Disentangling Environmental, Economic, and Technological Factors Driving Scallop (Argopecten purpuratus) Aquaculture in Chile
Abstract
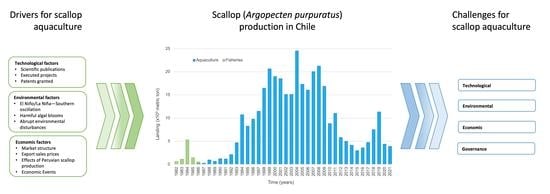
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Analysis of Economic Data
3. Analysis and Discussion
3.1. Scientific and Technological Development Factors and Scallop Production
3.1.1. Scientific Publications
3.1.2. Research and Development Projects
3.1.3. Patents
3.2. Relationship between Environmental Factors and Scallop Production
3.2.1. El Niño/La Niña—Southern Oscillation (EN/LN)
3.2.2. Harmful Algal Blooms
3.2.3. Environmental Disturbances Caused by Tsunami and Storms
3.3. Relationship between Economic and Commercial Factors and Scallop Production
3.3.1. Market Structure
3.3.2. Export Sales Prices
3.3.3. Effects of Peruvian Scallop Production
3.3.4. Economic and Environmental Events
3.4. Challenges for Argopecten Purpuratus Industry in Chile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kluger, L.C.; Taylor, M.H.; Wolff, M.; Stotz, W.; Mendo, J. From an Open-Access Fishery to a Regulated Aquaculture Business: The Case of the Most Important Latin American Bay Scallop (Argopecten purpuratus). Rev. Aquac. 2019, 11, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Uribe, E.; Sfeir, R.; Bakit, J. Informe Final FIPA 2017-12. In Análisis del Desarrollo Histórico y Colapso del Cultivo del Ostión del Norte como Herramienta para el Re-Impulso de la Actividad en la III y IV Regiones; Fondo Investigación de Pesca y Acuicultura; SUBPESCA: Coquimbo, Chile, 2018; p. 305. Available online: https://www.researchgate.net/profile/Jose-Bakit/research (accessed on 28 July 2022).
- Bakit, J.; Hurtado, A.; Bahamonde, C.; Uribe, E.; Sfeir, R. Transferencia Tecnológica del Cultivo Suspendido en la Bahía de Tongoy: Circulación y Cuestionamiento de la Acuicultura del Ostión del Norte. In Estudios y Propuestas para el Desarrollo de la Región de Coquimbo, 1st ed.; Henríquez, D., Moncayo, L., Eds.; Universidad Católica del Norte: Coquimbo, Chile, 2019; pp. 275–283. Available online: https://www.researchgate.net/profile/Jose-Bakit/research (accessed on 28 July 2022).
- Uriarte, I. Estado Actual Del Cultivo de Moluscos En Chile. In Proceedings of the Taller Técnico Regional de la FAO, Puerto Montt, Chile, 20–24 August 2007; Available online: https://www.vliz.be/en/imis?refid=133465 (accessed on 28 July 2022).
- von Brand, E.; Abarca, A.; Merino, G.E.; Stotz, W. Scallop Fishery and Aquaculture in Chile: A History of Developments and Declines. In Developments in Aquaculture and Fisheries Science; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 40, pp. 1047–1072. [Google Scholar] [CrossRef]
- Avendaño, M.; Cantillanez, M.; Le Pennec, M.; Thouzeau, G. Reproductive and Larval Cycle of the Scallop Argopecten purpuratus (Ostreoida: Pectinidae), during El Nino La Nina Events and Normal Weather Conditions in Antofagasta, Chile. Rev. Biol. Trop. 2008, 56, 121–132. Available online: https://www.scielo.sa.cr/scielo.php?pid=S0034-77442008000100008&script=sci_arttext (accessed on 28 July 2022). [CrossRef] [PubMed] [Green Version]
- Illanes, J.E.; Akaboshi, S.; Uribe, E. Efectos de la Temperatura en la Reproducción del Ostión del Norte Argopecten purpuratus en la Bahía de Tongoy durante el Fenómeno El Niño. Investig. Pesq. 1985, 32, 167–173. [Google Scholar]
- Wolff, M. Population Dynamics of the Peruvian Scallop Argopecten purpuratus during the Nino Phenomenon of 1983. Can. J. Fish. Aquat. Sci. 1987, 44, 1684–1691. [Google Scholar] [CrossRef]
- SUBPESCA Registro Pesquero 178/2018—Prorroga de La Suspensión Temporal Del Acceso a La Pesquería Del Recurso Ostión Del Norte (Argopecten purpuratus), 2018–2023; Subsecretaria de Pesca y Acuicultura—SUBPESCA: Valparaíso, Chile, 2018.
