Identification and Characterization of Heme Oxygenase-1 from Litopenaeus vannamei Involved in Antioxidant and Anti-Apoptosis under Ammonia Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shrimp Preparation, Challenge, and Sample Collection
2.2. RNA Isolation and cDNA Synthesis
2.3. Cloning and Sequence Analysis of Lv-HO-1
2.4. RNAi Assay
2.5. Ammonia Exposure Assay
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Histologic Analysis
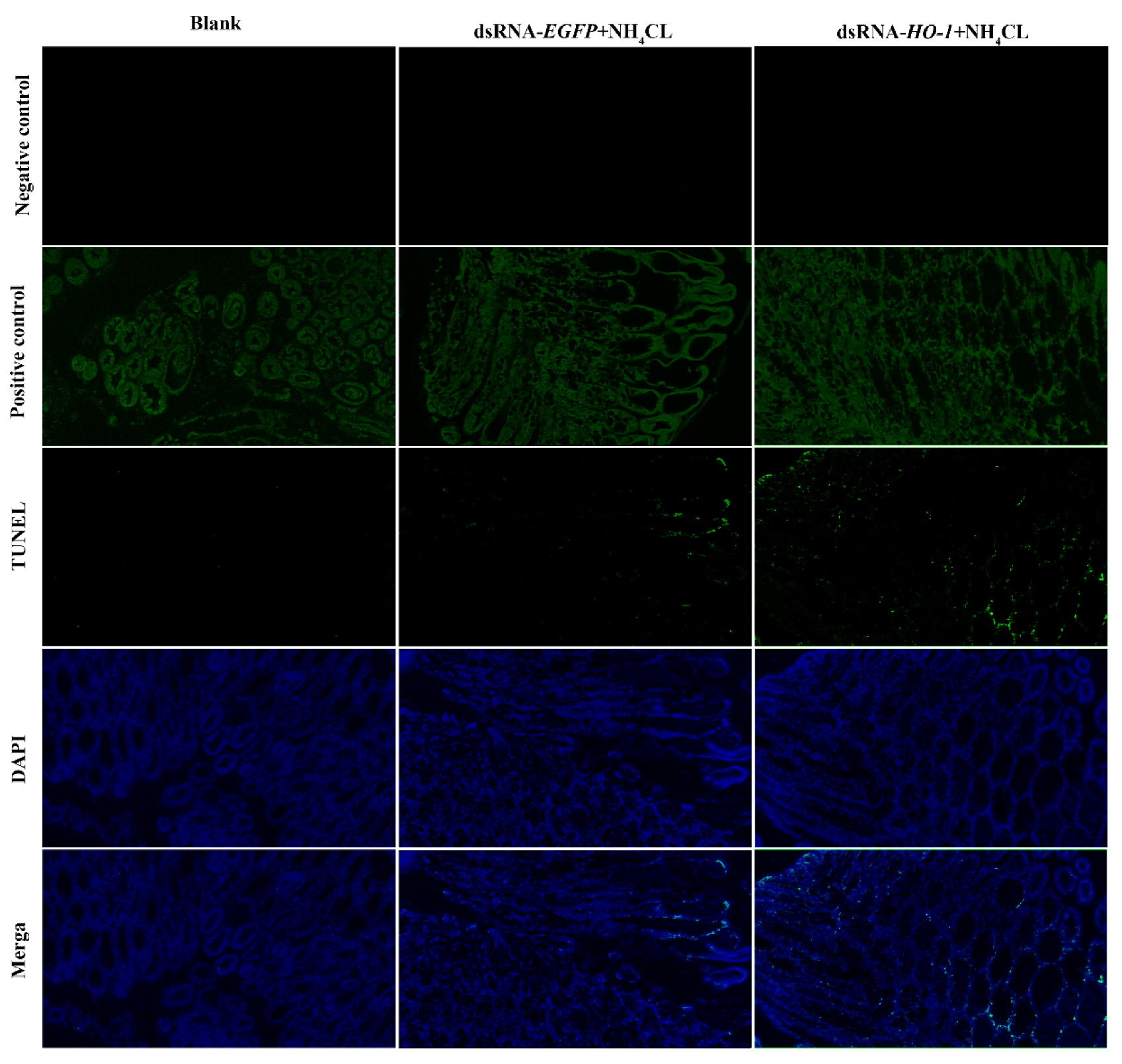
2.8. TUNEL Assay
2.9. Enzyme Activities Assays
2.10. Drawings and Statistical Analysis
3. Results
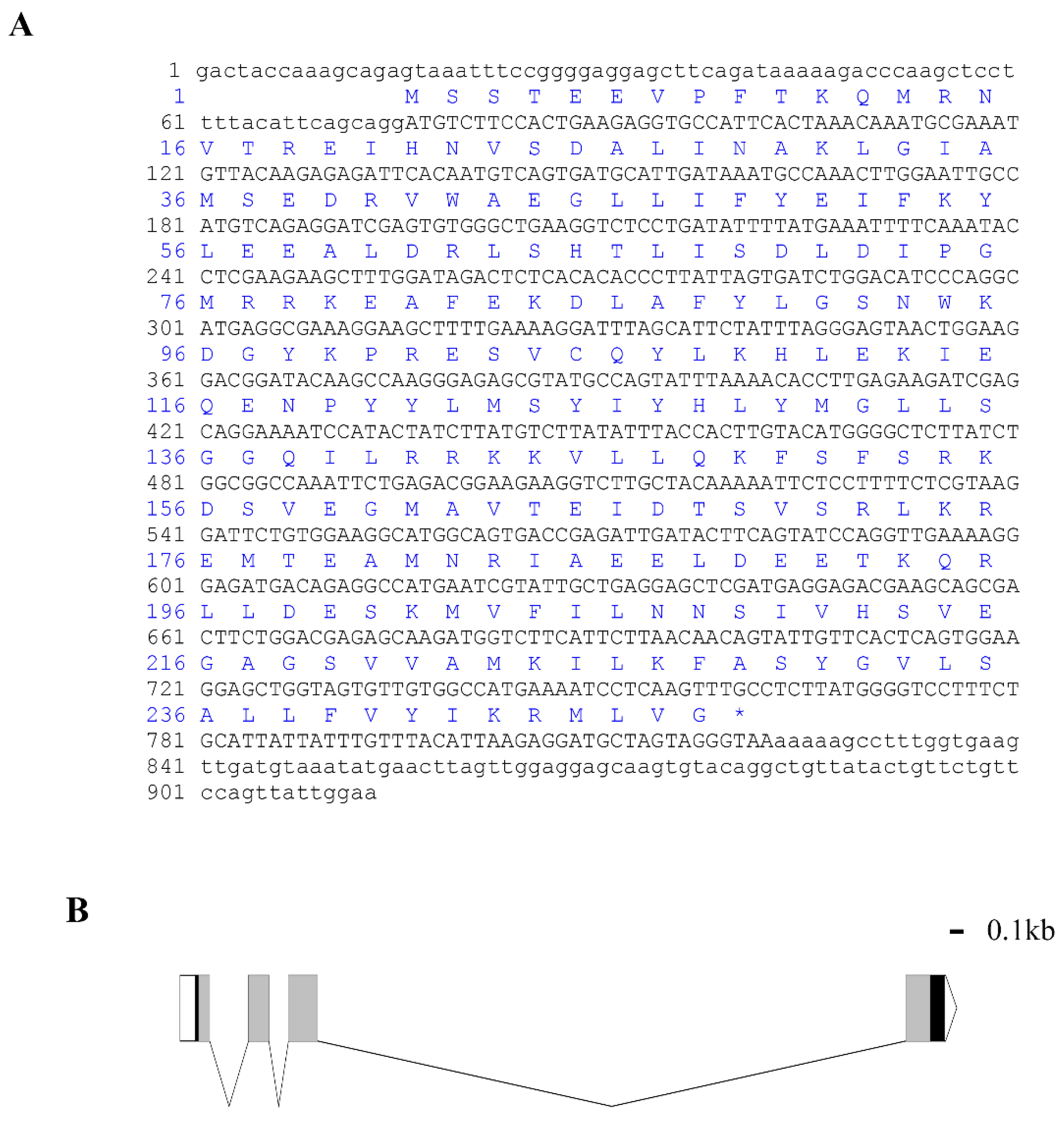
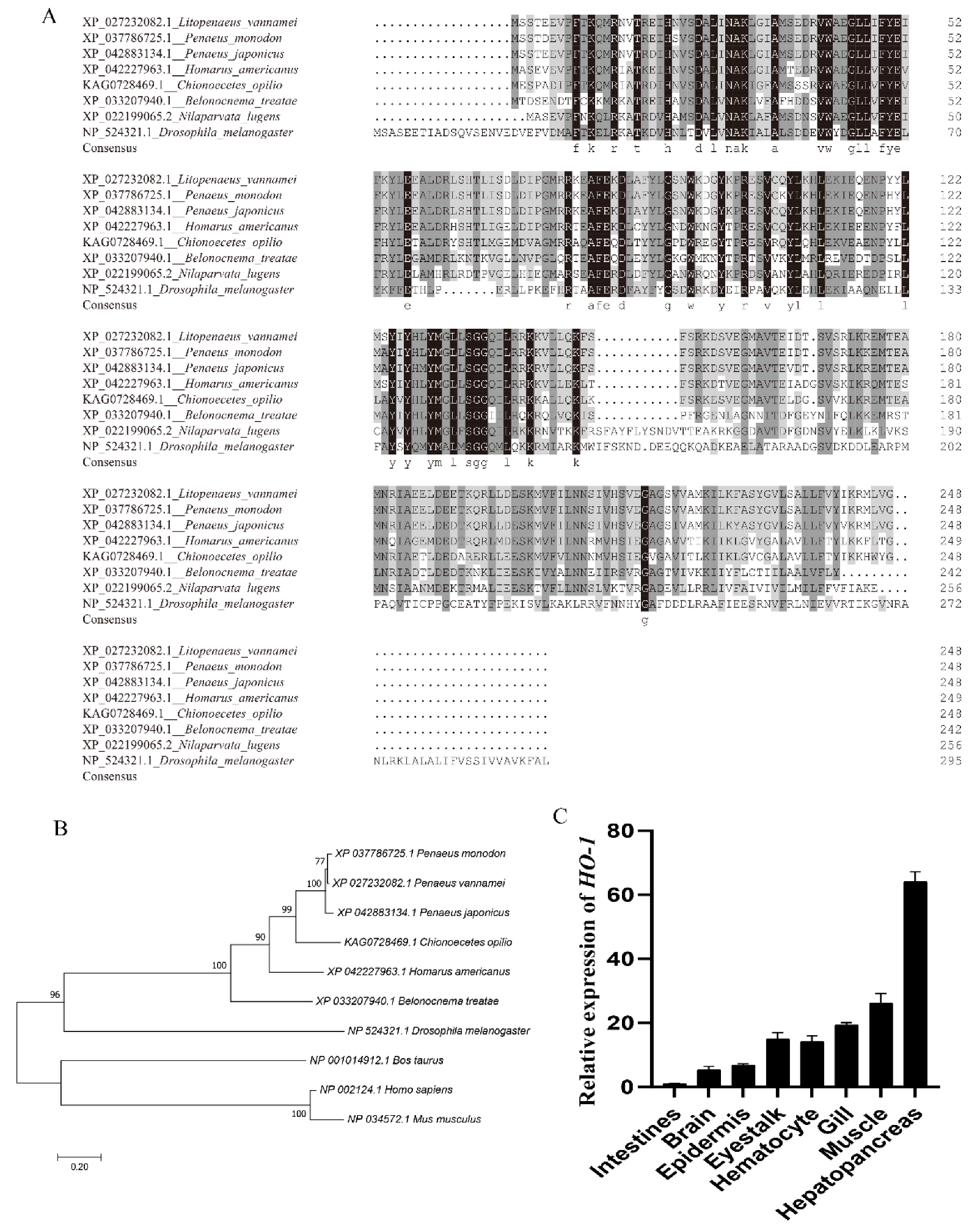
3.1. Cloning and Bioinformatics Analysis of Lv-HO-1
3.2. Expression Characteristics of Lv-HO-1 in Various Tissues
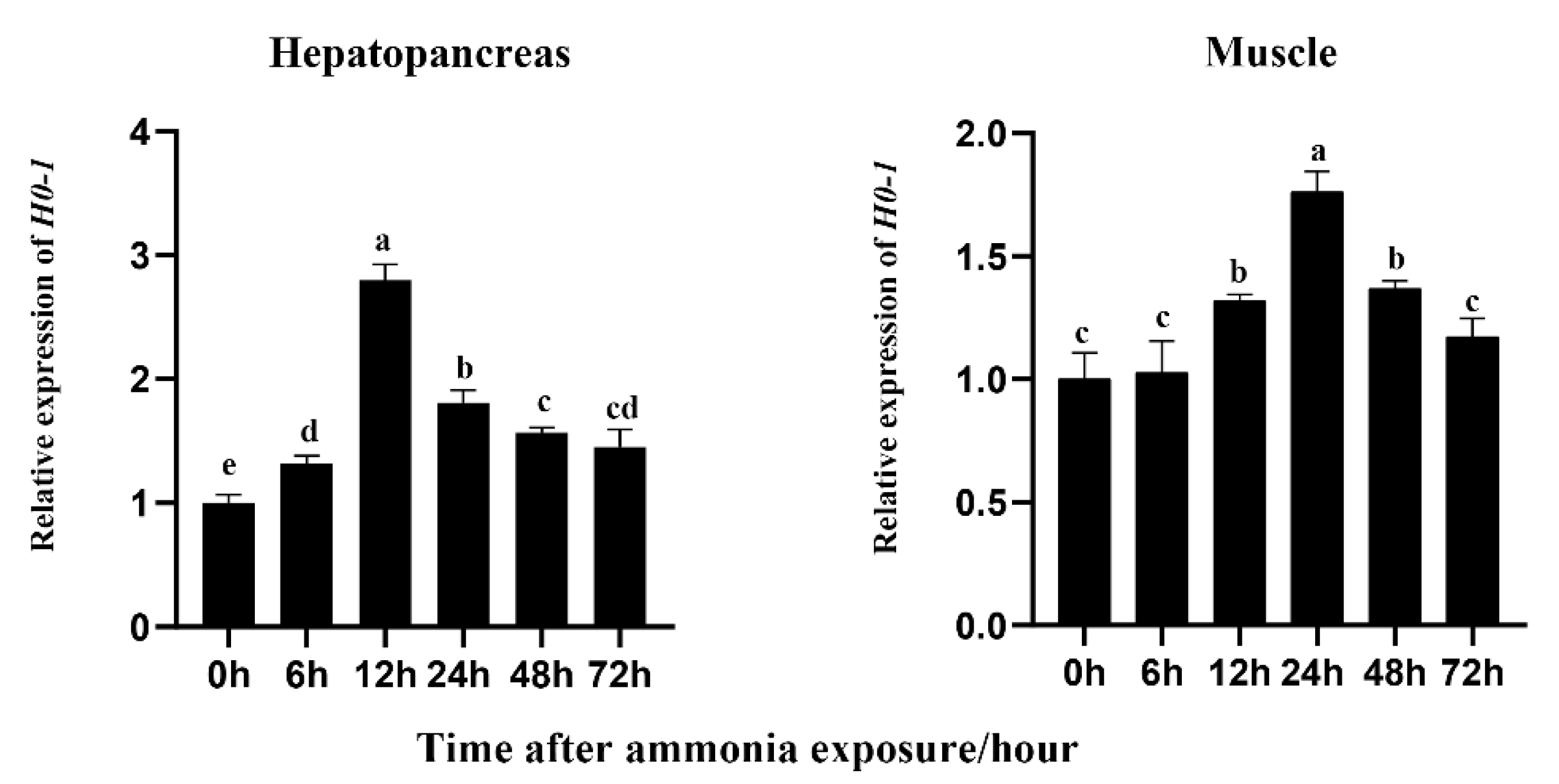
3.3. Effects of Ammonia Exposure on Expression of Lv-HO-1
3.4. Lv-HO-1 Silencing via RNAi
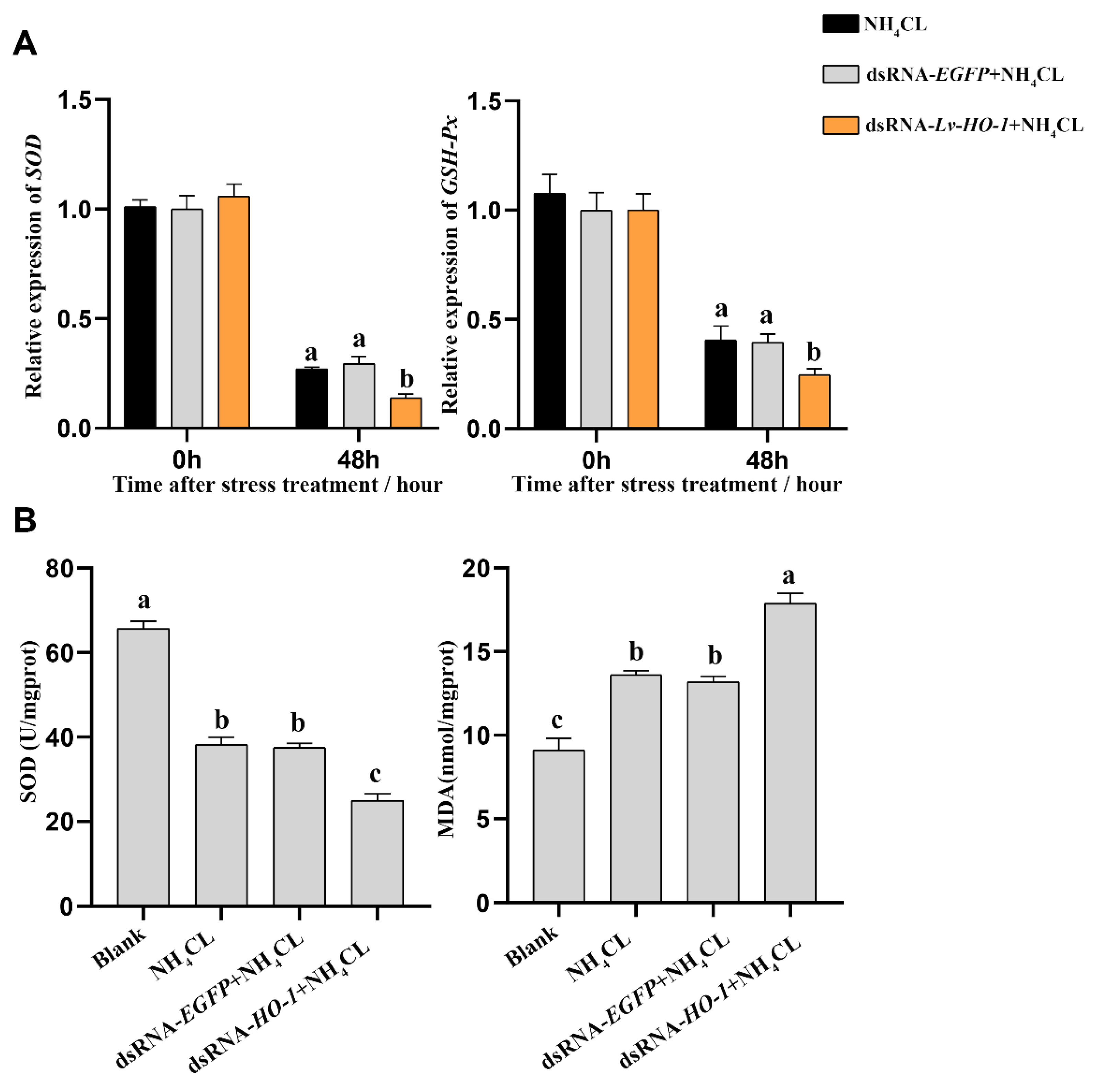
3.5. Effect of Silencing Lv-HO-1 on Antioxidant Capacity
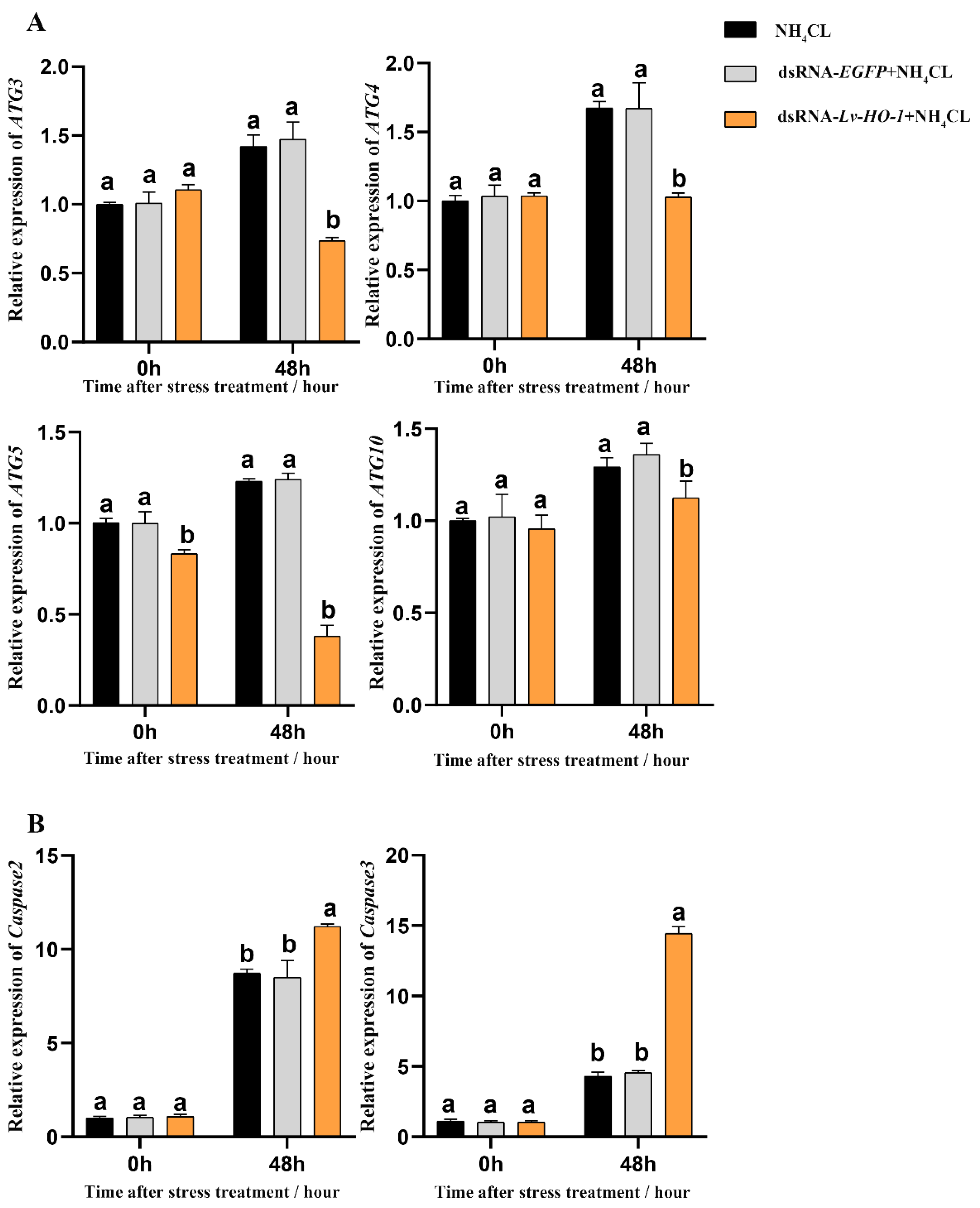
3.6. Effect of Silencing Lv-HO-1 on Autophagy and Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Nick, H.S. Renal response to tissue injury: Lessons from heme oxygenase-1 GeneAblation and expression. J. Am. Soc. Nephrol. 2000, 11, 965–973. [Google Scholar] [CrossRef]
- Maines, M.D.; Trakshel, G.M.; Kutty, R.K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986, 261, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R. Heme Oxygenase-1 As a Target for Drug Discovery. Antioxid. Redox Signal. 2014, 20, 1810–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Stocker, R. Heme oxygenase and iron: From bacteria to humans. Redox Rep. 2009, 14, 95–101. [Google Scholar] [CrossRef]
