Exposure to Polymethylmethacrylate Microplastics Induces a Particle Size-Dependent Immune Response in Mediterranean Mussel Mytilus galloprovincialis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microparticles and Chemicals
2.2. The Model Organism and Sampling Site
2.3. Experimental Setup and Sampling Procedure
2.4. Condition Index (CI)
2.5. Stress-on-Stress (SOS) Test
2.6. Haemolymph Analyses
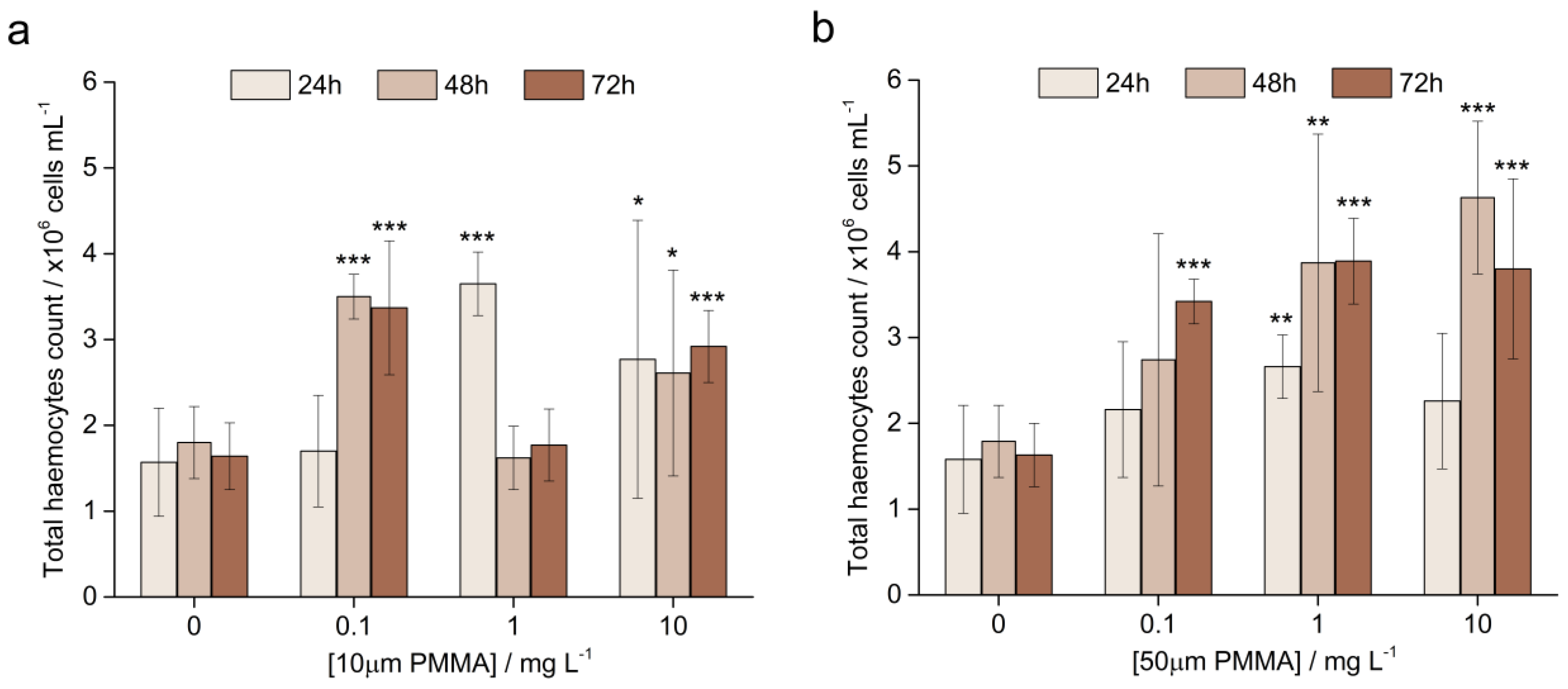
2.6.1. Total Haemocyte Count (THC)
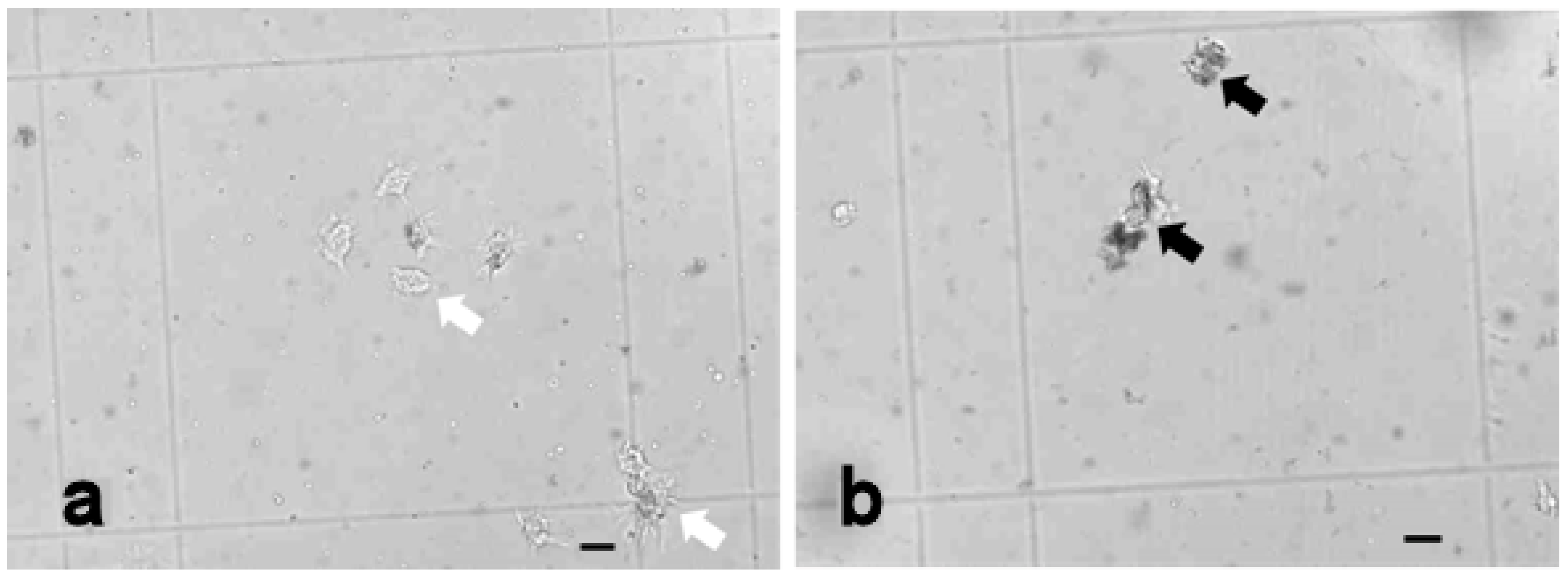
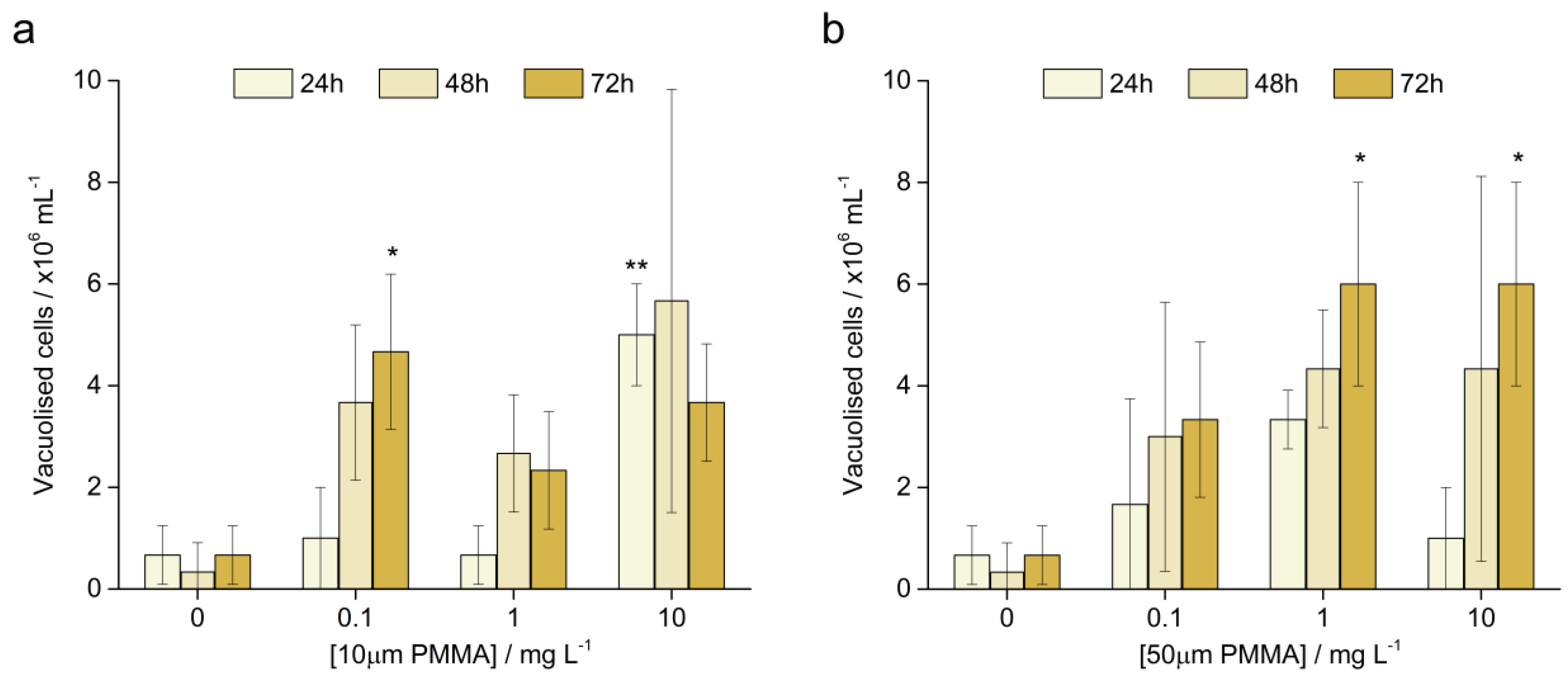
2.6.2. Vacuolised Haemocytes
2.6.3. Cell Viability
2.6.4. Lysosomal Membrane Stability
2.7. Histological Analysis
2.8. Biochemical Analyses—Enzymatic Activities
2.8.1. AcetylCholinesterase Activity (AChE)
2.8.2. The Activity of Catalase (CAT)
2.8.3. The Activity of Glutathione S–Transferase (GST)
2.8.4. The Activity of Glutathione Reductase (GR)
2.9. Statistics
3. Results
3.1. Condition Index (CI)
3.2. Stress-on-Stress (SOS) Test
3.3. Haemolymph Analysis
3.3.1. The Total Haemocyte Count (THC)
3.3.2. Cell Viability
3.3.3. Vacuolised Cells
3.4. Histological Analysis
3.5. Enzymatic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe, Plastics—The Facts 2021. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 30 August 2022).
- UNEP Marine Plastic Debris and Microplastics—Global Lessons and Research to Inspire Action and Guide Policy Change; United Nations Environment Programme: Nairobi, Kenya, 2016.
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.S.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine litter distribution and density in European seas, from the shelves to deep basins. PLoS ONE 2014, 9, e95839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 60, 1596–1605. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, P.J. (Ed.) GESAMP Sources, fate, and effects of microplastics in the marine environment: A global assessment. In IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection; Rep. Stud. GESAMP; FAO: Rome, Italy, 2015; No. 90; p. 96. [Google Scholar]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean plastic soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatinus, A.; Viršek, M.K.; Robič, U.; Grego, M.; Bajt, O.; Šiljić, J.; Suaria, G.; Liubartseva, S.; Coppini, G.; Peterlin, M. Marine litter in the Croatian part of the middle Adriatic Sea: Simultaneous assessment of floating and seabed macro and micro litter abundance and composition. Mar. Pollut. Bull. 2019, 139, 427–439. [Google Scholar] [CrossRef]
