Abstract
The present study investigated the macroscopical and histological features of two spontaneous seminomas developed by zebrafish adults. Two wild-type male zebrafish of AB/TU strain aged 2 and 3 years, respectively, developed gross pathological signs consisting of asymmetrical swollen abdomens. In one fish, named fish 1, the testicular alteration is referred to as one testis (the right one), while in fish 2, both testes were altered in their size and shape. No signs of invasion were reported. The histological analysis revealed an extensive differentiation of germ cells in various developmental stages without any oocyte, and both lesions were compatible with spermatocytic and intratubular seminomas. The etiology and the pathogenesis of the reported lesions could be related to an intricate and interconnected network of intrinsic and extrinsic factors, including the housing condition, diet, genetic background, and operator training.
1. Introduction
Zebrafish has emerged as a valuable vertebrate model system widely used to investigate the signaling pathways involved in the development of disease, including cancer [1]. This aquatic species has become a popular model organism for cancer biology due to several advantages, including its cost-effective maintenance, high fecundity, fast external development, optical clarity, and small embryo size [2]. Moreover, the genetic amenability of the zebrafish model modulates, through different techniques, specific gene-targeted mutations and stable transgenes [3]. However, the high level of sophistication of the genomic tools available for the zebrafish model is not followed by the acquisition of basic pathology data for this species. Although there is available information on the chemical and genetic induction of specific cancer types in zebrafish, little data are published regarding the incidences of spontaneous tumors or histologic lesions developed by wild-type zebrafish strains.
The most common neoplasm reported in the zebrafish model is the seminoma. The Zebrafish International Resource Center (ZIRC) diagnosticated 182 zebrafish seminomas out of 16,169 total fish examined by histology between 2006 and 2016 [4].
In Danio rerio, most of the potential seminomas that are described in the literature exhibit minimal deviation from normal testis tissue at the histological level, posing a challenge for their definition as a true neoplasm, rather than a proliferative process. In this study, we reported the clinical and histological alterations developed by two wild-type zebrafish bred for laboratory use, highlighting the complexity of the histological definition of these masses.
2. Materials and Methods
2.1. Zebrafish Maintenance
Adult zebrafish were bred in the facilities at the University of Teramo. The fish were kept in 3.5 L ZebTec tanks (Tecniplast S.p.a., Buguggiate, Italy) in a recirculating aquatic system. The physical parameters were standardized as follows: the temperature was 28 °C; the pH was 7 ± 0.2; the conductivity was 500 ± 100 μS cm−1; and the dissolved O2 was 6.1 mg L−1. The photoperiod was 14 h light–10 h dark. The chemical parameters were checked weekly and kept as follows: ammonia 0.02 mg L−1, nitrite 0.02 mg L−1, and nitrate 21.3 mg L−1. Animals were fed twice per day with live food (Artemia salina) and supplemented with Zebrafeed 400–600 (Sparos, Olhão, Portugal). The fish were not chemically treated; rather, they were reared for spawning.
2.2. Necropsy and Histological Analysis
Two wild-type male zebrafish of AB/TU strain and aged 2 and 3 years, respectively, developed asymmetrical swollen abdomens (Figure 1A and Figure 2A). That was the only abnormal clinical sign observed. The fish were euthanized with an overdose of tricaine methanesulfonate (MS-222) in accordance with the law of the country in which the study was performed (Italy, D.L. 26/2014) and the principle of the Humane Endpoint. Necropsy was performed to evaluate gross lesions.
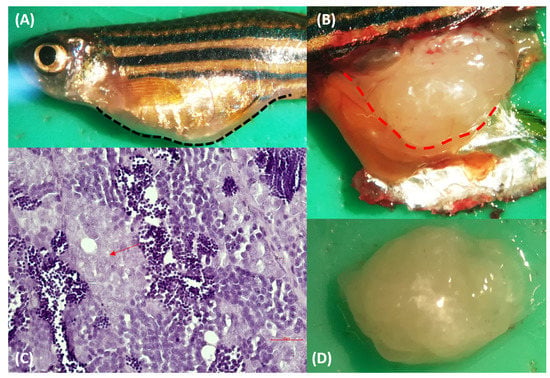
Figure 1.
