Detection of Macrobrachium rosenbergii Nodavirus (MrNV) of White Tail Disease (WTD) in Apparently Healthy Giant Freshwater Prawn, Macrobrachium rosenbergii in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and RNA Extraction
2.2. Reverse-Transcription (RT)—PCR, Sequencing, and Phylogenetic Analysis
2.3. Histopathology Analysis
3. Results
3.1. Collected Samples
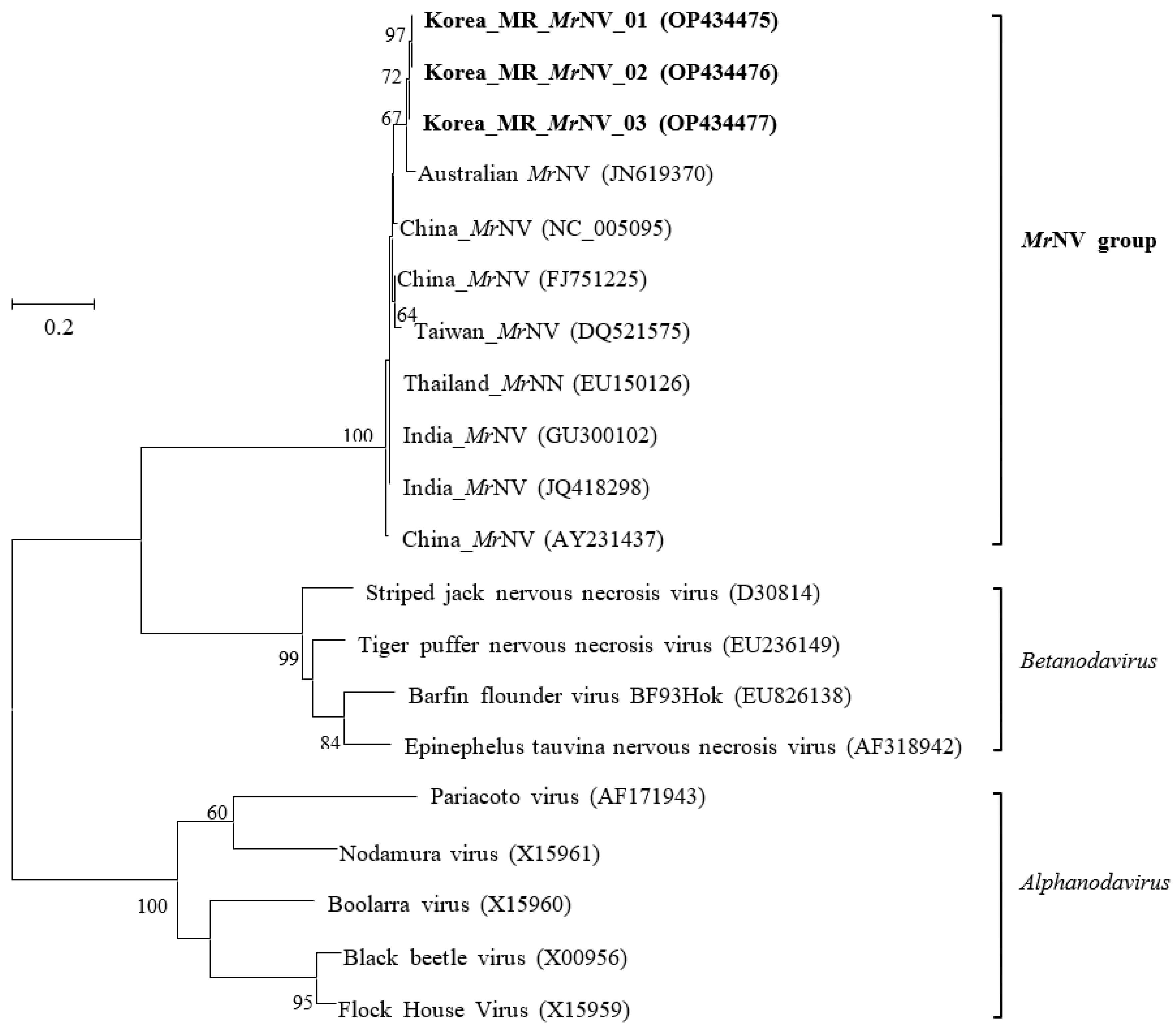
3.2. Sequence Analysis of MrNV
3.3. Microscopic Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- New, M.B. Freshwater prawn culture: A review. Aquaculture 1990, 88, 99–143. [Google Scholar]
- Sahul Hameed, A.S.; Bonami, J.R. White Tail Disease of Freshwater Prawn, Macrobrachium rosenbergii. Indian J. Virol. 2012, 23, 134–140. [Google Scholar]
- Gangnonngiw, W.; Bunnontae, M.; Phiwsaiya, K.; Senapin, S.; Dhar, A.K. In experimental challenge with infectious clones of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV), MrNV alone can cause mortality in freshwater prawn (Macrobrachium rosenbergii). Virology 2020, 540, 30–37. [Google Scholar]
- Sri Widada, J.; Durand, S.; Cambournac, I.; Qian, D.; Shi, Z.; Dejonghe, E.; Richard, V.; Bonami, J.R. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii: Dot-blot, in situ hybridization and RT-PCR. J. Fish Dis. 2003, 26, 583–590. [Google Scholar]
- Qian, D.; Shi, Z.; Zhang, S.; Cao, Z.; Liu, W.; Li, L.; Xie, Y.; Cambournac, I.; Bonami, J.R. Extra small virus-like particles (XSV) and nodavirus associated with whitish muscle disease in the giant freshwater prawn, Macrobrachium rosenbergii. J. Fish Dis. 2003, 26, 521–527. [Google Scholar]
- Sahul Hameed, A.S.; Yoganandhan, K.; Sri Widada, J.; Bonami, J.R. Studies on the occurrence and RT-PCR detection of Macrobrachium rosenbergii nodavirus and extra small virus like particles associated with white tail disease of Macrobrachium rosenbergii in India. Aquaculture 2004, 238, 127–133. [Google Scholar]
- Arcier, J.M.; Herman, F.; Lightner, D.V.; Redman, R.M.; Mari, J.; Bonami, J.R. A viral disease associated with mortalities in hatchery-reared post-larvae of the giant freshwater prawn Macrobrachium rosenbergii. Dis. Aquat. Org. 1999, 38, 177–181. [Google Scholar]