- Vianello, A.; Da Ros, L.; Boldrin, A.; Marceta, T.; Moschino, V. First evaluation of floating microplastics in the northwestern Adriatic Sea. Environ. Sci. Poll. Res. 2018, 25, 28546–28561. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2016, 128, 2–11. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health; a short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Andbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Brate, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Li, L.; Jabeen, K.; Shi, H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Luster, A. Marine Anthropogenic Litter, Chapter 10. In Microplastics in the Marine Environment: Distribution, Interactions and Effects; Springer Open: Cham, Switzerland, 2015; pp. 245–307. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 12, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Tosetto, L.; Williamson, J.E.; Browne, C. Trophic transfer of microplastics does not affect fish personality. Anim. Behav. 2017, 123, 159–167. [Google Scholar] [CrossRef]
- Laist, D.W. Overview of the biological effects of lost and discarded plastic debris in the marine environment. Mar. Pollut. Bull. 1987, 18, 319–326. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Microplastic—an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodkovicova, N.; Hollerova, A.; Caloudova, H.; Blahova, J.; Franc, A.; Garajova, M.; Lenz, J.; Tichy, F.; Faldyna, M.; Kulich, P.; et al. Do foodborne polyethylene microparticles affect the health of rainbow trout (Oncorhynchus mykiss)? Sci. Total Environ. 2021, 793, 148490. [Google Scholar] [CrossRef] [PubMed]
- Seth, K.; Shriwastav, A. Contamination of Indian sea salts with microplastics and a potential prevention strategy. Environ. Sci. Pollut. Res. 2018, 25, 30122–30131. [Google Scholar] [CrossRef]