(A) Macroscopic appearance of wild-type male zebrafish of AB/TU strain Fish 1. The abdomen was considerably distended. (B,D) The mass filled the coelom. (C) Hematoxylin & eosin-stained sections from formalin-fixed, paraffin-embedded zebrafish testis’ mass: proliferation of atypical pale-staining spermatogones (red arrow), with hyperchromatic nuclei and poorly defined eosinophilic cytoplasm. Spermatocytes and spermatids were present. Bar = 10 µm.
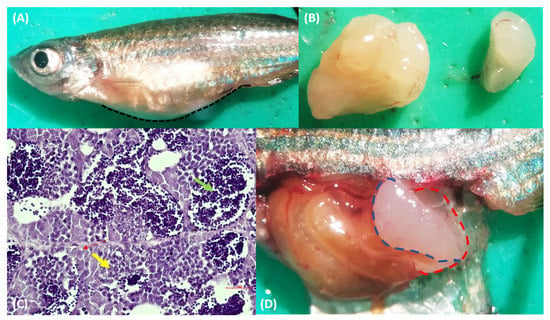
Figure 2.
(A) Macroscopic appearance of wild-type male zebrafish of AB/TU strain, fish 2. The abdomen was considerably distended. (B,D) The masses filled the abdominal cavity. (C) Hematoxylin and eosin-stained sections from the formalin-fixed, paraffin-embedded zebrafish testis’ mass. The cells were arranged in a lobular pattern, and spermatogones (red arrow), spermatocytes (yellow arrow), and spermatids (green arrow) proliferated. Bar = 10 µm.
For microscopic examination, whole testis samples were collected, fixed in a 10% neutral buffered formalin (NBF), and dehydrated by hand through an ascending series of graded ethanol solutions to minimize artifacts. The specimens were treated by modifying the standard procedure of hydration/dehydration in order to adapt them to manipulate very small pieces of tissue. The procedure steps are listed in Supplementary Table S1. In the next step, the tissues were cleared in xylene and embedded in a block of paraffin. The samples were then sectioned at 5 µm using a rotary microtome (Leica 2030 Biocut, Reichert-Jung, Bensheim, Germany), floated in a 37 °C water bath, and quickly mounted on glass slides (Super-Frost, Menzel-Gläser, Braunschweig, Germany). The slides were put in an incubation oven at 37 °C for 24 h, and then they were stained manually with Hematoxylin and Eosin (H&E), as described in Supplementary Table S2. After staining, the sections were protected by mounting a coverslip over the tissue using a mounting medium to adhere the coverslip to the slide, and they were also examined under a light microscope (Leika DM4000B, Milan, Italy). Digital photos were taken with an OLYMPUS-DP12 camera for the detection of histopathological alterations.
3. Results
3.1. Macroscopic Evaluation
The fish showed abdominal distension, and the skin overlaying the enlargement appeared thinned (Figure 1A and Figure 2A). Changes in the skin and fins were not detected. The visceral cavity was opened according to Kent et al. [5]. Specifically, the operculum was removed, and the first incision was made starting at the dorsal posterior of the opercular cavity and extending toward the anus. Then, a connect incision with a cut starting at the ventral posterior of the opercular cavity was done, and this cut was connected dorsally to the first one. The fish were opened with the right side down. When they were opened, the coelom was filled with a whitish lardaceous and a fleshy mass. In particular, in fish 1, a soft lobate mass, sized 5.5 × 6.5 × 3 mm with an uneven surface, was located dorsally to the intestine and caudally to the swim bladder (Figure 1B). The mass was confined to the right testis (Figure 1D); indeed, the left testis was regular in size and shape (Figure S1A). In fish 2, the coelom was filled by two white masses, with an irregular and unsmooth surface ascribed to the left and right testis (Figure 2D). The topography of the masses was the same as fish 1. The right mass (5 × 6 × 3 mm) was larger than the left one (3 × 4 × 2 mm) (Figure 2B). Both of the masses appeared soft, white, and lobulated, and they were confined to the testis. All the other visceral organs were macroscopically normal in size and shape.