- Hsieh, C.Y.; Wu, Z.B.; Tung, M.C.; Tu, C.; Lo, S.P.; Chang, C.D.; Chen, S.C.; Hsieh, Y.C.; Tsai, S.S. In situ hybridization and RT-PCR detection of Macrobrachium rosenbergii nodavirus in giant freshwater prawn, Macrobrachium rosenbergii (de Man), in Taiwan. J. Fish Dis. 2006, 29, 665–671. [Google Scholar]
- Yoganandhan, K.; Leartvibhas, M.; Sriwongpuk, S.; Limsuwan, C. White tail disease of the giant freshwater prawn Macrobrachium rosenbergii in Thailand. Dis. Aquat. Org. 2006, 69, 255–258. [Google Scholar]
- Owens, L.; La Fauce, K.; Juntunen, K.; Hayakijkosol, O.; Zeng, C. Macrobrachium rosenbergii nodavirus disease (White tail disease) in Australia. Dis. Aquat. Org. 2009, 85, 175–180. [Google Scholar]
- Saedi Tayebeh, A.; Moeini, H.; Wen Tan, S.; Yusoff, K.; Hassan Daud, M.; Kua Chu, B.; Soon Tan, G.; Bhassu, S. Detection and phylogenetic profiling of nodavirus associated with white tail disease in Malaysian Macrobrachium rosenbergii (de Man). Mol. Biol. Rep. 2012, 39, 5785–5790. [Google Scholar]
- Murwantoko, M.; Bimantara, A.; Roosmanto, R.; Kawaichi, M. Macrobrachium rosenbergii nodavirus infection in a giant freshwater prawn hatchery in Indonesia. Springerplus 2016, 5, 1729. [Google Scholar]
- Ball, L.A.; Hendry, D.A.; Johnson, J.E.; Rueckert, R.R.; Scotti, P.D. Family Nodaviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses. Seventh Report of the International Committee for the Taxonomy of Viruses; van Regenmortel, M.H.V., Fauquet, C.M., Bishop, D.H.L., Carstens, E.B., Estes, M.K., Lemon, S.M., Manilo¡, J., Mayo, M.A., McGeoch, D.J., Pringle, C.R., et al., Eds.; Academic Press: San Diego, CA, USA, 2000; pp. 747–755. [Google Scholar]
- NaveenKumar, S.; Shekar, M.; Karunasagar, I.; Karunasagar, I. Genetic analysis of RNA1 and RNA2 of Macrobrachium rosenbergii nodavirus (MrNV) isolated from India. Virus Res. 2013, 173, 377–385. [Google Scholar]
- Romestand, B.; Bonami, J.R. A sandwich enzyme linked immunosorbent assay (S-ELISA) for detection of MrNV in the giant freshwater prawn, Macrobrachium rosenbergii (de Man). J. Fish Dis. 2003, 26, 71–75. [Google Scholar]
- WOAH. Manual of Diagnostic Tests for Aquatic Animals. Chapter 2.2.6, Infection with Macrobrachium rosenbergii Nodavirus (White Tail Disease). 2021. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/ (accessed on 19 September 2022).
