Abstract
The study of natural selection and local adaptation is a thriving field of research. Local adaptation is driven by environment components and results in locally adapted phenotypes with higher fitness relative to other phenotypes from other locations in the species range. Tests of local adaptations have traditionally been done using transplant experiments, but the advent of next-generation sequencing methods have allowed the study of local adaptation to move from a phenotypic to a genomic approach. By using genome scans and state-of-the-art statistical tests, researchers can identify genes putatively under selection and study the genomic architecture of local adaptation, which often includes the observation of clustering of adaptive genes concentrated in fewer genomic regions known as “genomic islands of divergence”. The two species of North Atlantic eels, the European and the American eel, are excellent species for studying selection since they are panmictic and present large population sizes, show a wide distribution range across extremely heterogenous environments, and are subject to high mortalities. We reviewed studies of natural selection and local adaptation in American eel, European eel, between life cycle stages, between European and American eel. Finally, we discussed genome architecture in relation to local adaptation in eels and the role of both genetic (i.e., local adaptation) and non-genetic (i.e., phenotypic plasticity) in the survival of eels across their distribution range.
1. Introduction
In order to understand the selective pressures acting upon natural populations, it is crucial to identify which regions of the genome are under selection and discriminate between neutral vs. adaptive genetic differentiation [1]. A growing number of studies have examined the factors driving historical and contemporary evolution in natural populations, searching for gene-environment interactions leading to local adaptation [2]. In particular, species with a wide geographic distribution across heterogeneous habitats in terms of environment drivers (e.g., temperature, productivity, depth, salinity, oxygen, photoperiod) may experience spatially varying selective pressures, which can result in local adaptation of ecologically important traits [3]. A meta-analysis on salmonids estimated the frequency of local adaptation to be ca. 55–70%, with local populations having, on average, a 20% fitness advantage relative to foreign populations [4].
Population genomic studies predict the observation of highly differentiated genomic regions referred to as genomic islands of divergence, which arise as a consequence of genome hitchhiking [5]. Under the hitchhiking model [6], when a selectively favoured beneficial mutation rises to fixation, the neutral variants located nearby the selected mutation will also rise to fixation, which is known as hitchhiking. Consequently, we can observe genomic regions with higher-than-average genetic differentiation together with a change in genetic variability. The loss or reduction in genetic variation in genomic regions adjacent to causative variants that may occur in response to directional selection within one or more populations is referred to as a selective sweep [7]. Genomic islands of divergence have been observed in many taxa from insects [8] to humans [9], including many classic studies in fish, such as stickleback [10,11], Atlantic cod [12,13] or whitefish [14].
2. Excellent Species for the Study of Footprints of Selection
The two species of North Atlantic eels, the European eel Anguilla anguilla and the American eel Anguilla rostrata, are optimal species for the study of footprints of natural selection. First, both species are panmictic [15,16,17,18] and present large effective population sizes despite the acknowledged stock declines. Using an RAD-sequencing approach, Pujolar et al. [17] estimated an effective population size (Ne) for European eel from 100,000 to 1 million individuals. A large Ne was also suggested by PSMC (pairwise sequentially Markovian coalescent) analysis of effective population size back in time, with estimates of >1 million individuals in both species [19]. Such high Ne is important as it renders natural selection the major evolutionary force determining the changes in genetic composition and suggests a negligible role of random genetic drift in the evolution of North Atlantic eels. Second, North Atlantic eels show a wide distribution range and are present across extremely heterogenous environments [20]; for instance, in terms of temperature, the European eel is distributed from subtropical habitats in the Mediterranean to subarctic habitats in Iceland and Scandinavia, while the American eel is distributed from Venezuela to Greenland [21]. Eels are extremely plastic regarding salinity and are regarded as facultative catadromous, with some eels being freshwater residents, some being brackish and marine water residents, and some shifting between habitats [20]. In such highly heterogenous environments, selective pressures vary from region to region so that signatures of selection and local adaptation are likely region-specific. Similarly, Indo-Pacific eel species would also be suitable for selection studies due to their panmictic status and wide geographic distribution in a variety of habitats.
Moreover, there is high potential for selective responses due to high mortalities in both early and late life stages. Bonhommeau et al. [22] estimated a 10% survival rate in European eel glass eels, while Åström and Dekker [23] estimated a natural mortality rate of M = 0.14 per year and a fishery mortality rate of F = 0.54 per year. Many sources of mortality in eels are anthropogenic, including fisheries, habitat loss, migration barriers and human-introduced parasites and viruses [24]. Anthropogenic mortality in the early continental phase notably includes fisheries targeting glass eels and upstream migration barriers, while in the final continental years of the eel’s life cycle exploitation targets the last part of the yellow eel stage and the silver eel stage.
3. How Panmixia Affects the Detection of Signatures of Selection
North Atlantic eels are textbook examples of panmixia (i.e., the existence of one single randomly mating population), and together with the lack of larval homing, it has important implications for the type of signatures of selection we can detect in North Atlantic eel populations. Despite the wide distribution range of North Atlantic eels, there is conclusive evidence for panmixia in both species. In European eel, the comprehensive study of Als et al. [15] genotyped >1000 specimens collected across the entire distribution range in Europe at 21 microsatellites. A low nonsignificant genetic differentiation was found (FST = 0.00024), which was supported by the lack of substructuring found among larvae collected in the spawning site in the Sargasso Sea (FST = 0.00076) or when comparing Europe vs. Sargasso Sea samples (FST = −0.00012). Very low mean FST values were also reported in previous studies using low-density marker sets that included large numbers of sampling sites and individuals (e.g., FST = 0.0017, [25]; FST = 0.0014, [26]; FST = 0.0099, [27]; FST = −0.00003, [28]), the only exception being the study of Baltazar-Soares et al., [29] showing a microsatellite differentiation > 10 times more than reported in other studies (FST = 0.02). Panmixia has also been confirmed at the genomic level using reduced representation sequencing [17], showing again no differentiation between geographic areas consistent with a single panmictic population (FST = 0.00007) after analyzing 259 RAD-sequenced juvenile (glass eel) individuals from eight locations between 34 and 64° N at >450,000 SNPs. A recent paper analyzing full genome data reached the same conclusion of panmixia in European eel [18]. Similarly, Côté et al. [16] conducted the most comprehensive study on American eel, including a total of 2142 eels from 32 sampling locations genotyped at 18 microsatellite loci. Data showed all measures of genetic differentiation to be practically zero, providing decisive evidence for panmixia in American eel.