- Pavičić-Hamer, D.; Kovačić, I.; Koščica, L.; Hamer, B. Physiological indices of maricultured mussel Mytilus galloprovincialis Lamarck, 1819 in Istria, Croatia: Seasonal and transplantation effect. J. World Aquac. Soc. 2016, 47, 768–778. [Google Scholar] [CrossRef]
- Hamer, B.; Korlević, M.; Durmiši, E.; Nerlović, V.; Bierne, N. Nuclear marker Me 15-16 analyses of Mytilus galloprovincialis populations along the eastern Adriatic coast. Cah. Biol. Mar. 2012, 53, 35–44. [Google Scholar] [CrossRef]
- Goldberg, E.D. The mussel wach-a first step in global marine monitoring. Mar. Pollut. Bull. 1975, 6, 111–114. [Google Scholar] [CrossRef]
- Hamer, B.; Pavičić-Hamer, D.; Muller, W.E.G.; Batel, R. Stress-70 proteins in marine mussel Mytilus galloprovincialis as biomarkers of environmental pollution: A field study. Environ. Int. 2004, 30, 873–882. [Google Scholar] [CrossRef]
- Hamer, B.; Pavičić-Hamer, D.; Muller, W.E.G.; Zahn, R.; Batel, R. Detection of stress-70 proteins of mussel Mytilus galloprovincialis using 2-D gel electrophoresis:a proteomics approach. Fresenius Environ. Bull. 2005, 14, 605–611. [Google Scholar]
- Hamer, B.; Jaksic, Z.; Pavicic-Hamer, D.; Peric, L.; Medakovic, D.; Ivankovic, D.; Pavicic, J.; Zilberberg, C.; Schroder, H.C.; Muller, W.E.G.; et al. Effect of hypoosmotic stress by low salinity acclimation of Mediterranean mussels Mytilus galloprovincialison biological parameters used for pollution assessment. Aquat. Toxicol. 2008, 89, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Tsarpali, V.; Dailianis, S. Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Sci. Total Environ. 2018, 636, 220–229. [Google Scholar] [CrossRef]
- Höher, N.; Turja, R.; Köhler, A.; Lehtonen, K.K.; Broeg, K. Immunological responses in the mussel Mytilus trossulus transplanted at the coastline of the northern Baltic Sea. Mar. Environ. Res. 2015, 112, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.R.; Pipe, R.K. Mussels and environmental contaminants: Molecular and cellular aspects. In Developments in Aquaculture and Fisheries Science; Gosling, E.M., Ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1992; pp. 425–464. [Google Scholar]
- Famme, P.; Riisgård, H.U.; Jørgensen, C.B. On direct measurement of pumping rates in the mussel Mytilus edulis. Mar. Biol. 1986, 92, 323–327. [Google Scholar] [CrossRef]