3.2. Histopathological Analysis
In fish 1 the mass confined to the right testis was non-encapsulated and poorly demarcated, appeared highly cellular, and showed an extensive differentiation of germ cells in various developmental stages. In particular, the architecture of the lobule appeared overrun by a predominant proliferation of atypical pale-staining spermatogones, with hyperchromatic and centrally located nuclei of various sizes, a single nucleolus, and a poorly defined eosinophilic cytoplasm. There was moderate anisocytosis and anisokaryosis. Spermatocytes and spermatids were also present (Figure 1C). Orchitis, metastasis, or invasions of the cells were not observed. A microscopic evaluation of the left testis showed no evident microscopic alterations (Figure S1B). During the microscopic examination of fish 2, the masses were mildly cellular and, as in case 1, they contained the lobular proliferation of cell types that was expected in spermatogenesis (spermatogonia, spermatocytes, and spermatids) (Figure 2C). Both cases were diagnosed as seminoma, and particularly, the lower malignancy of the masses, the lack of undifferentiated seminal stem cells, and the presence of neoplastic cells resembling spermatogonia and primary spermatocytes led us to define it as a spermatocytic type, according to the WHO classification of testicular tumors in humans [6]. According to the WHO International Histological Classification of the Tumors of the Genital System of Domestic Animals, seminomas can be classified into two types: (i) an intratubular type or type free of infiltration, and (ii) a diffuse type [7]. In both our cases, the lack of invasion of the fibrous connective tissue prompts us to define our seminomas as intratubular.
4. Discussion
Published data from rodents and other laboratory animal species revealed as specific mutations and transgenes are critical to determine both spontaneous and carcinogen-induced neoplasm incidences as well as the histologic pattern of tumors [8]. In the context of testicular lesions, zebrafish carrying a heterozygous nonsense mutation in Leucine-Rich Repeat Containing protein 50 (LRRC50, also called DNAAF1), associated previously with ciliary function, are found to be highly susceptible to the formation of seminomas [9].
In the present case, adult male zebrafish that developed seminomas were not intentionally treated with chemicals, and they were not screened for the presence of genetic mutations. Spitsbergen et al. (2012) noted that seminomas are one of the most frequently diagnosed tumors associated with carcinogen exposure [10]. Zebrafish were responsive to the carcinogenic effects of N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) when they were treated as embryos or fry. The housing system and diet could influence the development of non-experimentally spontaneous tumor incidences in zebrafish [10]. One of the most pressing problems in the field of cancer research using the zebrafish model is the need to standardize aquaculture systems and diets to eliminate contamination by natural carcinogens and to minimize the potential tumor promoters that confound research studies [5]. For example, when spontaneous seminomas and other neoplasms have been diagnosed in older fish living in flow-through systems using semi-purified diets, these tumors are typically quite small (1–4 mm rather than 10–14 mm), as are many seminomas identified in fishes that come from recirculating systems using commercial diets [10].
Another alarming factor to consider in the field of zebrafish pathology is the critical role of genetic background in determining tumor incidences. To avoid inbreeding depression, each new generation should be produced by an outcross, and sibling mating should be performed only when necessary [11]. In the present study, it is difficult to establish the etiology and the pathogenesis of the reported lesions; however, the housing condition and the diet were highly standardized, and no other type of tumors were reported in the zebrafish colony. The potential underestimation of these lesions could also be related to the ability of the operator to recognize the alterations. While egg-associated inflammation of female zebrafish is a common and recognized lesion by animal care attendants, swollen abdomens in males related to seminoma are less frequently detected and notified.