The existence of single randomly mating panmictic populations for both North American eel species suggests there is no larval homing and larvae do not return to the parental original freshwater habitats. Despite panmixia, there have been suggestions of cryptic female philopatric behavior in this species based on analysis of mitochondrial DNA [29]. However, the conclusion is not supported as it has not been verified in other studies utilizing the same marker [30]. If larvae showed philopatry (i.e., if larvae from Mediterranean parents always returned to the Mediterranean), genetic differences would be expected to accumulate across regions over time. Hence, the lack of genetic differentiation found between geographic areas consistent with a single panmictic population suggests that larval migratory routes are random.
One direct consequence of panmixia and random dispersal of larvae is that heritable local adaptation is not possible in North Atlantic eels, despite the high potential for selection. If for instance, an individual thrives in the Mediterranean because its genetic composition makes it better adapted to survive the particular environmental conditions of the Mediterranean. However, due to lack of homing, its progeny might end up randomly in Iceland or Scandinavia, hence not benefiting from the pre-adapted alleles in their genetic composition. Therefore, all signatures of spatially varying selection in a given generation are expected to be lost in the next generation, which prevents heritable trans-generational local adaptation [31]. However, single-generation signatures of local selection should still be detectable [17,31].
We present now a summary of all selection studies to date on North Atlantic eels (Table 1), including studies on American eel, on European eel, between life cycle (juvenile vs. adult) stages, between the two species and finally studies discussing the role of genome architecture in relation with local adaptation, including epigenetic studies.

Table 1.
Summary of all selection studies on North Atlantic eels including species studied, genetic markers used, sampling details and main results.
4. Studies of Selection in American Eel
Using a candidate gene approach, Gagnaire et al. [31] studied the evolutionary effects of spatially varying selection in American eel. A panel of 100 candidate single nucleotide polymorphisms (SNPs) were genotyped in 992 individuals from 16 sampling sites at different life stages of the same cohort as well as in glass eels of the following cohort. Evidence for spatially varying selection was suggested at 13 coding genes in American eel showing significant correlations with environmental variables (latitude, longitude, and temperature) across the entire species range. Within glass eels, associations with environmental variables were found at eight loci using generalized linear models. Most loci under selection represented key metabolic genes involved in lipid metabolism (ACP, acyl carrier activity; ANX2, inhibition of phospholipase A2; GPX4, phospholipid–hydroperoxide glutathione peroxidase activity), saccharide metabolism (MDH, malate dehydrogenase activity; UGP2, UDP-glucose pyrophosphorylase activity) and protein biosynthesis (PRP-40, pre-mRNA–processing activity). The environmental variable showing the highest associations was temperature, arguably a key factor influencing enzymatic activities and metabolic pathways [38]. In fact, a decreased metabolism has been observed below certain threshold temperatures in both North Atlantic eels [39,40] and early-life history traits such as glass eel upstream migration have been shown to be temperature-related [41]. Using RAD sequencing, Pavey et al. [34] studied natural selection between American eels inhabiting freshwater vs. brackish/saltwater habitats. Out of 42,424 SNPs analyzed, 331 were associated with habitat, located in genes representing vascular and morphological development, calcium ion regulation, growth and transcription factors, and olfactory receptors. This points to the existence of differential selective pressures in American eel in the two distinct habitats.
5. Studies of Selection in European Eel
Early studies of adaptive evolution in European eel focused on the detection of signatures of local selection in glass eels. Ulrik et al. [32] used a panel of 80 coding-gene SNPs previously analyzed in American eel [31] to genotype individuals collected from eight locations across Europe. Signatures of selection were found at 11 coding-gene SNPs, four from outlier tests and seven from environmental correlations. Most genes were involved in major metabolic functions, including GAPDH (Glyceraldehyde 3-phosphate dehydrogenase, glycolysis pathway, catalyzes the conversion of glyceraldehyde 3-phosphate to D-glycerate 1,3-bisphosphate), ALDH2 (Aldehyde dehydrogenase 2, major oxidative pathway of alcohol metabolism, catalyzes acetaldehyde to acetic), and ALD_R (Aldose reductase, polyol pathway of glucose metabolism, catalyzes the reduction of glucose to sorbitol). None of the above genes linked to metabolic pathways was correlated with temperature, despite its known importance for enzymatic activities and metabolism.
When comparing the results from Ulrik et al. [32] on European eel with Gagnaire et al. [31] on American eel using the same SNP panel, no genes putatively under selection were shared across studies. The contrasting pattern found suggested no apparent parallel footprints of selection in North Atlantic eels. It also suggested no common genetic-by-environment associations between European and American eel.
As an alternative to the candidate gene approach, Pujolar et al. [17] tested for footprints of natural selection in glass eels at the genome level using a total of 50,354 SNPs generated by RAD sequencing. A total of 754 potentially locally selected SNPs were identified using FST-based outlier tests and significant correlations with environmental variables. Candidate genes for local selection constituted a wide array of functions, including calcium signalling, neuroactive ligand-receptor interaction, and circadian rhythm. One of the candidate genes identified was the circadian clock gene Period, possibly related to differences in local photoperiod associated with the >30° difference in latitude between localities (34 and 64° N).