- Decreto-Ley Decreto con Fuerza de Ley 5/1983. Legisla sobre la Industria Pesquera y sus Derivados 1983; p. 31. Available online: https://www.bcn.cl/leychile/navegar?idNorma=3676 (accessed on 28 July 2022).
- LGPA Ley General de Pesca y Acuicultura 18.892/1991 1991; p. 222. Available online: https://www.bcn.cl/leychile/navegar?idNorma=30265 (accessed on 28 July 2022).
- Molina, R.; Cerda, R.; González, E.; Hurtado, F. Simulation Model of the Scallop (Argopecten purpuratus) Farming in Northern Chile: Some Applications in the Decision Making Process. Lat. Am. J. Aquat. Res. 2012, 40, 679–693. [Google Scholar] [CrossRef]
- Stotz, W. When Aquaculture Restores and Replaces an Overfished Stock: Is the Conservation of the Species Assured? The Case of the Scallop Argopecten purpuratus in Northern Chile. Aquac. Int. 2000, 8, 237–247. [Google Scholar] [CrossRef]
- Acuicultura, C. Compendio de La Acuicultura En Chile. In Aquanoticias Internacional; TechnoPress: Santiago, Chile, 1998; p. 244. [Google Scholar]
- Edgar, G.J.; Samson, C.R. Catastrophic Decline in Mollusc Diversity in Eastern Tasmania and Its Concurrence with Shellfish Fisheries. Conserv. Biol. 2004, 18, 1579–1588. [Google Scholar] [CrossRef]
- Kirby, M.X. Fishing down the Coast: Historical Expansion and Collapse of Oyster Fisheries along Continental Margins. Proc. Natl. Acad. Sci. USA 2004, 101, 13096–13099. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, M.; Cordisco, C.A.; Giovanardi, O. The Long-Term Decline of the Chamelea gallina L. (Bivalvia: Veneridae) Clam Fishery in the Adriatic Sea: Is a Synthesis Possible? Acta Adriat. 2009, 50, 171–205. Available online: https://www.researchgate.net/publication/242575745_The_long_term_decline_of_the_Chamelea_gallina_L_Bivalvia_Veneridae_clam_fishery_in_the_Adriatic_Sea_is_a_synthesis_possible (accessed on 28 July 2022).