- Czibik, G.; Derumeaux, G.; Sawaki, D.; Valen, G.; Motterlini, R. Heme oxygenase-1: An emerging therapeutic target to curb cardiac pathology. Basic Res. Cardiol. 2014, 109, 450. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [Green Version]
- Maines, M.D. The Heme Oxygenase System: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Omata, Y.; Sakamoto, H.; Higashimoto, Y.; Hara, T.; Sagara, Y.; Noguchi, M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004, 336, 241–250. [Google Scholar] [CrossRef]
- Silva, R.C.M.C.; Travassos, L.H.; Paiva, C.N.; Bozza, M.T. Heme oxygenase-1 in protozoan infections: A tale of resistance and disease tolerance. PLoS Pathog. 2020, 16, e1008599. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, T.; Zhang, L.; Yang, R.; Li, Y.; Zhang, M.; Dong, Y.; Zhou, Y.; Xu, C.; Yang, B.; et al. Heme oxygenase-1 prevents heart against myocardial infarction by attenuating ischemic injury-induced cardiomyocytes senescence. eBioMedicine 2019, 39, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Deshane, J.; Jozkowicz, A.; Agarwal, A. Heme Oxygenase-1 and Carbon Monoxide in Vascular Pathobiology. Circulation 2008, 117, 231–241. [Google Scholar] [CrossRef]
- Nath, K. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006, 70, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Castilho, F.; Aveleira, C.A.; Leal, E.C.; Simões, N.F.; Fernandes, C.R.; Meirinhos, R.I.; Baptista, F.I.; Ambrósio, A.F. Heme Oxygenase-1 Protects Retinal Endothelial Cells against High Glucose- and Oxidative/Nitrosative Stress-Induced Toxicity. PLoS ONE 2012, 7, e42428. [Google Scholar] [CrossRef] [Green Version]