6. Signature of Selection between Life-Cycle Stages
Eels present a complex life cycle organized into morphologically distinct phases separated by abrupt metamorphic transitions (metamorphosis). Life stages represent alternative adaptations for optimal food and niche exploitation (e.g., growth, feeding, growth, dispersal) as well as specific tasks (e.g., reproduction) [42]. North Atlantic eels present a particularly complex life cycle that includes two metamorphoses. After spawning in partial sympatry in the Sargasso Sea, larvae are transported by currents to the coasts of Europe (European eel) and North America (American eel). On reaching the continental shelf, eels undergo a first metamorphosis from larvae into glass eels (juvenile stage). After an extensive period of feeding and growth as yellow eels, eels undergo a second metamorphosis into silver eels (adult stage). The latter encompasses modifications both at the morphological (skin colour, eye size, body length and weight) and physiological level (loss of digestive tract, development of gonads). The second metamorphosis prepares animals for the spawning migration back to the Sargasso Sea, where eels reproduce once and die [21]. Given the drastic changes associated with metamorphic transitions, selective pressures should differ before and after metamorphosis and different genes and pathways should be under selection at different life stages. Hence North Atlantic eels provide an excellent opportunity to study the genetic associations between life cycle stages.
Pujolar et al. [35] compared juvenile glass eels vs. adult silver eels in European eel using two different sets of markers to test for selection: the same panel of functional genes developed by Gagnaire et al. [31] for American eel and a new set of ca. 150,000 SNPs generated by RAD sequencing. A total of 2413 (1.57%) candidate SNPs were identified with signal transduction pathway as the most over-represented group of genes, including MAPK signalling, calcium signalling and GnRH (gonadotropin-releasing hormone) signalling. The majority of the over-represented pathways were related to growth, while others could result from the different conditions that eels inhabit during their life cycle.
The observation of many different genes and pathways under selection when comparing juvenile and adult eels supports the adaptive decoupling hypothesis for the benefits of metamorphosis [43]. The hypothesis states that partitioning the life cycle into discrete morphological phases may be overall beneficial as it allows the different life stages to respond independently to their unique selection pressures. In turn, this might translate into a more effective use of niche resources and a better performance of phase-specific tasks (e.g., feeding and growth in juveniles, migration, and reproduction in adults).
7. Signatures of Selection between European and American Eel
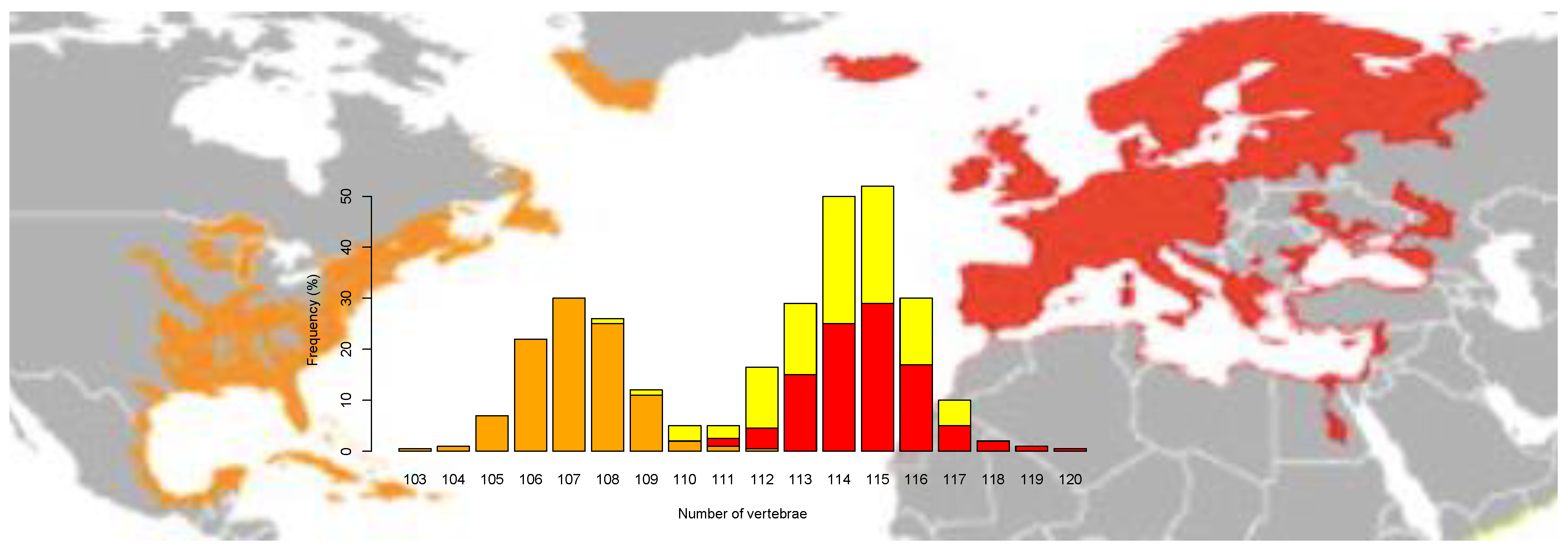
The European and American eel overlap in many morphological parameters and only differ in vertebral counts (Figure 1) [44,45,46] but are considered as sister species based on mitogenomic differences [47]. Divergence time between the two species is estimated to range between 1.3–2.4 Mya [19], based on the joint allele frequency spectrum (JAFS) and PSMC plots, and 3.8 Mya [30], based on the mitogenome. Surprisingly, genetic differentiation between the two species is low/moderate as shown by SNP data, with an overall FST = 0.087 [36], which confirmed earlier studies using AFLP and microsatellites [15,25,48,49]. This is a likely result of gene flow between species, which are known to breed together in partial sympatry in the Sargasso Sea [50]. Several genetic studies have detected hybrid individuals in larvae, juvenile and adult samples, with the highest signatures of admixture detected in Iceland on the basis of vertebrae counts (Figure 1) and especially molecular data [15,44,49,51,52,53].
