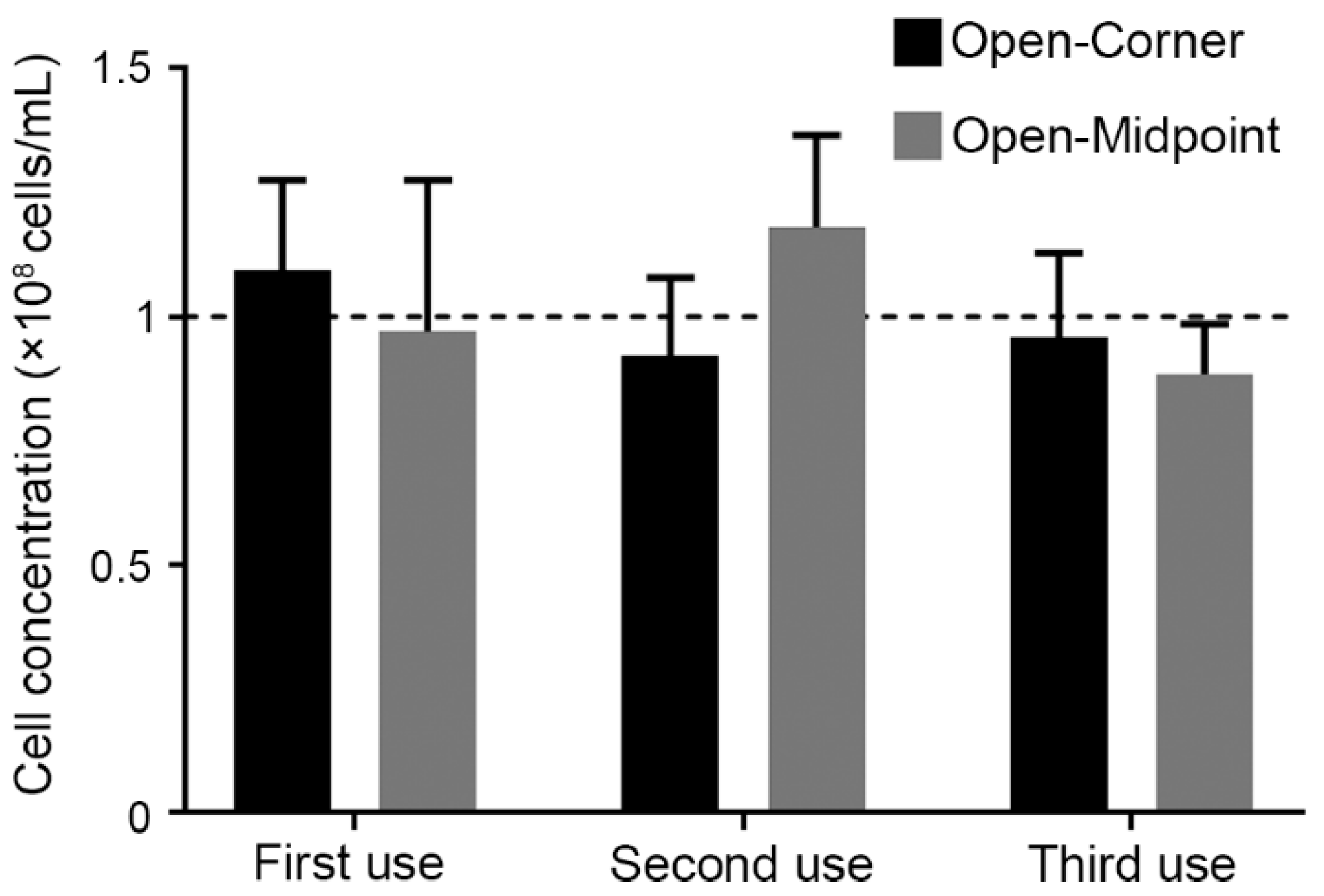
3.2.3. Feasibility of SSCC Reusability
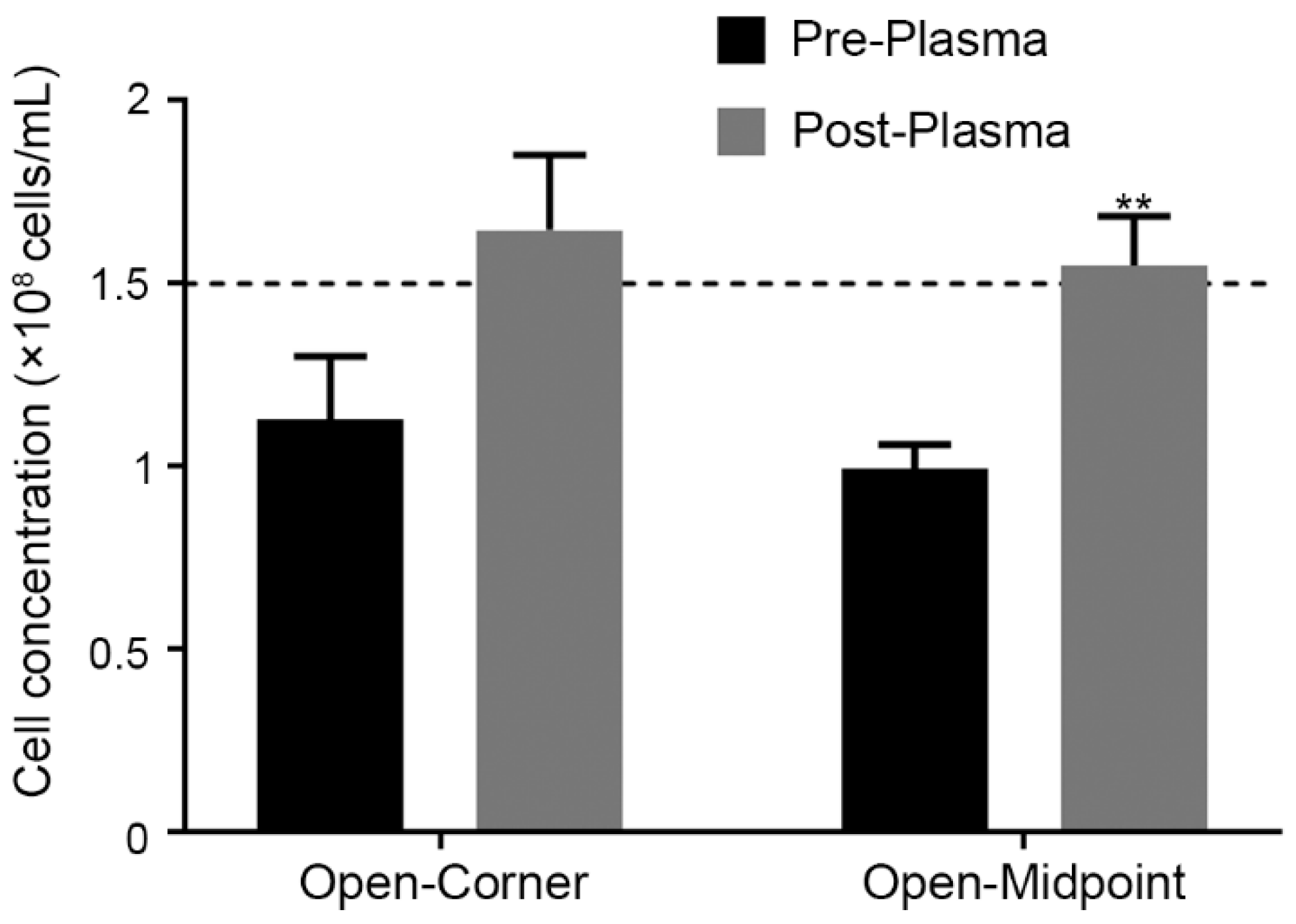
3.2.4. Determining the Effect of Plasma Bonding on SSCC Counting Accuracy
3.3. Accuracy Comparison of Performance Prototypes and Commercial Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Blackburn, H.; Taylor, S.S.; Tiersch, T.R. Development of germplasm repositories to assist conservation of endangered fishes: Examples from small-bodied livebearing fishes. Theriogenology 2019, 135, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Liu, Y.; Guitreau, A.; Yang, H.; Tiersch, T.R. Challenges in development of sperm repositories for biomedical fishes: Quality control in small-bodied species. Zebrafish 2017, 14, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Tiersch, T.R. Process pathways for cryopresevation research. In Cryopreservation in Aquatic Species; Tiersch, T.C.C., Ed.; The World Aquaculture Society: Louisiana, LA, USA, 2011; pp. 646–671. [Google Scholar]
- Judycka, S.; Cejko, B.I.; Dryl, K.; Dobosz, S.; Grudniewska, J.; Kowalski, R.K. The effect of supplementation of a trehalose-based extender with KCl on rainbow trout (Oncorhynchus mykiss) sperm freezability and post-thaw motility. Aquaculture 2016, 465, 303–310. [Google Scholar] [CrossRef]
- Nynca, J.; Judycka, S.; Liszewska, E.; Dobosz, S.; Ciereszko, A. Standardization of spermatozoa concentration for cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 2017, 477, 23–27. [Google Scholar] [CrossRef]
- Dong, Q.; Huang, C.; Tiersch, T.R. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: Evidence from sperm agglutination in oysters. Cryobiology 2007, 54, 87–98. [Google Scholar] [CrossRef]
- Liu, Y.; Torres, L.; Tiersch, T.R. Quality evaluation of sperm from livebearing fishes: Standardized assessment of sperm bundles (spermatozeugmata) from Xenotoca eiseni (Goodeidae). Theriogenology 2018, 107, 50–56. [Google Scholar] [CrossRef]
- Torres, L.; Hu, E.; Tiersch, T.R. Cryopreservation in fish: Current status and pathways to quality assurance and quality control in repository development. Reprod. Fertil. Dev. 2016, 28, 1105–1115. [Google Scholar] [CrossRef]