Seminoma is the most common germ cells tumor type (GCT) in humans, accounting for 30–50% of GCT [12]. In humans, three subtypes of seminoma have been described: typical (85% of the cases), anaplastic (5% to 10%), and spermatocytic (2% to 10%). The anaplastic and typical subtypes are associated with the worst prognosis for the patient. Indeed, spermatocytic seminoma usually presents later in the patient’s life and its prognosis is excellent, and cases of metastasis are extremely rare [13].
Seminomas are also common tumors in mammals that have been reported in canine, bull, boar, stallion, mule, ram, buck, tomcat, and bull camelids testes [14]. They occur in older animals, and they are disproportionately common in cryptorchid testes. Their phenotype is usually spermatocytic, and there is subclassification into classical and spermatocytic types based on immunohistochemical biomarkers, such as the placental alkaline phosphatase (PLAP) staining [14]. The main macroscopic presenting sign is testicular enlargement, and microscopically, intratubular, and diffuse types are recognized. The earliest development of the tumor is intratubular, then rupture of the tubules occurs, and growth becomes confluent, forming broad sheets of closely packed cells with scant supporting stroma [14]. Seminomas in horses are thought to behave more aggressively than those in other domestic animal species, with a similar tendency to metastasize [15]. In a recent case report, an 18-year-old Salernitano stallion presented with enlargement of the left testicle with no other clinical signs, and the sonoelastographic examination showed parenchymal changes with deformation of the normal testis, and based on gross, microscopic, and immunohistochemical findings, a definitive diagnosis of diffuse seminoma was confirmed [16]. Moreover, the invasion of tumor cells throughout the testicular parenchyma has been reported in malignant seminomas of rats, and there was also a description of metastasis in canine seminomas [17,18].
Spontaneous testicular tumors of gonadic germ cells have been identified in barbel, African lungfish species, black seabass, yellow perch, koi carp, goldfish, and medaka [19,20,21,22,23,24]. The histological features of fish seminoma are very diversified. The two spermatocytic seminomas described by Nigrelli and Jakowska (1953) [19] and Masahito et al. (1984) [20] in African lungfish were characterized by neoplastic spermatocytes in various stages of maturation. Like in our cases, they maintained the normal spermatogenetic stages, while the seminoma described by Sirri et al. (2010) [23] in koi carp showed undifferentiated neoplastic cells that were consistent with the immature neoplastic elements typical of classical seminoma but not of the spermatocytic type. The seminoma described by Hubbard & Fletcher (1985) [20] in African lungfish consisted of well-differentiated populations of large neoplastic cells with a high mitotic rate and no remarkable inflammation and necrosis. Finally, the seminoma described by Palikova et al. (2007) [22] in barbel displayed poorly differentiated neoplastic germ cells. In Japanese medaka, a recent case report reported the diagnosis of two spontaneous seminomas that exhibited different histologic characteristics. One tumor had partly invaded the dorsal muscular tissue and metastasized to the liver, kidney, and eye, and the tumor cells were arranged in solid and cord patterns and resembled spermatogonia, spermatocytes, and spermatids. The spermatogonia-like cells had round nuclei with prominent nucleoli and moderate amounts of cytoplasm. Additionally, a small number of large cells resembling oocytes were scattered within the tumor [24]. Indeed, the other medaka testicular tumor was not detected during the macroscopic examination, and it did not exhibit local invasion or metastasis. As for the first case, spermatocyst-like structures comprising the same type of tumor cells (spermatogonia-, spermatocyte-, or spermatid-like cells) were characteristically present in this tumor [24]. However, no clear suspicious definition of the type of seminomas (e.g., spermatocytic vs. classical or diffuse vs. intratubular) was reported by these authors.