Figure 1.
Vertebrae counts in European eel (red), American eel (orange) and Icelandic individuals (yellow) after re-examining data from Avise et al. [44]. Geographic distribution for each species is adapted from Jacoby et al. [54].
When testing for positive selection between European and American eel, Jacobsen et al. [33] found that candidate SNPs were located within genes related to development and phosphorylation, consistent with the hypothesis that larval phase duration and migration loops play a key role in the speciation of North Atlantic eels, which was also confirmed recently in the re-analysis of Pujolar et al. [36]. The different footprints of selection between species could be due to distinct selection pressures associated with the much longer larval migration for European eel (from seven months to two years) relative to American eel (from six to 12 months) [22]. The potential extra year spent in the open sea could impose a stronger selective pressure on European eel larvae relative to American eel. Similarly, the migration loop of adults returning to the Sargasso Sea for spawning is ca. 5000 km for European eel vs. ca. 2000 km for American eel [21]. Hence the distinct signatures of selection between North American eels could be attributable to the different metabolic and energetic requirements in larval and adult migration between species. The same argument was also used to explain the contrasting pattern of spatially varying selection in American eel [31] and European eel [32] using the same panel of candidate SNPs.
8. Genomic Islands of Divergence
Early studies aiming at finding signatures of selection in eels had the disadvantage of utilizing a European eel draft genome assembled into a large number of small contigs and scaffolds [55], which was the only eel genome available at the time. This meant that the existence of regions of elevated differentiation across the genome (or genomic islands of divergence) could not be properly tested. However, a new high-quality reference European eel genome has been recently released, assembled at the chromosome level [56].
Taking advantage of the newly assembled and annotated European eel genome, Enbody et al. [18] searched for genetic footprints of differentiation within European eel using whole genome resequencing data. The study compared samples collected in the Baltic and Mid-Atlantic (England, Ireland, France) and reported little evidence for islands of selection, finding only a small region under selection located on chromosome 1 (covering ca. 6 kb around 81.2 Mbp) and two other regions on chromosomes 13 and 15.
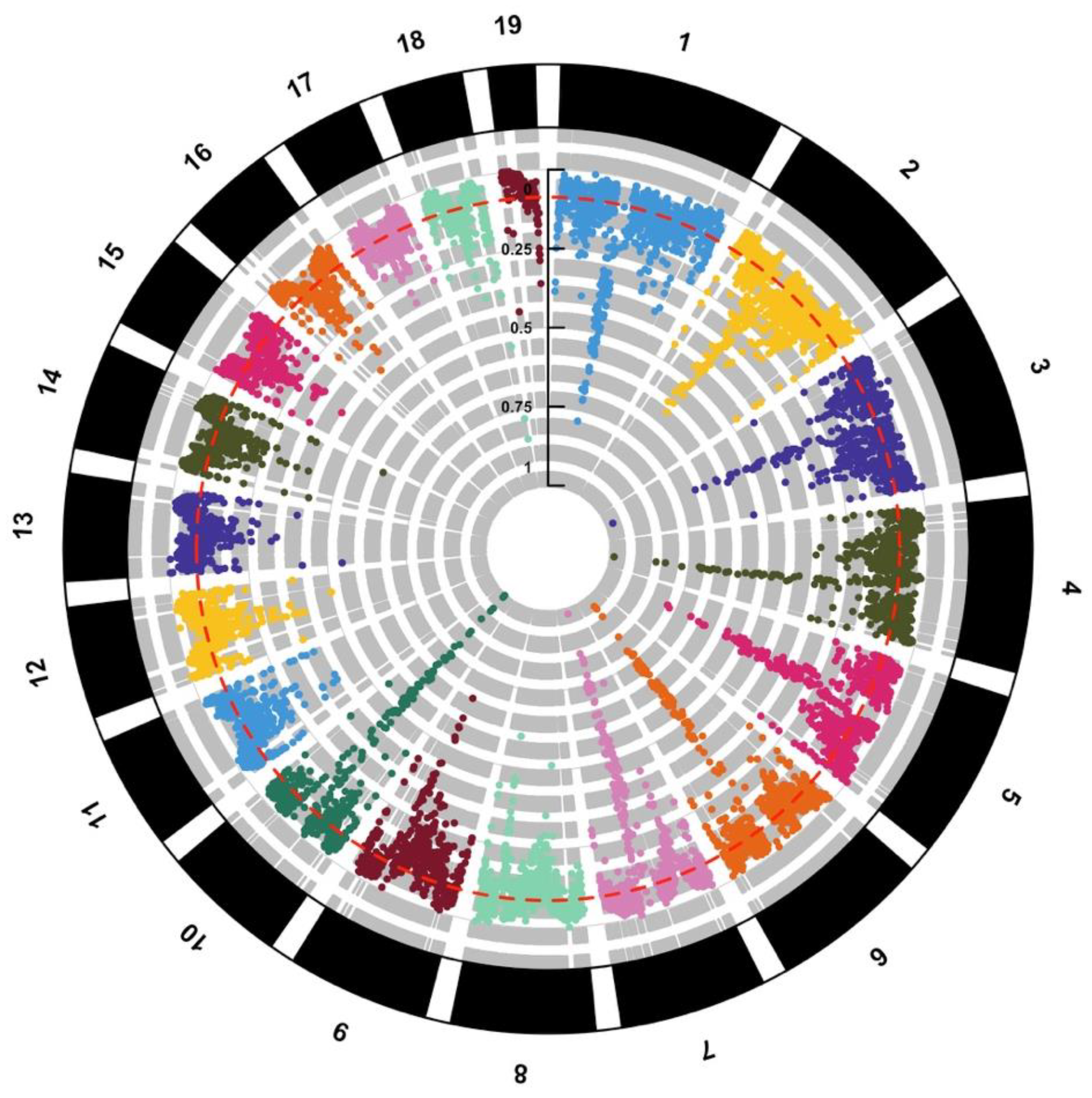
Similarly, Pujolar et al. [36] used genome-wide data from a total of 359 RAD-sequenced individuals retrieved from the Sequence Nucleotide Archive (SNA) to identify genomic islands of selection between North Atlantic eels using the new European eel genome as reference. State-of-the-art statistic tests were used, including methods based on higher population differentiation than under neutral expectation (FST value) and measures of linkage disequilibrium (iHS, XP-EHH) [57]. First, two between-population methods (FST and XP-EHH) were used to identify islands of selection between North Atlantic eels. Larger regions or islands were observed in a total of seven chromosomes (2, 4, 5, 6, 7, 9 and 10), most recognizable on chromosome 6 with a 3.6 Mbp region from 31.45 to 35.10 Mbp and on chromosome 10 with a 950 kb region from 28.80 to 29.75 Mbp (Figure 2). The two between-population methods are best at detecting complete or nearly complete signatures of selection. Due to the long time required until fixation is reached, FST and XP-EHH are expected to identify older selection events between populations in the more distant past [58]. In this sense, both FST and XP-EHH are powerful tools to detect “hard selective sweeps”, which occur when a new mutation arises and spreads quickly to fixation due to natural selection [6]. Other scenarios might be more difficult to detect, especially when selection leads to changes in allele frequencies without reaching fixation. Genes included in the islands of selection detected in the study showed significant enrichment for terms related mainly to ATP phosphorylation and development. This is in accordance with previous studies comparing European vs. American eel [31,33], suggesting different selective pressures in relation to metabolism and energetics.
Figure 2.
Circular Manhattan Plot showing sliding-window FST between European and American eel at 19 chromosomes after re-examining data from Pujolar et al. [36]. Average FST (red dotted line) is reported.