- Torres, L.; Tiersch, T.R. Addressing reproducibility in cryopreservation, and considerations necessary for commercialization and community development in support of genetic resources of aquatic species. J. World Aquac. Soc. 2018, 49, 644–663. [Google Scholar] [CrossRef]
- Hagedorn, M.; Varga, Z.; Walter, R.B.; Tiersch, T.R. Workshop report: Cryopreservation of aquatic biomedical models. Cryobiology 2019, 86, 120–129. [Google Scholar] [CrossRef]
- Islam, M.S.; Akhter, T. Tale of fish sperm and factors affecting sperm motility: A review. Adv. Life Sci. 2012, 1, 11–19. [Google Scholar] [CrossRef]
- Ginzberg, M.B.; Kafri, R.; Kirschner, M. Cell biology. On being the right (cell) size. Science 2015, 348, 1245075. [Google Scholar] [CrossRef] [PubMed]
- Makler, A. The improved ten-micrometer chamber for rapid sperm count and motility evaluation. Fertil. Steril. 1980, 33, 337–338. [Google Scholar] [CrossRef]
- Yang, H.; Daly, J.; Tiersch, T.R. Determination of sperm concentration using flow cytometry with simultaneous analysis of sperm plasma membrane integrity in zebrafish (Danio rerio). Cytom. A 2016, 89, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Yang, H.; Tiersch, T.R. Determination of sperm concentration for small-bodied biomedical model fishes by use of microspectrophotometry. Zebrafish 2010, 7, 233–240. [Google Scholar] [CrossRef]
- Chan, S.Y.; Wang, C.; Song, B.L.; Lo, T.; Leung, A.; Tsoi, W.L.; Leung, J. Computer-assisted image analysis of sperm concentration in human semen before and after swim-up separation: Comparison with assessment by haemocytometer. Int. J. Androl. 1989, 12, 339–345. [Google Scholar] [CrossRef]
- Liu, Y.; Chesnut, M.; Guitreau, A.; Beckham, J.; Melvin, A.; Eades, J.; Tiersch, T.R.; Monroe, W.T. Microfabrication of low-cost customisable counting chambers for standardised estimation of sperm concentration. Reprod. Fertil. Dev. 2020, 32, 873–878. [Google Scholar] [CrossRef]
- Childress, W.M.; Liu, Y.; Tiersch, T.R. Design, alpha testing, and beta testing of a 3-D printed open-hardware portable cryopreservation device for aquatic species. J. Appl. Aquac. 2021, 241, 34927. [Google Scholar] [CrossRef]