- Standal, D. The Rise and Decline of Blue Whiting Fisheries-Capacity Expansion and Future Regulations. Mar. Policy 2006, 30, 315–327. [Google Scholar] [CrossRef]
- Medina, B.; Guzman, H.M.; Mair, J.M. Failed Recovery of a Collapsed Scallop Argopecten ventricosus Fishery in Las Perlas Archipelago, Panama. J. Shellfish Res. 2007, 26, 9–15. [Google Scholar] [CrossRef]
- Murawski, S.A.; Brown, R.; Lai, H.L.; Rago, P.J.; Hendrickson, L. Large-Scale Closed Areas as a Fishery-Management Tool in Temperate Marine Systems: The Georges Bank Experience. Bull. Mar. Sci. 2000, 66, 775–798. [Google Scholar]
- Pacheco, A.; Stotz, W.B. Will Providing a Filamentous Substratum in the Water Column and Shell Litter on the Bottom Increase Settlement and Post-Larval Survival of the Scallop Argopecten purpuratus? J. Exp. Mar. Biol. Ecol. 2006, 333, 27–39. [Google Scholar] [CrossRef]
- Stotz, W.; Mendo, J. Pesquerías, repoblamiento y manejo de bancos naturales de pectínidos en Iberoamérica: Su interacción con la acuicultura. In Los Moluscos Pectínidos de Iberoamérica: Ciencia y Acuicultura, 1st ed.; Maeda-Martínez, A.N., Ed.; Editorial Limusa: México, Mexico, 2001; pp. 357–374. ISBN 968-18-6385-2. [Google Scholar]
- Oreska, M.P.J.; Truitt, B.; Orth, R.J.; Luckenbach, M.W. The Bay Scallop (Argopecten irradians) Industry Collapse in Virginia and Its Implications for the Successful Management of Scallop-Seagrass Habitats. Mar. Policy 2017, 75, 116–124. [Google Scholar] [CrossRef]
- Wolff, M.; Taylor, M.; Mendo, J.; Yamashiro, C. A Catch Forecast Model for the Peruvian Scallop (Argopecten purpuratus) Based on Estimators of Spawning Stock and Settlement Rate. Ecol. Modell. 2007, 209, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, S.; Turrubiates-Morales, J.; Hall, K. Decline of the Abalone Fishery at La Natividad, Mexico: Overfishing or Climate Change? J. Shellfish Res. 1998, 17, 839–846. [Google Scholar]
- Stotz, W.; González, S. Abundance, Growth, and Production of the Sea Scallop Argopecten purpuratus (Lamarck 1819): Bases for Sustainable Exploitation of Natural Scallop Beds in North-Central Chile. Fish. Res. 1997, 32, 173–183. [Google Scholar] [CrossRef]
- Avendaño, R.; Riquelme, C.; Escribano, R.; Reyes, N. Sobrevivencia y Crecimiento de Post-Larvas de Argopecten purpuratus (Lamarck, 1819) en Bahía Inglesa, Chile: Efectos del Origen, Distribución en la Bahía y Bacterioflora Larval. Rev. Chil. Hist. Nat. 2001, 74, 669–679. [Google Scholar] [CrossRef]
- Pérez, E.P.; Arancibia, M. Relaciones Funcionales entre la Temperatura del Agua de Mar y el Coeficiente de Brody en Cultivos Suspendidos de Argopecten purpuratus. Lat. Am. J. Aquat. Res. 2016, 44, 355–362. [Google Scholar] [CrossRef]
- Uribe, E.; Blanco, J.L. Carrying Capacity of Aquatic Systems to Support Intensive Scallop Aquaculture; the Case of Argopecten pururatus, Bahia Tongoy, Chile. In Los Moluscos Pectínidos de Iberoamerica: Ciencia y Acuicultura; Maeda-Martínez, A.N., Ed.; Editorial Limusa: México, Mexico, 2001; pp. 233–248. ISBN 968-18-6385-2. [Google Scholar]
- Uribe, E.; Moraga, J.; Zuñiga, S.; Rosales, S.; Álvarez, G.; Ávalos, P.; Chirino, S. Informe Final FIP 2006-37. In Establecimiento de un Protocolo de Seguimiento Ambiental para la Determinación de la Capacidad de Carga para el Cultivo del Ostión del Norte; Fondo de Investigación Pesquera, SUBPESCA: Valparaíso, Chile, 2008; p. 184. [Google Scholar]
- Bustos-Gallardo, B.; Prieto, M. Nuevas Aproximaciones Teóricas a las Regiones-Commodity desde la Ecología Política. EURE 2019, 45, 153–175. [Google Scholar] [CrossRef] [Green Version]
- Norambuena, R.; González, L. National Aquaculture Sector Overview—Chile; Departamento de Pesca y Acuicultura de la FAO: Roma, Italy, 2005; Available online: http://www.fao.org/fishery/countrysector/naso_chile/es (accessed on 28 July 2022).