Second, shared signatures of selection within European eel and American eel were detected using iHS at a total of 11 chromosomes. Regions were generally small (100–300 kb) except for two large regions, a region of 800 kb on chromosome 8 from 52.35 to 52.75 Mbp and a region of 850 kb on chromosome 16 from 2.45 to 3.30 Mbp. Unlike the two between-population methods (FST and XP-EHH), the iHS test has higher statistical power when selected alleles are at intermediate frequencies that have not yet reached fixation [57]. Hence, it can detect signatures of recent and even ongoing selective sweeps [59]. Those scenarios include “soft selective sweeps”, in which multiple haplotypes harboring advantageous mutations are all favoured [60]. Several hypotheses might account for the detection of shared islands of selection in North Atlantic eels, including parallel evolution due to adaptation to similar habitats and introgression.
9. Making Sense of Genomic Islands of Divergence
Islands of genomic divergence are regions in which differentiation between populations or species is the highest and can in some cases also constitute “islands of speciation” [61]. Such islands represent regions in the genome in which a selective sweep leads to the increase and fixation of adaptive mutations, resulting in a reduction of genetic variability in the region nearby the favorable allele due to genetic hitchhiking [62].
While many studies on genomic regions of elevated differentiation focus on searching for speciation genes, there is an increasing realization that selection might be acting mainly on regulation and expression rather than functional changes. In this sense, it is interesting that Jacobsen et al. [33] found upstream regions, likely involved in regulation, to include a lower percentage of outlier SNPs (FST = 1) compared to the rest of the genome. This suggests a conserved and important role of these regions in both species [33]. The recent paper of Pujolar et al. [36] looked at the genomic location and effects of each candidate SNP under selection when comparing North Atlantic eels using genome-wide RAD sequencing data. Only 1.5% of SNPs were in coding regions (exons), with most variants found in introns (66.6%) and intergenic regions (25.6%). Mutations in the exons were mostly synonymous and only two mutations had a moderate effect producing a different amino acid. Given that most SNPs putatively under selection in the study were found in noncoding regions, this possibly reflects regulatory differences between North Atlantic eels. This is also supported by gene expression analysis performed on American and European eel leptocephali larvae collected in the Sargasso Sea, which suggests differential timing of gene expression regulation during early development [63]. Similarly, when comparing marine and freshwater stickleback populations, Jones et al. [11] reported up to 83% of SNPs under selection located in noncoding regions with an assumed regulatory role. It should also be taken into account that within an island of genomic differentiation, most mutations are likely to be hitchhiking and not the direct target of selection, making it difficult to distinguish between neutral and adaptive variation. On this account, searching for candidate genes within genomic islands of differentiation can only be used as an indication of putative selection and functional validation (i.e., gene expression analyses or QTL mapping from genetic crosses) would be required to demonstrate causality.
Finally, epigenetic variation as a mechanism of adaptive plasticity could play a role in local adaptation of North Atlantic eels to the heterogenous habitat conditions they experience throughout their life cycle. Epigenetics mechanisms, including DNA methylation, are defined as DNA modifications affecting gene expression without changing the DNA sequence [64]. Emerging evidence suggests significant methylation differences of functional importance associated with environmental variation [65]. When studying methylation variation in European eel, Liu et al. [37] reported differentially methylated regions including genes involved in developmental processes, particularly Hox genes. Overall, methylation results highlight the importance of epigenetics in the adaptation and resilience of eels and suggest interactions between habitat, development, and epigenetic variation. The importance of epigenetic variation as a mechanism of adaptive plasticity in eels merits further research.