- Ward, J.E.; Shumway, S.E. Separating the grain from the chaff: Particle selection in suspension- and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300, 83–130. [Google Scholar] [CrossRef]
- Gonçalves, C.; Martins, M.; Sobral, P.; Costa, P.M.; Costa, M.H. An assessment of the ability to ingest and excrete microplastics by filter-feeders: A case study with the Mediterranean mussel. Environ. Pollut. 2019, 245, 600–606. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Birger Oysaed, K.; Fabi, G. First occurrence and composition assessment of microplastics in native mussels collected from coastal and offshore areas of the northern and central Adriatic Sea. Environ. Sci. Pollut. Res. 2019, 26, 24407–24416. [Google Scholar] [CrossRef]
- Qu, X.; Su, L.; Li, H.; Liang, M.; Shi, H. Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci. Total. Environ. 2018, 621, 679–686. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef] [Green Version]
- Rist, S.; Baun, A.; Almeda, R.; Hartmann, N.B. Ingestion and effects of micro- and nanoplastics in blue mussel (Mytilus edulis) larvae. Mar. Pollut. Bull. 2019, 140, 423–430. [Google Scholar] [CrossRef]
- von Moos, N.; Burkhardt-Holm, P.; Kohler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Détrée, C.; Gallardo-Escárate, C. Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2018, 83, 52–60. [Google Scholar] [CrossRef]
- Tomazic-Jezic, V.J.; Merritt, K.; Umbreit, T.H. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J. Biomed. Mater. Res. 2001, 55, 523–529. [Google Scholar] [CrossRef]
- FAO. Microplastics in Fisheries and aquaculture. In FAO Fisheries and Aquaculture Technical Paper 615; FAO: Rome, Italy, 2017. [Google Scholar]
- O’Neill, P.; Barrett, A.; Sullivan, T.; Regan, F.; Brabazon, D. Rapid Prototyped Biomimetic Antifouling Surfaces for Marine Applications. Mater. Today 2016, 3, 527–532. [Google Scholar] [CrossRef]