- Rivas Arzaluz, C.; Ayala, M.E.; Aragón Martínez, A. A new open-source hardware device to measure vertical sperm motility and concentration. Cytometry Part. A 2021, 99, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Harmon, E.R.; Liu, Y.; Shamkhalichenar, H.; Browning, V.; Savage, M.; Tiersch, T.R.; Monroe, W.T. An Open-Hardware Insemination Device for Small-Bodied Live-Bearing Fishes to Support Development and Use of Germplasm Repositories. Animals 2022, 12, 961. [Google Scholar] [CrossRef]
- Liu, Y.; Eskridge, M.; Guitreau, A.; Beckham, J.; Chesnut, M.; Torres, L.; Tiersch, T.R.; Monroe, W.T. Development of an open hardware 3-D printed conveyor device for continuous cryopreservation of non-batched samples. Aquac. Eng. 2021, 95, 102202. [Google Scholar] [CrossRef]
- Zuchowicz, N.C.; Belgodere, J.A.; Liu, Y.; Semmes, I.; Monroe, W.T.; Tiersch, T.R. Low-cost resin 3-D printing for rapid prototyping of microdevices: Opportunities for supporting aquatic germplasm repositories. Fishes 2022, 7, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Belgodere, J.A.; Monroe, W.T.; Choi, J.; Gutierrez-Wing, M.T.; Tiersch, T.R. The emerging role of open technologies for community-based improvement of throughput and quality management of sperm cryopreservation for repository development in aquatic species. Anim. Reprod. Sci. 2021, 2021, 106871. [Google Scholar] [CrossRef]
- Beckham, J.; Alam, F.; Omojola, V.; Scherr, T.; Guitreau, A.; Melvin, A.; Park, D.S.; Choi, J.W.; Tiersch, T.R.; Todd Monroe, W. A microfluidic device for motility and osmolality analysis of zebrafish sperm. Biomed. Microdevices 2018, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Plecis, A.; Chen, Y. Fabrication of microfluidic devices based on glass–PDMS–glass technology. Microelectron. Eng. 2007, 84, 1265–1269. [Google Scholar]
- Borok, A.; Laboda, K.; Bonyar, A. PDMS bonding technologies for microfluidic applications: A review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef]
- Mathews, J.; Murphy, J.; Carmichael, C.; Varga, Z. ZIRC E400/RMMB Sperm Cryopreservation & IVF Protocol; Zebrafish International Resource Center: Eugene, OR, USA, 2017. [Google Scholar]
- Peng, N.; Zou, X.; Li, L. Comparison of different counting chambers using a computer-assisted semen analyzer. Syst. Biol. Reprod. Med. 2015, 61, 307–313. [Google Scholar] [CrossRef]
- Lu, J.C.; Yue, R.Q.; Feng, R.X.; Kong, L.Z.; Xu, Y.C. Accuracy evaluation of the depth of six kinds of sperm counting chambers for both manual and computer-aided semen analyses. Int. J. Fertil. Steril. 2016, 9, 527–533. [Google Scholar] [CrossRef]