Author Contributions
J.M.P. wrote the manuscript with contribution from F.B. and M.W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stapley, J.; Reger, J.; Feulner, P.G.D.; Smadja, C.; Galindo, J.; Ekblom, R.; Bennison, C.; Ball, A.D.; Beckerman, A.P.; Slate, J. Adaptation genomics: The next generation. Trends Ecol. Evol. 2010, 25, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Bourret, V.; Dionne, M.; Kent, M.P.; Lien, S.; Bernatchez, L. Landscape genomics in Atlantic salmon (Salmo salar): Searching for gene-environment interactions driving local adaptation. Evolution 2013, 67, 3469–3487. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Fraser, D.J.; Weir, L.K.; Bernatchez, L.; Hansen, M.M.; Taylor, E.B. Extend and scale of local adaptation in salmonid fishes: Review and meta-analysis. Heredity 2011, 106, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Feder, J.L.; Nosil, P. The efficacy of divergence hitchhiking in generating genomic islands during ecological speciation. Evolution 2010, 64, 1729–1747. [Google Scholar] [CrossRef] [PubMed]
- Maynard Smith, J.; Haigh, J. The hitch-hiking effect of a favourable gene. Genet. Res. 1974, 23, 23–35. [Google Scholar] [CrossRef]
- Stephan, W. Selective sweeps. Genetics 2019, 211, 5–13. [Google Scholar] [CrossRef]
- Nosil, P.; Egan, S.P.; Funk, D.J. Heterogenous genomic differentiation between walking-stick ecotypes: “Isolation by adaptation” and multiple roles for divergent selection. Evolution 2008, 62, 316–336. [Google Scholar] [CrossRef]
- Hoffer, T.; Foll, M.; Excoffier, L. Evolutionary forces shaping genomic islands of population differentiation in humans. BMC Genomics 2012, 13, 107. [Google Scholar] [CrossRef]
- Hohenlohe, P.A.; Basshan, S.; Etter, P.D.; Stiffler, N.; Johnson, E.A.; Cresko, W.A. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 2010, 6, e1000862. [Google Scholar] [CrossRef]
- Jones, F.C.; Grabherr, M.G.; Chan, Y.F.; Russell, P.; Mauceli, E.; Johnson, J.; Swofford, R.; Pirun, M.; Zody, M.C.; White, S.; et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 2012, 484, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hemmer-Hansen, J.; Nielsen, E.E.; Therkildsen, N.J.; Taylor, M.I.; Ogden, R.; Geffen, A.J.; Bekkevold, D.; Helyar, S.; Pampoulie, C.; Johansen, T.; et al. A genomic island linked to ecotype divergence in Atlantic cod. Mol. Ecol. 2013, 22, 2653–2667. [Google Scholar] [CrossRef]
- Rodríguez-Ramilo, S.T.; Baranski, M.; Moghadam, H.; Grove, H.; Lien, S.; Goddard, M.E.; Meuwissen, T.H.E.; Sonesson, A.K. Strong selection pressures maintain divergence on genomic islands in Atlantic cod (Gadus morhua) populations. Genet. Sel. Evol. 2019, 51, 61. [Google Scholar] [CrossRef] [PubMed]
- Gagnaire, P.A.; Pavey, S.A.; Normandeau, E.; Bernatchez, L. The genetic architecture of reproductive isolation during speciation-with-gene-flow in lake whitefish species pairs assessed by RAD sequencing. Evolution 2013, 67, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Als, T.D.; Hansen, M.M.; Maes, G.E.; Castonguay, M.; Riemann, L.; Aerestrup, K.; Munk, P.; Sparholt, T.; Hanel, R.; Bernatchez, L. All roads lead to home: Panmixia of European eel in the Sargasso Sea. Mol. Ecol. 2011, 20, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Côté, C.; Gagnaire, P.A.; Bourret, V.; Verrault, G.; Castonguay, M.; Bernatchez, L. Population genetics of the American eel (Anguilla rostrata): FST = 0 and North Atlantic Oscillation effects on demographic fluctuations of a panmictic species. Mol. Ecol. 2013, 22, 1763–1776. [Google Scholar] [CrossRef]
- Pujolar, J.M.; Jacobsen, M.W.; Als, T.D.; Frydenberg, J.; Munch, K.; Jónsson, B.; Jiang, X.; Cheng, L.; Maes, G.E.; Bernatchez, L.; et al. Genome-wide signatures of within-generation local selection in the panmictic European eel. Mol. Ecol. 2014, 23, 2514–2528. [Google Scholar] [CrossRef]
- Enbody, E.D.; Petterson, M.E.; Sprehn, C.G.; Palm, S.; Wickstrom, H.; Andersson, L. Ecological adaptation in European eels is based on phenotypic plasticity. Proc. Natl. Acad. Sci. USA 2021, 118, e2022620118. [Google Scholar] [CrossRef]
- Nikolic, N.; Liu, S.; Jacobsen, M.W.; Jónsson, B.; Bernatchez, L.; Gagnaire, P.A.; Hansen, M.M. Speciation history of European (Anguilla Anguilla) and American eel (A. rostrata), analysed using genomic data. Mol. Ecol. 2020, 29, 565–577. [Google Scholar] [CrossRef]
- Daverat, F.; Limburg, K.E.; Thibaut, I.; Shiao, J.C.; Dodson, J.J.; Caron, F.; Tzeng, W.N.; Iizuka, Y.; Wickstrom, H. Phenotypic plasticity of habitat use by three temperate eel species Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Progr. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Tesch, F. The Eel; Blackwell Science Ltd.: Oxford, UK, 2003. [Google Scholar]