- Wiesel, P.; Patel, A.P.; DiFonzo, N.; Marria, P.B.; Sim, C.U.; Pellacani, A.; Maemura, K.; LeBlanc, B.W.; Marino, K.; Doerschuk, C.M.; et al. Endotoxin-Induced Mortality Is Related to Increased Oxidative Stress and End-Organ Dysfunction, Not Refractory Hypotension, in Heme Oxygenase-1–Deficient Mice. Circulation 2000, 102, 3015–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Balla, J.; Alam, J.; Croatt, A.J.; Nath, K.A. Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995, 48, 1298–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, K.A.; Haggard, J.J.; Croatt, A.J.; Grande, J.P.; Poss, K.D.; Alam, J. The Indispensability of Heme Oxygenase-1 in Protecting against Acute Heme Protein-Induced Toxicity in Vivo. Am. J. Pathol. 2000, 156, 1527–1535. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, F.; Curtis, L.M.; Truong, L.; Poss, K.; Visner, G.A.; Madsen, K.; Nick, H.S.; Agarwal, A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am. J. Physiol. Physiol. 2000, 278, F726–F736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzaneva, V.; Perry, S.F. Heme Oxygenase-1 (Ho-1) Mediated Respiratory Responses to Hypoxia in the Goldfish. Carassius Auratus. Respir. Physiol. Neurobiol. 2014, 199, 1–8. [Google Scholar] [CrossRef]
- Abaquita, T.A.L.; Damulewicz, M.; Bhattacharya, D.; Pyza, E. Regulation of Heme Oxygenase and Its Cross-Talks with Apoptosis and Autophagy under Different Conditions in Drosophila. Antioxidants 2021, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, L.; Liao, M.; Dong, W.; Liu, C.; Zhuang, X.; Liu, Y.; Yin, X.; Liang, Q.; Wang, W. Pva-miR-252 participates in ammonia nitrogen-induced oxidative stress by modulating autophagy in Penaeus vannamei. Ecotoxicol. Environ. Saf. 2021, 225, 112774. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Xu, L.; Si, L. Effects of ammonia-N exposure on the concentrations of neurotransmitters, hemocyte intracellular signaling pathways and immune responses in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 75, 48–57. [Google Scholar] [CrossRef]
- Si, L.; Pan, L.; Wang, H.; Zhang, X. Ammonia-N exposure alters neurohormone levels in the hemolymph and mRNA abundance of neurohormone receptors and associated downstream factors in the gills of Litopenaeus vannamei. J. Exp. Biol. 2019, 222, jeb.200204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Huang, Y.; Chen, B.; Liu, H.; Huang, Y.; Cai, S.; Jian, J. Protective effects of sulphoraphane on oxidative damage caused by ammonia in Litopenaeus vannamei. Aquac. Res. 2022, 53, 1197–1204. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Fan, W.; Huang, Y.; Niu, J.; Luo, G.; Liu, X.; Huang, Y.; Jian, J. LECT2 Protects Nile Tilapia (Oreochromis niloticus) Against Streptococcus agalatiae Infection. Front. Immunol. 2021, 12, 667781. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Huang, Y.; Jian, J. SP protects Nile tilapia (Oreochromis niloticus) against acute Streptococcus agalatiae infection. Fish Shellfish Immunol. 2022, 123, 218–228. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shibahara, S.; Müller, R.; Taguchi, H.; Yoshida, T. Cloning and expression of cDNA for rat heme oxygenase. Proc. Natl. Acad. Sci. USA 1985, 82, 7865–7869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, C.O.; Healey, J.F.; Greene, Y.; Bonkovsky, H.L. Cloning, sequencing