- Sudhakaran, R.; Syed Musthaq, S.; Haribabu, P.; Mukherjee, S.C.; Gopal, C.; Sahul Hameed, A.S. Experimental transmission of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) in three species of marine shrimp (Penaeus indicus, Penaeus japonicus and Penaeus monodon). Aquaculture 2006, 257, 136–141. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kim, B.S.; Jang, G.I.; Kim, S.M.; Kim, Y.S.; Jeon, Y.G.; Oh, Y.K.; Hwang, J.Y.; Kwon, M.G. First Report of Enterocytozoon hepatopenaei Infection in Pacific Whiteleg Shrimp (Litopenaeus vannamei) Cultured in Korea. Animals 2021, 11, 3150. [Google Scholar]
- Bonami, J.R.; Shi, Z.; Qian, D.; Sir Widada, J. White tail disease of the giant freshwater prawn, Macrobrachium rosenbergii: Separation of the associated virions and characterization of MrNV as a new type of nodavirus. J. Fish. Dis. 2005, 28, 23–31. [Google Scholar]
- Sri Widada, J.; Bonami, J.R. Characterization of the monocistronic genome of extra small virus, a virus-like particle associated with Macrobrachium rosenbergii nodavirus: Possible candidate for a new species of satellite virus. J. Gen. Virol. 2004, 85, 643–646. [Google Scholar]
- Zhang, H.; Wang, J.; Yuan, J.; Li, L.; Zhang, J.; Bonami, J.R.; Shi, Z. Quantitative relationship of two viruses (MrNV and XSV) in white tail disease of Macrobrachium rosenbergii. Dis. Aquat. Org. 2006, 71, 11–17. [Google Scholar]
- Pasookhush, P.; Hindmarch, C.; Sithigorngul, P.; Longyant, S.; Bendena, W.G.; Chaivisuthangkura, P. Transcriptomic analysis of Macrobrachium rosenbergii (giant fresh water prawn) post-larvae in response to M. rosenbergii nodavirus (MrNV) infection: De novo assembly and functional annotation. BMC Genom. 2019, 20, 762. [Google Scholar]
- Shekhar, M.S.; Sahoo, P.K.; Dillikumar, M.; Das, A. Cloning, expression and sequence analysis of Macrobrachium rosenbergii nodavirus genes: Indian isolate. Aquac. Res. 2011, 42, 1778–1788. [Google Scholar]
- Tang, K.F.; Pantoja, C.R.; Redman, R.M.; Lightner, D.V. Development of in situ hybridization and RT-PCR assay for the detection of a nodavirus (PvNV) that causes muscle necrosis in Penaeus vannamei. Dis. Aquat. Org. 2007, 75, 183–190. [Google Scholar]
- Park, K.H.; Seong, I.W. Electron Microscopic Study on the Replcation of Hantaan Virus in Vero-E6 Cells. J. Korean Soc. Virol. 1999, 29, 201–209. [Google Scholar]
- O’Quigley, J.; Hughes, M.D.; Fenton, T. Dose-finding designs for HIV studies. Biometrics 2001, 57, 1018–1029. [Google Scholar]