- Bonhommeau, S.; Blanke, B.; Tréguier, A.M.; Grima, N.; Rivot, E.; Vermand, Y.; Greiner, E.; Le Pape, O. How fast can the European eel (Anguilla anguilla) larvae cross the Atlantic Ocean? Fish. Oceanogr. 2009, 18, 371–385. [Google Scholar] [CrossRef]
- Åström, M.; Dekker, W. When will the eel recover? A full life cycle model. ICES J. Mar. Sci. 2007, 64, 1491–1498. [Google Scholar] [CrossRef]
- Van den Thillart, G.; Rankin, J.C.; Dufour, S. Spawning Migration of the European Eel: Reproduction Index, a Useful Tool for Conservation Management; Springer: Dordecht, The Netherlands, 2009. [Google Scholar]
- Wirth, T.; Bernatchez, L. Decline of Atlantic eels: A fatal synergy? Proc. Biol. Sci. 2003, 270, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Dannewitz, J.; Maes, G.E.; Johansson, L.; Wickström, H.; Volckaert, F.A.M.; Jarvi, T. Panmixia in the European eel: A matter of time. Proc. Biol. Sci. 2005, 272, 1129–1137. [Google Scholar] [CrossRef]
- Maes, G.E.; Pujolar, J.M.; Hellemans, B.; Volckaert, F.A.M. Evidence for isolation by time in the European eel (Anguilla Anguilla). Mol. Ecol. 2006, 15, 2095–2107. [Google Scholar] [CrossRef]
- Palm, S.; Dannewitz, J.; Prestegaard, T.; Wickström, H. Panmixia in European eel revisited: No genetic difference between maturing adults from southern and northern Europe. Heredity 2009, 103, 82–89. [Google Scholar] [CrossRef]
- Baltazar-Soares, M.; Biastoch, A.; Harrod, C.; Hanel, R.; Marohn, L.; Prigge, E.; Evans, D.; Bodles, K.; Behrens, E.; Böning, C.W.; et al. Recruitment collapse and population structure of the European eel shaped by local ocean current dynamics. Curr. Biol. 2014, 24, 104–108. [Google Scholar] [CrossRef]
- Jacobsen, M.W.; Pujolar, J.M.; Gilbert, M.T.P.; Moreno-Mayar, J.V.; Bernatchez, L.; Als, T.D.; Lobón-Cervià, J.; Hansen, M.M. Speciation and demographic history of Atlantic eels (Anguilla anguilla and A. rostrata) revealed by mitogenome sequencing. Heredity 2014, 113, 432–442. [Google Scholar] [CrossRef]
- Gagnaire, P.A.; Normandeau, E.; Côté, C.; Hansen, M.M.; Bernatchez, L. The genetic consequences of spatially varying selection in the panmictic American eel (Anguilla rostrata). Genetics 2012, 190, 725–736. [Google Scholar] [CrossRef]
- Ulrik, M.G.; Pujolar, J.M.; Ferchaud, A.L.; Jacobsen, M.W.; Als, T.D.; Gagnaire, P.A.; Frydenberg, J.; Bøcher, P.K.; Jónsson, B.; Bernatchez, L.; et al. Do North Atlantic eels show parallel patterns of spatially varying selection? BMC Evol. Biol. 2014, 14, 138. [Google Scholar] [CrossRef]
- Jacobsen, M.W.; Pujolar, J.M.; Bernatchez, L.; Munch, K.; Jian, J.; Niu, Y.; Hansen, M.M. Genomic footprints of speciation in Atlantic eels Anguilla anguilla and A. rostrata. Mol. Ecol. 2014, 23, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Pavey, S.A.; Gaudin, J.; Normandeau, E.; Dionne, M.; Castonguay, M.; Audet, C.; Bernatchez, L. RAD sequencing highlights polygenic discrimination of habitat ecotypes in the panmictic American eel. Curr. Biol. 2015, 25, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Pujolar, J.M.; Jacobsen, M.W.; Bekkevold, D.; Lobon-Cervià, J.; Jónsson, B.; Bernatchez, L.; Hansen, M.M. Signatures of natural selection between life cycles stages separated by metamorphosis in European eel. BMC Genom. 2015, 16, 600. [Google Scholar] [CrossRef] [PubMed]
- Pujolar, J.M.; Jacobsen, M.W.; Bertolini, F. Comparative genomics and signatures of selection in North Atlantic eels. Mar. Genom. 2022, 62, 100933. [Google Scholar] [CrossRef]
- Ling, S.; Tengstedt, A.N.B.; Jacobsen, M.W.; Pujolar, J.M.; Jónsson, B.; Lobón-Cervià, J.; Bernatchez, L.; Hansen, M.M. Genome-wide methylation in the panmictic European eel (Anguilla anguilla). Mol. Ecol. 2022, 31, 4286–4306. [Google Scholar] [CrossRef]
- Somero, G.N. Adaptation of enzymes to temperature: Searching for basic “strategies”. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 321–333. [Google Scholar] [CrossRef]
- Nyman, L. Some effects of temperature on eel (Anguilla) behaviour. Rep. Inst. Freshw. Res. Drottingholm 1972, 52, 90–102. [Google Scholar]
- Walsh, P.J.; Foster, G.D.; Moon, T.W. The effects of temperature and metabolism of the American eel Anguilla rostrata: Compensation in the summer and torpor in the winter. Physiol. Zool. 1983, 56, 532–540. [Google Scholar] [CrossRef]
- Linton, E.D.; Jónsson, B.; Noakes, D.L.G. Effects of water temperature on the swimming and climbing behavior of glass eels Anguilla spp. Environ. Biol. Fishes 2007, 78, 189–192. [Google Scholar] [CrossRef]
- Heyland, A.; Moroz, L.L. Signalling mechanisms underlying metamorphic transitions in animals. Integr. Comp. Biol. 2006, 46, 743–759. [Google Scholar] [CrossRef]
- Moran, N.A. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Syst. 1994, 25, 573–600. [Google Scholar] [CrossRef]