In the present study, the suspected diagnosis of spermatocytic and intratubular seminomas was supported by the histological features of the lesions. To the best of our knowledge, there have been no reports on the distinction between benign and malignant seminomas in fishes. The age of the sampled animals, who live approximately 3 years in laboratory conditions, is in agreement with the development of spermatocytic seminomas in human older patients. The relative older age of the sampled zebrafish also made the prediction of tumor development complex. At the time of sampling, it was ascribed as an intratubular type, without invasion of testis stroma, but this stage could just be prodromic to the diffusion of the neoplastic cells out of the seminiferous tubules.
Moreover, our results showed the complexity of defining this mass as a true neoplasm or just as testicular hyperplasia. There is a fine line between benign neoplasia and hyperplasia, particularly because hyperplasia generally precedes and predisposes the development of neoplasia. While this neoplasm is defined and called seminoma, the presence of normal tissue could indicate that, rather than a neoplastic process of testicular tissue, it is, in fact, diffuse testis hyperplasia [4]. Evidence supporting the hypothesis is that neoplasia is related to the fact that this mass is presented as a large, ovoid, multinodular, and expensive mass in which the overall architecture of the testis is profoundly distorted, although there is a progression of spermatogenic process, and there is no sign of cellular atypia. Usually, hyperplastic lesions theoretically regress following the removal of the stimulus of proliferation. However, to date, the spontaneous regression of this lesion has not been documented, but this could be related to the fact that these masses are diagnosed by post-mortem histology of healthier zebrafish. Further studies should ascertain the pathogenetic mechanisms at the basis of the observed neoplastic background. Previous studies have found germ cell tumors in zebrafish that are associated with mutations in the gene alk6b/bmpr1bb, encoding a type IB BMP receptor, and nonsense mutations in the llrc50 gene [25]. The diagnostical interest of those mutations is undoubtful, as they can be the target of specific molecular tests. Additionally, in human pathology, new tissue-based immunohistochemical and molecular biomarkers, including zing-finger transcription factors, have demonstrated potential value for the diagnosis and prognostication of testicular lesions, which need to be used in combination and interpreted according to the general neoplastic background [26].
5. Conclusions
The results of the present preliminary study showed the spontaneous development of testicular tumors in the zebrafish model. In contrast with other mammal models, such as mice, there has been relatively little documentation or control of subclinical disease in zebrafish research facilities, and several infectious and non-infectious conditions are frequently detected by histopathology in apparently healthy fish. To date, there is little information on the impact of these conditions on experimental procedures involving sub-clinically affected fish, but there is reason to believe that they should be considered as a potentially significant confounding factor leading to non-protocol variation of experimental plans. Further investigations are warranted to evaluate the presence of genetic variants in the zebrafish population. Moreover, the immunohistochemical characterization of the lesions could be helpful to elucidate the potential pathways involved in the development of these alterations and to make a definitive diagnosis. The education of animal care attendants and the execution of good health monitoring practices for laboratory fish could also contribute to the diagnosis of testicular lesions, avoiding the underestimation of this pathology in the zebrafish colony.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7060314/s1. Figure S1: (A) macroscopic appearance of fish 1 left testis (B) hematoxylin and eosin-stained sections from formalin-fixed, paraffin-embedded zebrafish fish 1 left testis. Bar = 80 µm; Table S1: Tissue processing steps; Table S2: Haematoxylin and eosin staining steps.