- Schurman, R.A. Snails, Southern Hake and Sustainability: Neoliberalism and Natural Resource Exports in Chile. World Dev. 1996, 24, 1695–1709. [Google Scholar] [CrossRef]
- Avendaño, M.; Cantillánez, M.; Thouzeau, G. Evidence of Clandestine Harvest and Failure of Conservation Policies for Argopecten purpuratus in the Rinconada Marine Reserve (Chile). Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 588–603. [Google Scholar] [CrossRef]
- Avendaño, M.; Cantillánez, M.; Thouzeau, G. Effects of Water Depth on Survival and Growth of Argopecten purpuratus (Lamarck, 1819) Spat in Northern Chile. Aquac. Int. 2008, 16, 377–391. [Google Scholar] [CrossRef]
- Bravo, S.; Silva, M.T.; Lagos, C. Informe Final FIP 2005-24. Diagnóstico de La Proyección de La Investigación En Ciencia y Tecnología de La Acuicultura Chilena; Fondo de Investigación Pesquera, SUBPESCA: Valparaíso, Chile, 2007; p. 332. [Google Scholar]
- Pérez, E.P. Efectos Económicos de la Estrategia de Cosechas Múltiples en el Cultivo del Ostión del Norte Argopecten purpuratus: Una Oportunidad para Mejorar la Competitividad. Lat. Am. J. Aquat. Res. 2014, 42, 180–191. [Google Scholar] [CrossRef]
- Alarcón, E.; Illanes, J.; Pereira, L. Crecimiento de Argopecten purpuratus, en la Zona Norte de Chile. In Proceedings of the IV Congreso Latinoamericano de Ciencias del Mar, Coquimbo, Chile, 30 September–4 October 1991; p. 70. [Google Scholar]
- Mendo, J.; Ysla, L.; Orrego, H.; Miglio, M.; Gil, P.; Del Solar, A. Manual Técnico para el Repoblamiento de Concha Abanico en la Bahía de Sechura; UNALM: Lima, Peru, 2011; pp. 1–100. [Google Scholar]
- Ruiz, S.; Zuniga-Jara, S. Reviewing Capital Cost Estimations in Aquaculture. Aquac. Econ. Manag. 2018, 22, 72–93. [Google Scholar] [CrossRef]
- Zúñiga, S.; Soria, K. Costo del Capital en el Sector Pesquero-Acuícola Chileno. Interciencia 2009, 34, 543–550. [Google Scholar]
- Mendo, J.; Wolff, M.; Mendo, T.; Ysla, L. Scallop Fishery and Culture in Peru. In Developments in Aquaculture and Fisheries Science; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 40, pp. 1089–1109. ISBN 978-0-444-62710-0. [Google Scholar]
- Wolff, M.; Mendo, J. Management of the Peruvian Bay Scallop (Argopecten purpuratus) Metapopulation with Regard to Environmental Change. Aquat. Conserv. Mar. Freshw. Ecosyst. 2000, 10, 117–126. [Google Scholar] [CrossRef]
- Villasante, S.; Tubío, A.; Gianelli, I.; Pita, P.; García-Allut, A. Ever Changing Times: Sustainability Transformations of Galician Small-Scale Fisheries. Front. Mar. Sci. 2021, 8, 712819. [Google Scholar] [CrossRef]
- FAO Statistical Series FAO. Fisheries and Aquaculture Information and Statistics Branch; Food Agricultural Organization: Roma, Italy, 2018. [Google Scholar]
- NOAA/National Weather Service, Climate Prediction Center. Available online: https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php (accessed on 28 July 2022).