- Kühn, S.; Schaafsma, F.L.; van Werven, B.; Flores, H.; Bergmann, M.; Egelkraut-Holtus, M.; Tekman, M.B.; van Franeker, J.A. Plastic ingestion by juvenile polar cod (Boreogadus saida) in the Arctic Ocean. Polar Biol. 2018, 41, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.J.; Oral, R.; Pagano, G.; Tez, S.; Toscanesi, M.; Ranieri, P.; Trifuoggi, M.; Lyons, D.M. Mild toxicity in Paracentrotus lividus early life stages may indicate species-specific sensitivity to polystyrene and polymethylmethacrylate microplastics. Mar. Environ. Res. 2020, 161, 105132. [Google Scholar] [CrossRef]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Pavičić-Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-induced damage in early embryonal development of sea urchin Sphaerechinus granularis. Environ. Res. 2019, 179, 108815. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, E.; Pompe, R.; Besseling, E.; Koelmans, A.A. Detection of low numbers of microplastics in North Sea fish using strict quality assurance criteria. Mar. Pollut. Bull. 2017, 122, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Canesi, L.; Pertica, M.; Mancinelli, G.; Accomando, R.; Smaal, A.C.; Orunesu, M. Stress on stress response: A simple monitoring tool in the assessment of a general stress syndrome in mussels. Mar. Environ. Res. 1995, 39, 245–248. [Google Scholar] [CrossRef]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN project: Detailed description of the scoring criteria for the cytokinesis block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Pagano, M.; Porcino, C.; Briglia, M.; Fiorino, E.; Vazzana, M.; Silvestro, S.; Faggio, C. The influence of exposure of cadmium chloride and zinc chloride on haemolymph and digestive gland cells from Mytilus galloprovincialis. Int. J. Environ. Res. 2017, 11, 207–216. [Google Scholar] [CrossRef]
- Kovačić, I.; Pavičić-Hamer, D.; Phannkuchen, A.M.; Usich, M. Mytilus galloprovincialis (Lamarck, 1819) as host of Mytillcola orientalis (Mori, 1935) in the northern Adriatic Sea: Presence and effect. Aquac. Int. 2016, 25, 211–221. [Google Scholar] [CrossRef]
- Thomas, P.J.; Perono, G.; Tommasi, F.; Pagano, G.; Oral, R.; Burić, P.; Kovačić, I.; Toscanesi, M.; Trifuoggi, M.; Lyons, D.M. Resolving the effects of environmental micro- and nanoplastics exposure in biota: A knowledge gap analysis. Sci. Total Environ. 2021, 780, 146534. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.J. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Orban, E.; Di Lena, G.; Nevigato, T.; Casini, I.; Marzetti, A.; Caproni, R. Seasonal changes in meat content, condition index and chemical composition of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem. 2002, 77, 57–65. [Google Scholar] [CrossRef]