- Christensen, P.; Stryhn, H.; Hansen, C. Discrepancies in the determination of sperm concentration using Bürker-Türk, Thoma and Makler counting chambers. Theriogenology 2005, 63, 992–1003. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Huang, Z.-W.; Tsai, F.-S.; Chen, C.-Y.; Lin, C.-M.; Wo, A.M. Analysis of sperm concentration and motility in a microfluidic device. Microfluid. Nanofluidics 2011, 10, 59–67. [Google Scholar] [CrossRef]
- Park, D.S.; Egnatchik, R.A.; Bordelon, H.; Tiersch, T.R.; Monroe, W.T. Microfluidic mixing for sperm activation and motility analysis of pearl Danio zebrafish. Theriogenology 2012, 78, 334–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherr, T.; Quitadamo, C.; Tesvich, P.; Park, D.S.; Tiersch, T.; Hayes, D.; Choi, J.W.; Nandakumar, K.; Monroe, W.T. A Planar Microfluidic Mixer Based on Logarithmic Spirals. J. Micromech. Microeng. 2012, 22, 55019. [Google Scholar] [CrossRef]
- Kulik, E.A.; Calahan, P. Laser profilometry of polymeric materials. Cells Mater. 1997, 7, 3. [Google Scholar]
- Leksycki, K.; Królczyk, J.B. Comparative assessment of the surface topography for different optical profilometry techniques after dry turning of Ti6Al4V titanium alloy. Measurement 2021, 169, 108378. [Google Scholar] [CrossRef]
- Hynes, M. The distribution of luecocytes on the counting chamber. J. Clin. Pathol. 1947, 1, 25. [Google Scholar]
- Nielson, L.; Smyth, G.; Greenfield, P. Hemacytometer cell count distributions: Implications of non-poisson behavior. Biotechnol. Prog. 1991, 7, 560–563. [Google Scholar] [CrossRef]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A practical guide to rapid-prototyping of PDMS-based microfluidic devices: A tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Gage, M.J.; Freckleton, R.P. Relative testis size and sperm morphometry across mammals: No evidence for an association between sperm competition and sperm length. Proc. Biol. Sci. 2003, 270, 625–632. [Google Scholar] [CrossRef]
- Prakash, S.; Prithiviraj, E.; Suresh, S.; Lakshmi, N.V.; Ganesh, M.K.; Anuradha, M.; Ganesh, L.; Dinesh, P. Morphological diversity of sperm: A mini review. Iran. J. Reprod. Med. 2014, 12, 239–242. [Google Scholar]