- Prochile. Available online: https://cdc.prochile.cl/?post_type=documento&s=ostión (accessed on 28 July 2022).
- Aduanas, Estadísticas Exportación. Available online: www.aduana.cl/Exportacion-Por-Codigo-Arancelario/Aduana/2018-12-14/095532.html (accessed on 28 July 2022).
- Sfeir, R.; Zuñiga, S.; Soria, K.; Illanes, J.E.; Stotz, W. Informe Final 2002-24. Diagnóstico Económico y Social de la Acuicultura en Chile; Fondo de Investigación Pesquera, SUBPESCA: Valparaíso, Chile, 2005; Volume 2, pp. 1–291. [Google Scholar]
- Perú, Ministerio de la Producción. Anuario Estadístico Pesca y Acuícola 2018; Produce: Lima, Perú, 2018; pp. 1–199. Available online: https://ogeiee.produce.gob.pe/index.php/en/shortcode/oee-documentos-publicaciones/publicaciones-anuales/item/901-anuario-estadistico-pesquero-y-acuicola-2018 (accessed on 28 July 2022).
- Perú, Ministerio de la Producción. Anuario Estadístico Pesca y Acuícola 2020; Produce: Lima, Perú, 2020; pp. 1–182. Available online: https://ogeiee.produce.gob.pe/index.php/en/shortcode/oee-documentos-publicaciones/publicaciones-anuales/item/1001-anuario-estadisticoo-pesquero-y-acuicola-2020 (accessed on 28 July 2022).
- Fernández-Polanco, J.; Llorente, I. Price Transmission in the Spanish Fresh Wild Fish Market. Aquac. Econ. Manag. 2015, 19, 104–124. [Google Scholar] [CrossRef]
- Johansen, S. Statistical analysis of cointegration vectors. J. Econ. Dyn. Control 1988, 12, 231–254. [Google Scholar] [CrossRef]
- Johansen, S. Estimation and hypothesis testing of cointegration vectors in gaussian vector autoregressive models. Econometrica 1991, 59, 1551–1580. [Google Scholar] [CrossRef]
- Trapletti, A.; Hornik, K. Tseries: Time Series Analysis and Computational Finance. R Package Version 0.10-52. 2022. Available online: https://CRAN.R-project.org/package=tseries (accessed on 20 October 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; ISBN 3-900051-07-0. Available online: http://www.r-project.org/ (accessed on 20 October 2022).
- Nardi, G.; Prickett, R.; Meeren, T.; Boyce, D.; Moir, J. Atlantic Cod Aquaculture: Boom, Bust, and Rebirth? J. World Aquac. Soc. 2021, 52, 672–690. [Google Scholar] [CrossRef]
- Barton, J.R.; Román, Á.; Rehner, J. Responsible Research and Innovation (RRI) in Chile: From a Neostructural Productivist Imperative to Sustainable Regional Development? Eur. Plan. Stud. 2019, 27, 2510–2532. [Google Scholar] [CrossRef]
- Huffman, W.; Just, R. The Organization of Agricultural Research in Western Developed Countries. Agric. Econ. 1999, 21, 1–18. [Google Scholar] [CrossRef]