- Lucas, A.; Beninger, P.G. The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 1985, 44, 187–200. [Google Scholar] [CrossRef]
- Okumuş, İ.; Stirling, H.P. Seasonal variations in the meat weight, condition index and biochemical composition of mussels (Mytilus edulis L.) in suspended culture in two Scottish sea lochs. Aquaculture 1998, 159, 249–261. [Google Scholar] [CrossRef]
- Kanduč, T.; Šlejkovec, Z.; Falnoga, I.; Mori, N.; Budič, B.; Kovačić, I.; Pavičić-Hamer, D.; Hamer, B. Ecological status of the Istrian marine environment (NE Adriatic Sea, Croatia): Insights from mussel Mytilus galloprovincialis condition indices, stable isotopes and metal(loid)s. Marine Pollution Bulletin 2017, 126, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Thrush, S.F.; Hewitt, J.E.; Hickey, W.C.; Kelly, S. Multiple stressor effects identified from species abundance distributions: Interactions between urban contaminants and species habitat relationships. J. Exp. Mar. Biol. Ecol. 2008, 366, 160–168. [Google Scholar] [CrossRef]
- Andrade, M.; Soares, A.; Figueira, E.; Freitas, R. Biochemical changes in mussels submitted to different time periods of air exposure. Environ. Sci. Pollut. Res. 2018, 25, 8903–8913. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.E.; Thrush, S.F. Do Species’ Abundances become More Spatially Variable with Stress? Open J. Ecol. 2009, 10, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Carballal, M.J.; Lopez, M.C.; Azevedo, C.; Villalba, A. Hemolymph cell types of the mussel Mytilus galloprovincialis. Dis. Aquat. Org. 1997, 29, 127–135. [Google Scholar] [CrossRef] [Green Version]
- King, M.A. Detection of dead cells and measurement of cell killing by flow cytometry. J. Immunol. Methods 2000, 243, 155–166. [Google Scholar] [CrossRef]