and expression of cDNA for chick liver haem oxygenase. Comparison of avian and mammalian cDNAs and deduced proteins. Biochem. J. 1991, 273, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, I.; Baisvar, V.S.; Singh, M.; Kumar, R.; Srivastava, P.; Kushwaha, B.; Pathak, A.K. Isolation and characterization of hypoxia inducible heme oxygenase 1 (HMOX1) gene in Labeo rohita. Genomics 2020, 112, 2327–2333. [Google Scholar] [CrossRef]
- Wilks, A.; Ikeda-Saito, M. Heme Utilization by Pathogenic Bacteria: Not All Pathways Lead to Biliverdin. Acc. Chem. Res. 2014, 47, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zheng, S.; Tang, F.; Yang, L.; Wei, X.; Kong, D.; Sun, W.; Li, W. Heme oxygenase 1 plays a crucial role in swamp eel response to oxidative stress induced by cadmium exposure or Aeromonas hydrophila infection. Fish Physiol. Biochem. 2020, 46, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
- Prevot-D’Alvise, N.; Pierre, S.; Gaillard, S.; Gouze, E.; Gouze, J.-N.; Aubert, J.; Richard, S.; Grillasca, J.-P. cDNA sequencing and expression analysis of Dicentrarchus labrax heme oxygenase-1. Cell Mol. Biol. 2008, 54, Ol1046–O11054. [Google Scholar]
- Xie, J.; He, X.; Fang, H.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Identification of heme oxygenase-1 from golden pompano (Trachinotus ovatus) and response of Nrf2/HO-1 signaling pathway to copper-induced oxidative stress. Chemosphere 2020, 253, 126654. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Wanner, G.A.; Rensing, H.; Alte, C.; Miescher, E.A.; Wolf, B.; Pannen, B.H.J.; Clemens, M.G.; Bauer, M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology 1998, 27, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wilson, A.T.; Mathahs, M.M.; Wen, F.; Brown, K.E.; Luxon, B.A.; Schmidt, W.N. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology 2008, 48, 1430–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.C.; Gimenez, A.V.F.; Mendiara, S.N.; Fenucci, J.L. Antioxidant Activity in Hepatopancreas of the Shrimp (Pleoticus muelleri) by Electron Paramagnetic Spin Resonance Spectrometry. J. Agric. Food Chem. 2004, 52, 3189–3193. [Google Scholar] [CrossRef]
- Liu, M.-J.; Guo, H.-Y.; Liu, B.; Zhu, K.-C.; Guo, L.; Liu, B.-S.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.-C. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, M.M.; Attia, Z.I.; Ashour, O.A. Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 2010, 99, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Braggins, P.; Trakshel, G.; Kutty, R.; Maines, M. Characterization of two heme oxygenase isoforms in rat spleen: Comparison with the hematin-induced and constitutive isoforms of the liver. Biochem. Biophys. Res. Commun. 1986, 141, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Fredenburgh, L.E.; Merz, A.A.; Cheng, S. Haeme oxygenase signalling pathway: Implications for cardiovascular disease. Eur. Heart J. 2015, 36, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhang, B.H.; Chen, L.; An, W. Overexpression of heme oxygenase-1 protects smooth muscle cells against oxidative injury and inhibits cell proliferation. Cell Res. 2002, 12, 123–132. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Gur, C.; Kandemir, O.; Kandemir, F.M. Investigation of the effects of hesperidin administration on abamectin-induced testicular toxicity in rats through oxidative stress, endoplasmic reticulum stress, inflammation, apoptosis, autophagy, and JAK2/STAT3 pathways. Environ. Toxicol. 2022, 37, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Li, Z.; Zhang, S.; Zhang, H.; Teng, X. miR-187-5p/apaf-1 axis was involved in oxidative stress-mediated apoptosis caused by ammonia via mitochondrial pathway in chicken livers. Toxicol. Appl. Pharmacol. 2019, 388, 114869. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Xiong, X.; Xu, Y.; Zhang, H.; Huang, C.; Tian, Y.; Jiao, C.; Wang, X.; Li, X. Remote Ischemic Preconditioning Protects against Liver Ischemia-Reperfusion Injury via Heme Oxygenase-1-Induced Autophagy. PLoS ONE 2014, 9, e98834. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Traylor, A.M.; Kim, J.; Joseph, R.; Ricart, K.; Landar, A.; Agarwal, A. Heme Oxygenase-1 Inhibits Renal Tubular Macroautophagy in Acute Kidney Injury. J. Am. Soc. Nephrol. 2010, 21, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Basu, A.; Wegiel, B.; Otterbein, L.E.; Mizumura, K.; Gasser, M.; Waaga-Gasser, A.M.; Choi, A.M.; Pal, S. Heme Oxygenase-1 Promotes Survival of Renal Cancer Cells through Modulation of Apoptosis- and Autophagy-regulating Molecules. J. Biol. Chem. 2012, 287, 32113–32123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shan, P.; Jiang, D.; Noble, P.W.; Abraham, N.G.; Kappas, A.; Lee, P.J. Small Interfering RNA Targeting Heme Oxygenase-1 Enhances Ischemia-Reperfusion-induced Lung Apoptosis. J. Biol. Chem. 2004, 279, 10677–10684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.-B.; Zhou, F.; Yao, S.