- Hall, B.H.; Griliches, Z.; Hausman, J.A. Patents and R and D: Is there a Lag? Int. Econ. Rev. 2019, 27, 265–283. Available online: https://econpapers.repec.org/paper/nbrnberwo/1454.htm (accessed on 28 July 2022). [CrossRef]
- Arntz, W.; Tarazona, J. Effects of El Niño 1982–83 on Benthos, Fish and Fisheries off the South American Pacific Coast. In Global Ecological Consequences of the 1982–83 El Niño—Southern Oscillation; Glynn, P., Ed.; Elsevier Oceanography: Amsterdam, The Netherlands, 1990; Volume 52, pp. 323–360. ISBN 978-85-7811-079-6. [Google Scholar]
- Disalvo, L.H.; Alarcón, E.; Martinez, E.; Uribe, E. Progress in Mass Culture of Chlamys (Argopecten) Purpura Lamarck (1819) with Notes on its Natural History. Rev. Chil. Hist. Nat. 1984, 57, 35–45. [Google Scholar]
- Tarazona, J.; Espinoza, R.; Solís, M.; Arntz, W. Crecimiento y Producción Somática de la Concha de Abanico (Argopecten purpuratus) en Bahía Independencia, Pisco (Perú) Comparados entre Eventos El Niño y La Niña. Rev. Biol. Mar. Oceanogr. 2007, 42, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Lodeiros, C.; Maeda-Martínez, A.N.; Freites, L.; Uribe, E.; Lluch-cota, D.B.; Sicard, M.T. Ecophysiology of Scallops from Iberoamérica. In Los Moluscos Pectínidos de Iberoamérica: Ciencia y Acuicultura; Maeda-Martínez, A.N., Ed.; Editorial Limusa: Mexico, Mexico, 2001; pp. 77–88. ISBN 968-18-6385-2. [Google Scholar]
- Uribe, E.; Lodeiros, C.; Félix-Pico, E.; Etchepare, I. Fouling in Iberoamerican Scallops. In Los Moluscos Pectínidos de Iberoamérica: Ciencia y Acuicultura; Maeda-Martínez, A.N., Ed.; Editorial Limusa: Mexico, Mexico, 2001; pp. 249–266. ISBN 968-18-6385-2. [Google Scholar]
- Uribe, E.; Etchepare, I. Effects of Biofouling by Ciona Intestinalis on Suspended Culture of Argopecten purpuratus in Bahía Inglesa. Bull. Aquacul. Assoc. Canada 2002, 102, 93–95. Available online: https://www.researchgate.net/profile/Eduardo-Uribe/research (accessed on 28 July 2022).
- Thiel, M.; Macaya, E.C.; Acuña, E.; Arntz, W.E.; Bastias, H.; Brokordt, K.; Camus, P.A.; Castilla, J.C.; Castro, L.R.; Cortés, M.; et al. The Humboldt Current System of Northern and Central Chile—Oceanographic Processes, Ecological Interactions and Socioeconomic Feedback. Oceanogr. Mar. Biol. 2007, 45, 195–344. Available online: http://www.bedim.cl/publications/thieletalOMBAR2007.pdf (accessed on 28 July 2022).
- SUBPESCA Registro Pesquero 135/2012—Veda Extractiva y Suspensión de Inscripciones para Recurso Ostión del Norte (Argopecten Purpuratus) en el Área Marítima Comprendida entre las Regiones de Arica-Parinacota y Coquimbo (XV-IV Regiones); Subsecretaria de Pesca y Acuicultura—SUBPESCA: Valparaíso, Chile, 2012.