| Methods | Primers | Sequences | Product Size | References |
|---|---|---|---|---|
| MrNV (1st RT-PCR) | Forward | 5′-GCG-TTA-TAG-ATG-GCA-CAA-GG-3′ | 425 bp | [4,6] |
| Reverse | 5′-AGC-TGT-GAA-ACT-TCC-ACT-GG-3′ | |||
| XSV (1st RT-PCR) | Forward | 5′-CGC-GGA-TCC-GAT-GAA-TAA-GCG-CAT-TAA-TAA-3′ | 546 bp | |
| Reverse | 5′-CCG-GAA-TTC-CGT-TAC-TGT-TCG-GAG-TCC-CAA-3′ | |||
| MrNV (nested RT-nPCR) | Forward | 5′-GAT-GAC-CCC-AAC-GTT-ATC-CT-3′ | 205 bp | [17] |
| Reverse | 5′-GTG-TAG-TCA-CTT-GCA-AGA-GG-3′ | |||
| XSV (nested RT-nPCR) | Forward | 5′-ACA-TTG-GCG-GTT-GGG-TCA-TA-3′ | 236 bp | |
| Reverse | 5′-GTG-CCT-GTT-GCT-GAA-ATA-CC-3′ |
| Samples | No. of Specimen | No. of Zenker’s Necrosis and Myolysis | Expression Rate of Histopathological Changes |
|---|---|---|---|
| Adult prawn (Farm 1) | 17 | 8 | 47% |
| Adult prawn (Farm 2) | 10 | 5 | 50% |
| Post-larval prawn (Farm 3) | 3 | 2 | 67% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, G.I.; Kim, B.S.; Kim, S.M.; Oh, Y.K.; Kim, J.O.; Hwang, J.Y.; Lee, S.J.; Hong, S.Y.; Kwon, M.G. Detection of Macrobrachium rosenbergii Nodavirus (MrNV) of White Tail Disease (WTD) in Apparently Healthy Giant Freshwater Prawn, Macrobrachium rosenbergii in Korea. Fishes 2022, 7, 294. https://doi.org/10.3390/fishes7050294
Jang GI, Kim BS, Kim SM, Oh YK, Kim JO, Hwang JY, Lee SJ, Hong SY, Kwon MG. Detection of Macrobrachium rosenbergii Nodavirus (MrNV) of White Tail Disease (WTD) in Apparently Healthy Giant Freshwater Prawn, Macrobrachium rosenbergii in Korea. Fishes. 2022; 7(5):294. https://doi.org/10.3390/fishes7050294
Chicago/Turabian StyleJang, Gwang Il, Bo Seong Kim, Su Mi Kim, Yun Kyeong Oh, Jae Ok Kim, Jee Youn Hwang, Soon Jeong Lee, Sung Youl Hong, and Mun Gyeong Kwon. 2022. "Detection of Macrobrachium rosenbergii Nodavirus (MrNV) of White Tail Disease (WTD) in Apparently Healthy Giant Freshwater Prawn, Macrobrachium rosenbergii in Korea" Fishes 7, no. 5: 294. https://doi.org/10.3390/fishes7050294
APA StyleJang, G. I., Kim, B. S., Kim, S. M., Oh, Y. K., Kim, J. O., Hwang, J. Y., Lee, S. J., Hong, S. Y., & Kwon, M. G. (2022). Detection of Macrobrachium rosenbergii Nodavirus (MrNV) of White Tail Disease (WTD) in Apparently Healthy Giant Freshwater Prawn, Macrobrachium rosenbergii in Korea. Fishes, 7(5), 294. https://doi.org/10.3390/fishes7050294