Author Contributions
Conceptualization, A.T. and C.M.; methodology, A.T. and R.L.; validation, M.A., G.C. (Giuseppe Crescenzo) and M.P.; formal analysis, G.C. (Giulia Caioni); investigation, A.T. and C.M.; resources, M.A., G.C. (Giuseppe Crescenzo) and M.P.; data curation, N.P. and C.Z.; writing—original draft preparation, A.T., G.C. (Giulia Caioni) and C.M.; writing—review and editing, N.P. and C.Z.; visualization, N.P. and C.Z.; supervision, M.A., G.C. (Giuseppe Crescenzo) and M.P.; project administration, M.A., G.C. (Giuseppe Crescenzo) and M.P.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes and with the Italian D. Lgs n. 26/2014 “Attuazione della direttiva 2010/63/UE sulla protezione degli animali utilizzati a fini scientifici.” The euthanized animals spontaneously developed pathological conditions, and they were treated according to the Humane Endpoint principles. Ethical review and approval were waived for this study due to the fact that sampled fish were euthanized in the context of the routine monitoring program of the University of Teramo Zebrafish Facility (code 041TE294).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Davide Barbetta for his technical support in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stoletov, K.; Klemke, R. Catch of the Day: Zebrafish as a Human Cancer Model. Oncogene 2008, 27, 4509–4520. [Google Scholar] [CrossRef] [PubMed]
- Hason, M.; Bartůněk, P. Zebrafish Models of Cancer-New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Etchin, J.; Kanki, J.P.; Look, A.T. Zebrafish as a Model for the Study of Human Cancer. Methods Cell Biol. 2011, 105, 309–337. [Google Scholar] [CrossRef]
- Spagnoli, S.T.; Murray, K.N. Chapter 43—Nonexperimentally Induced Neoplastic and Proliferative Lesions in Laboratory Zebrafish. In The Zebrafish in Biomedical Research; Cartner, S.C., Eisen, J.S., Farmer, S.C., Guillemin, K.J., Kent, M.L., Sanders, G.E., Eds.; American College of Laboratory Animal Medicine: Chester, NH, USA; Academic Press: Cambridge, MA, USA, 2020; pp. 527–546. ISBN 978-0-12-812431-4. [Google Scholar]
- Kent, M.L.; Wall, E.S.; Sichel, S.; Watral, V.; Stagaman, K.; Sharpton, T.J.; Guillemin, K. Pseudocapillaria Tomentosa, Mycoplasma Spp., and Intestinal Lesions in Experimentally Infected Zebrafish Danio Rerio. Zebrafish 2021, 18, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, F.K.; Sesterhenn, I.A. Histological classification of testis tumours. In Histological Typing of Testis Tumours; Springer: Berlin/Heidelberg, Germany, 1998; pp. 3–5. [Google Scholar]
- Kennedy, P.C.; Cullen, J.M.; Edwards, J.F.; Goldschmidt, M.H.; Larsen, S.; Munson, L.; Nielsen, S. Histological classifications of tumors of the genital system of domestic animals. In World Health Organisation International Histological Classification of Tumors of Domestic Animals; Schulman, F.Y., Ed.; 2nd series; Armed Forces Institute of Pathology: Washington, DC, USA, 1998; Volume 4, pp. 17–18. [Google Scholar]
- Szymanska, H.; Lechowska-Piskorowska, J.; Krysiak, E.; Strzalkowska, A.; Unrug-Bielawska, K.; Grygalewicz, B.; Skurzak, H.M.; Pienkowska-Grela, B.; Gajewska, M. Neoplastic and Nonneoplastic Lesions in Aging Mice of Unique and Common Inbred Strains Contribution to Modeling of Human Neoplastic Diseases. Vet. Pathol. 2014, 51, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Basten, S.G.; Davis, E.E.; Gillis, A.J.M.; van Rooijen, E.; Stoop, H.; Babala, N.; Logister, I.; Heath, Z.G.; Jonges, T.N.; Katsanis, N.; et al. Mutations in LRRC50 Predispose Zebrafish and Humans to Seminomas. PLOS Genet. 2013, 9, e1003384. [Google Scholar] [CrossRef] [PubMed]