-L.; Tang, Z.-H.; Wang, M.; Chen, H.-R. Effect of heme oxygenase-1 on the kidney during septic shock in rats. Transl. Res. 2009, 153, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Wang, X.; Lee, S.-J.; Huang, M.-H.; Wan, Y.; Ryter, S.W.; Choi, A.M. Autophagic proteins regulate cigarette smoke induced apoptosis: Protective role of heme oxygenase-1. Autophagy 2008, 4, 887–895. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, Q.; Yang, S.; Yuan, Y.; Zhang, Z.; Jiang, B.; Lv, J.; Zhong, J.; Jian, J. Identification and Characterization of Heme Oxygenase-1 from Litopenaeus vannamei Involved in Antioxidant and Anti-Apoptosis under Ammonia Stress. Fishes 2022, 7, 356. https://doi.org/10.3390/fishes7060356
Huang Y, Li Q, Yang S, Yuan Y, Zhang Z, Jiang B, Lv J, Zhong J, Jian J. Identification and Characterization of Heme Oxygenase-1 from Litopenaeus vannamei Involved in Antioxidant and Anti-Apoptosis under Ammonia Stress. Fishes. 2022; 7(6):356. https://doi.org/10.3390/fishes7060356
Chicago/Turabian StyleHuang, Yongxiong, Qi Li, Shiping Yang, Yunhao Yuan, Zhiqiang Zhang, Baijian Jiang, Jing Lv, Jian Zhong, and Jichang Jian. 2022. "Identification and Characterization of Heme Oxygenase-1 from Litopenaeus vannamei Involved in Antioxidant and Anti-Apoptosis under Ammonia Stress" Fishes 7, no. 6: 356. https://doi.org/10.3390/fishes7060356
APA StyleHuang, Y., Li, Q., Yang, S., Yuan, Y., Zhang, Z., Jiang, B., Lv, J., Zhong, J., & Jian, J. (2022). Identification and Characterization of Heme Oxygenase-1 from Litopenaeus vannamei Involved in Antioxidant and Anti-Apoptosis under Ammonia Stress. Fishes, 7(6), 356. https://doi.org/10.3390/fishes7060356