- Jenkins, G. Rapid prototyping of PDMS devices using SU-8 lithography. Methods Mol. Biol. 2013, 949, 153–168. [Google Scholar] [CrossRef]
- Park, M.J.; Lim, M.Y.; Park, H.J.; Park, N.C. Accuracy comparison study of new smartphone-based semen analyzer versus laboratory sperm quality analyzer. Investig. Clin. Urol. 2021, 62, 672–680. [Google Scholar] [CrossRef]
- Tiersch, C.J.; Liu, Y.; Tiersch, T.R.; Monroe, W.T. 3-D Printed Customizable Vitrification Devices for Preservation of Genetic Resources of Aquatic Species. Aquac. Eng. 2020, 90, 102097. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, A.; Tiersch, T.R.; Monroe, W.T. A 3D Printed Vitrification Device for Storage in Cryopreservation Vials. Appl. Sci. 2021, 11, 7977. [Google Scholar]
- Liu, Y.; Dong, J.; Tiersch, T.R.; Wu, Q.; Monroe, W.T. An open hardware 3-D printed device for measuring tensile properties of thermoplastic filament polymers at cryogenic temperatures. Cryogenics 2022, 121, 103409. [Google Scholar]


| Item ** | Cost (USD) * | Unit |
|---|---|---|
| Glass slide | 8 | 72 pack |
| Glass coverslip | 5 | 100 pack |
| PDMS | 126 | 500 g + 50 g |
| Photomask | 177 | 1 mask |
| Wafer | 475 | 50 pack |
| SU-8 | 497 | 500 mL |
| SU-8 developer | 133 | 4 L |
| Material costs for 1 cast | 16 | per unit |
| Material costs for 10 casts | 2 | per unit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belgodere, J.A.; Liu, Y.; Reich, E.L.; Eades, J.; Tiersch, T.R.; Monroe, W.T. Development of a Single-Piece Sperm Counting Chamber (SSCC) for Aquatic Species. Fishes 2022, 7, 231. https://doi.org/10.3390/fishes7050231
Belgodere JA, Liu Y, Reich EL, Eades J, Tiersch TR, Monroe WT. Development of a Single-Piece Sperm Counting Chamber (SSCC) for Aquatic Species. Fishes. 2022; 7(5):231. https://doi.org/10.3390/fishes7050231
Chicago/Turabian StyleBelgodere, Jorge A., Yue Liu, Elizabeth L. Reich, Jason Eades, Terrence R. Tiersch, and William Todd Monroe. 2022. "Development of a Single-Piece Sperm Counting Chamber (SSCC) for Aquatic Species" Fishes 7, no. 5: 231. https://doi.org/10.3390/fishes7050231
APA StyleBelgodere, J. A., Liu, Y., Reich, E. L., Eades, J., Tiersch, T. R., & Monroe, W. T. (2022). Development of a Single-Piece Sperm Counting Chamber (SSCC) for Aquatic Species. Fishes, 7(5), 231. https://doi.org/10.3390/fishes7050231