- Franzellitti, S.; Capolupo, M.; Wathsala, R.H.G.R.; Valbonesi, P.; Fabbri, E. The multixenobiotic resistance system as a possible protective response triggered by microplastic ingestion in Mediterranean mussels (Mytilus galloprovincialis): Larvae and adult stages. Comp. Biochem. Physiol. C 2019, 219, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Brate, L.N.; Hurley, R.; Iversen, K.; Beyer, J.; Thomas, K.V.; Steindal, C.C.; Green, N.W.; Olsen, M.; Lusher, A. Mytilus spp. as sentinels for monitoring microplastic pollution in Norwegian coastal waters: A qualitative and quantitative study. Environ. Pollut. 2018, 243, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]

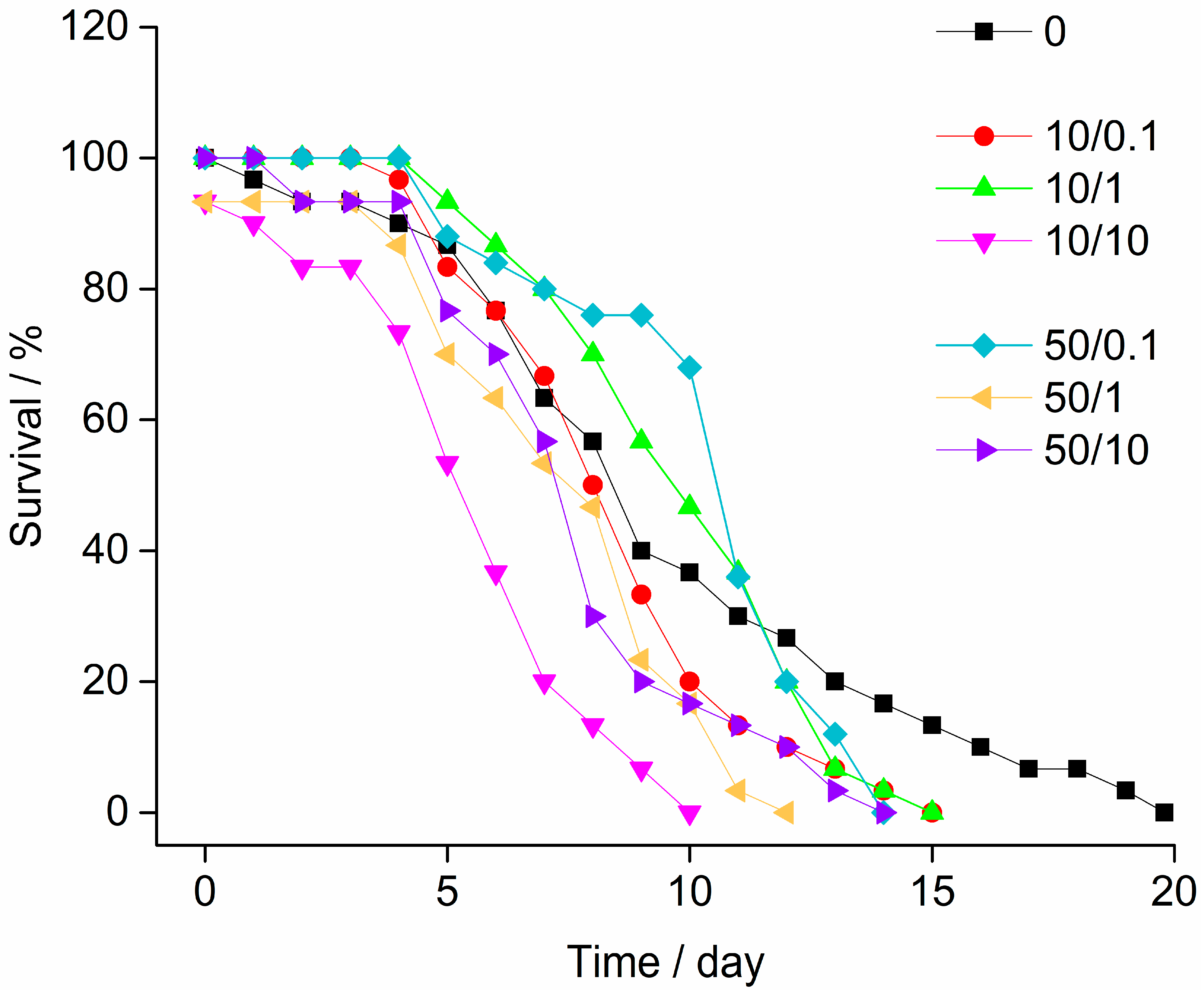



| LT50 Regression Analysis | Control | 10/0.1 | 10/1 | 10/10 | 50/0.1 | 50/1 | 50/10 |
|---|---|---|---|---|---|---|---|
| Median survival time LT50 | 8.32 | 7.86 | 9.29 | 4.35 | 9.55 | 6.48 | 6.76 |
| Lower confidence limit (95%) | 6.47 | 7.38 | 8.80 | 2.98 | 8.37 | ND | 5.47 |
| Upper confidence limit (95%) | 9.98 | 8.31 | 9.77 | 5.58 | 10.88 | ND | 7.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavičić-Hamer, D.; Kovačić, I.; Sović, T.; Marelja, M.; Lyons, D.M. Exposure to Polymethylmethacrylate Microplastics Induces a Particle Size-Dependent Immune Response in Mediterranean Mussel Mytilus galloprovincialis. Fishes 2022, 7, 307. https://doi.org/10.3390/fishes7060307
Pavičić-Hamer D, Kovačić I, Sović T, Marelja M, Lyons DM. Exposure to Polymethylmethacrylate Microplastics Induces a Particle Size-Dependent Immune Response in Mediterranean Mussel Mytilus galloprovincialis. Fishes. 2022; 7(6):307. https://doi.org/10.3390/fishes7060307
Chicago/Turabian StylePavičić-Hamer, Dijana, Ines Kovačić, Tamara Sović, Matea Marelja, and Daniel Mark Lyons. 2022. "Exposure to Polymethylmethacrylate Microplastics Induces a Particle Size-Dependent Immune Response in Mediterranean Mussel Mytilus galloprovincialis" Fishes 7, no. 6: 307. https://doi.org/10.3390/fishes7060307
APA StylePavičić-Hamer, D., Kovačić, I., Sović, T., Marelja, M., & Lyons, D. M. (2022). Exposure to Polymethylmethacrylate Microplastics Induces a Particle Size-Dependent Immune Response in Mediterranean Mussel Mytilus galloprovincialis. Fishes, 7(6), 307. https://doi.org/10.3390/fishes7060307