- Spitsbergen, J.M.; Buhler, D.R.; Peterson, T.S. Neoplasia and Neoplasm-Associated Lesions in Laboratory Colonies of Zebrafish Emphasizing Key Influences of Diet and Aquaculture System Design. ILAR J. 2012, 53, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and Husbandry Recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Waheeb, R.; Hofmann, M.-C. Human Spermatogonial Stem Cells: A Possible Origin for Spermatocytic Seminoma. Int. J. Androl. 2011, 34, e296–e305. [Google Scholar] [CrossRef]
- Huyghe, E. Testicular Cancer. In International Encyclopedia of Public Health; Heggenhougen, H.K.K., Quah, S., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 309–318. ISBN 978-0-12-373960-5. [Google Scholar]
- Foster, R.A. Chapter 5—Male Genital System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 3, 6th ed.; Maxie, M.G., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 465–510.e1. ISBN 978-0-7020-5319-1. [Google Scholar]
- Beck, C.; Charles, J.A.; Maclean, A.A. Ultrasound Appearance of an Equine Testicular Seminoma. Vet. Radiol. Ultrasound 2001, 42, 355–357. [Google Scholar] [CrossRef]
- Giangaspero, B.A.; Bucci, R.; Del Signore, F.; Vignoli, M.; Hattab, J.; Quaglione, G.R.; Petrizzi, L.; Carluccio, A. Ultrasound Examination of Unilateral Seminoma in a Salernitano Stallion. Animals 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Grieco, V.; Riccardi, E.; Rondena, M.; Ciampi, V.; Finazzi, M. Classical and Spermatocytic Seminoma in the Dog: Histochemical and Immunohistochemical Findings. J. Comp. Pathol. 2007, 137, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Creasy, D.; Bube, A.; de Rijk, E.; Kandori, H.; Kuwahara, M.; Masson, R.; Nolte, T.; Reams, R.; Regan, K.; Rehm, S.; et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Male Reproductive System. Toxicol. Pathol. 2012, 40, 40S–121S. [Google Scholar] [CrossRef] [PubMed]
- Nigrelli, R.F.; Jakowska, S. Spontaneous neoplasms in Fishes. VII. A spermatocytoma and renal melanoma in an African lungfish, Protopterus annectens (Owen). Zool. Sci. Contrib. N. Y. Zool. Soc. 1953, 38, 109–112. [Google Scholar] [CrossRef]
- Masahito, P.; Ishikawa, T.; Takayama, S. Spontaneous Spermatocytic Seminoma in African Lungfish, Protopterus aethiopicus Heckel. J. Fish Dis. 1984, 7, 169–172. [Google Scholar] [CrossRef]
- Hubbard, G.B.; Fletcher, K.C. A Seminoma and a Leiomyosarcoma in an Albino African Lungfish (Protopterus dolloi). J. Wildl. Dis. 1985, 21, 72–74. [Google Scholar] [CrossRef]
- Palíková, M.; Navratil, S.; Svobodova, Z.; Tichy, F.; Recek, L.; Pikula, J. Skin and Gonadal Tumours in a Barbel (Barbus barbus)—A Case Report. Bull. Eur. Assoc. Fish Pathol. 2007, 27, 234–238. [Google Scholar]
- Sirri, R.; Mandrioli, L.; Grieco, V.; Bacci, B.; Brunetti, B.; Sarli, G.; Schmidt-Posthaus, H. Seminoma in a Koi Carp Cyprinus carpio: Histopathological and Immunohistochemical Findings. Dis. Aquat. Organ. 2010, 92, 83–88. [Google Scholar] [CrossRef]
- Kuroda, Y.; Hoshikawa, Y.; Irie, K.; Kimura, M.; Takeuchi, K.; Furukawa, S. Spontaneous Seminoma in Medaka (Oryzias latipes). J. Toxicol. Pathol. 2022, 35, 95–98. [Google Scholar] [CrossRef]
- Sanchez, A.; Amatruda, J.F. Zebrafish Germ Cell Tumors. Adv. Exp. Med. Biol. 2016, 916, 479–494. [Google Scholar] [CrossRef]
- Siegmund, S.E.; Mehra, R.; Acosta, A.M. An Update on Diagnostic Tissue-Based Biomarkers in Testicular Tumors. Hum. Pathol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).