- Avise, J.C.; Nelson, W.S.; Arnold, J.; Koehn, R.K.; Williams, G.C.; Thorsteinsson, V. The evolutionary genetic status of Icelandic eels. Evolution 1990, 44, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Lecomte-Finiger, R. The genus Anguilla: Current state of knowledge and questions. Rev. Fish. Biol. Fish. 2003, 13, 265–279. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.T.; Maes, G.E. The European eel (Anguilla Anguilla), its life cycle, evolution and reproduction: A literature review. Rev. Fish. Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- Mineguishi, Y.; Aoyama, J.; Inoue, J.G.; Miya, M.; Nishida, M.; Tsukamoto, K. Molecular phylogeny and evolution of the freshwater eels genus Anguilla based on the whole mitochondrial genome sequences. Mol. Phylogenet. Evol. 2005, 34, 134–146. [Google Scholar] [CrossRef]
- Mank, J.E.; Avise, J.C. Microsatellite variation and differentiation in North Atlantic eels. J. Hered. 2003, 94, 30–34. [Google Scholar] [CrossRef][Green Version]
- Gagnaire, P.A.; Albert, V.; Jónsson, B.; Bernatchez, L. Natural selection influences AFLP intraspecific variability and introgression patterns in Atlantic eels. Mol. Ecol. 2009, 18, 1678–1691. [Google Scholar] [CrossRef]
- Miller, M.J.; Bonhommeau, S.; Munk, P.; Castonguay, M.; Hanel, R.; McCleave, J.D. A century of research on the larval distribution of the Atlantic eels: A re-examination of the data. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1035–1064. [Google Scholar] [CrossRef]
- Albert, V.; Jónsson, B.; Bernatchez, L. Natural hybrids in Atlantic eels (Anguilla anguilla, A. rostrata): Evidence for successful reproduction and fluctuating abundance in space and time. Mol. Ecol. 2006, 15, 1903–1916. [Google Scholar] [CrossRef]
- Pujolar, J.M.; Jacobsen, M.W.; Als, T.D.; Frydenberg, J.; Magnussen, E.; Jónsson, B.; Jiang, X.; Cheng, L.; Bekkevold, D.; Maes, G.E.; et al. Assessing patterns of hybridization between North Atlantic eels using diagnostic single nucleotide polymorphisms. Heredity 2014, 112, 627–637. [Google Scholar] [CrossRef]
- Jacobsen, M.; Smedegaard, L.; Sørensen, S.; Pujolar, J.M.; Munk, P.; Jónsson, B.; Magnussen, E.; Hansen, M.M. Assessing pre- and post-zygotic barriers between North Atlantic eels (Anguilla anguilla and A. rostrata). Heredity 2017, 118, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, D.M.P.; Casselman, J.M.; Crooks, V.; Delucia, M.B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.C.; Smith, K.; et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Henkel, C.V.; Burgerhout, E.; de Wijze, D.L.; Dirks, R.P.; Minegishi, Y.; Jansen, H.J.; Spaink, H.P.; Dufour, S.; Weltzien, F.A.; Tsukamoto, K.; et al. Primitive duplicate Hox clusters in the European eel’s genome. PLoS ONE 2012, 7, e32231. [Google Scholar] [CrossRef] [PubMed]
- Rhie, A.; McCarthy, S.A.; Fedrigo, O.; Damas, J.; Formenti, G.; Koren, S.; Uliano-Silva, M.; Chow, W.; Fungtammasan, A.; Kim, J.; et al. Towards complete and error-free genome assemblies of all vertebrate species. Nature 2021, 592, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ding, X.; Qanbari, S.; Weigend, S.; Zhang, Q.; Simianer, H. Properties of different selection signature statistics and a new strategy to combine them. Heredity 2015, 115, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Cadzow, M.; Boocock, J.; Nguyen, H.T.; Wilcox, P.; Merriman, T.R.; Black, M.A. A bioinformatics workflow for detecting signatures of selection in genomic data. Front. Genet. 2014, 5, 293. [Google Scholar] [CrossRef]
- Voight, B.F.; Kudaravalli, S.; Wen, X.; Pritchard, J.K. A map of recent positive selection in the human genome. PLoS Biol. 2006, 4, e72. [Google Scholar] [CrossRef]
- Hermisson, J.; Pennings, P.S. Soft sweeps: Molecular population genetics of adaptation from standing genetic variation. Genetics 2005, 169, 2335–2352. [Google Scholar] [CrossRef]
- Via, S.; West, J. The genetic mosaic suggests a new role for hitchhiking in ecological speciation. Mol. Ecol. 2008, 17, 4334–4345. [Google Scholar] [CrossRef]
- Storz, J.F. Using genome scans of DNA polymorphism to infer adaptive population divergence. Mol. Ecol. 2005, 14, 671–688. [Google Scholar] [CrossRef]
- Bernatchez, L.; St-Cyr, J.; Normandeau, E.; Maes, G.E.; Als, T.D.; Kalujnaia, S.; Cramb, G.; Castonguay, M.; Hansen, M.M. Differential timing of gene expression between leptocephali of the two Anguilla eel species in the Sargasso Sea. Ecol. Evol. 2011, 1, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Dupond, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, mechanisms and clinical perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Srikant, T.; Drost, H.H. How stress facilitates phenotypic innovation through epigenetic diversity. Front. Plant Sci. 2020, 11, 606800. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).