- Ortiz, M.; Jesse, S.; Stotz, W.; Wolff, M. Feeding Behaviour of the Asteroid Meyenaster Gelatinosus in Response to Changes in Abundance of the Scallop Argopecten purpuratus in Northern Chile. Arch. Fur. Hydrobiol. 2003, 157, 213–225. [Google Scholar] [CrossRef]
- Hilborn, R. Moving to Sustainability by Learning from Successful Fisheries. Ambio 2007, 36, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Álvarez, G.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of Harmful Algal Blooms on the Aquaculture Industry: Chile as a Case Study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, G.; Pizarro, G.; Blanco, J.; Reguera, B. Lipophilic Toxins in Chile: History, Producers and Impacts. Mar. Drugs 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Isla, B.; López, A.; Hernández, C.; Clément, A.; Guzmán, L. Impacto Económico de las Floraciones de Microalgas Nocivas en Chile y Datos Recientes sobre la Ocurrencia de Veneno Amnésico de los Mariscos. In Floraciones Algales Nocivas En el Cono sur Americano; Sar, E., Ferrairo, M., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, España, 2002; pp. 257–268. [Google Scholar]
- Álvarez, G.; Uribe, E.; Vidal, A.; Ávalos, P.; González, F.; Mariño, C.; Blanco, J. Paralytic Shellfish Toxins in Argopecten purpuratus and Semimytilus Algosus from Northern Chile. Aquat. Living Resour. 2009, 22, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, G.; Uribe, E.; Díaz, R.; Braun, M.; Mariño, C.; Blanco, J. Bloom of the Yessotoxin Producing Dinoflagellate Protoceratium Reticulatum (Dinophyceae) in Northern Chile. J. Sea Res. 2011, 65, 427–434. [Google Scholar] [CrossRef]
- Marín, A.; Bodin, Ö.; Gelcich, S.; Crona, B. Social Capital in Post-Disaster Recovery Trajectories: Insights from a Longitudinal Study of Tsunami-Impacted Small-Scale Fisher Organizations in Chile. Glob. Environ. Chang. 2015, 35, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.; Lara, A.; Torres, M. Community Resilience in Response to the 2010 Tsunami in Chile: The Survival of a Small-Scale Fishing Community. Int. J. Disaster Risk Reduct. 2019, 33, 376–384. [Google Scholar] [CrossRef]
- Winckler, P.; Contreras-López, M.; Campos-Caba, R.; Beyá, J.F.; Molina, M. The storm of August 8, 2015 in the regions of Valparaíso and Coquimbo, central Chile. Lat. Am. J. Aquat. Res. 2017, 45, 622–648. [Google Scholar] [CrossRef]
- Girard, S.; Mariojouls, C. French Consumption of Oysters and Mussels Analysed within the European Market. Aquac. Econ. Manag. 2003, 7, 319–333. [Google Scholar] [CrossRef]
- Lesur-Irichabeau, G.; Guyader, O.; Frésard, M.; Leroy, C.; Latouche, K.; Le Grel, L. Information on Sellers and Buyers Characteristics: Added Value to Explain Price Formation at Primary Fish Markets in Managed French Scallop Fisheries. Appl. Econ. 2016, 48, 2078–2092. [Google Scholar] [CrossRef] [Green Version]
- Parrondo, M.; López, S.; Aparicio-Valencia, A.; Fueyo, A.; Quintanilla-García, P.; Arias, A.; Borrell, Y.J. Almost Never You Get What You Pay for: Widespread Mislabeling of Commercial “Zamburiñas” in Northern Spain. Food Control 2021, 120, 107541. [Google Scholar] [CrossRef]
- Barrett, G.; Apostle, R. Formal and Informal Economic Ties between Fishing Boat Captains and Fish Buyers in Nova Scotia. Can. J. Sociol./Cah. Can. Sociol. 1989, 14, 1–23. [Google Scholar] [CrossRef]
- Kinnucan, H.; Sullivan, G. Monopsonistic Food Processing and Farm Prices: The Case of the West Alabama Catfish Industry. South. J. Agric. Econ. 1986, 18, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Perekhozhuk, O.; Glauben, T.; Teuber, R.; Grings, M. Regional-Level Analysis of Oligopsony Power in the Ukrainian Dairy Industry. Can. J. Agric. Econ. 2015, 63, 43–76. [Google Scholar] [CrossRef] [Green Version]
- Freebairn, J. Effects of Supermarket Monopsony Pricing on Agriculture. Aust. J. Agric. Resour. Econ. 2018, 62, 548–562. [Google Scholar] [CrossRef]
- Gordon, D.V.; Hussain, S. Price Determination and Demand Flexibilities in the Ex-Vessel Market for Tuna in the Republic of Maldives. Aquac. Econ. Manag. 2015, 19, 8–28. [Google Scholar] [CrossRef]
- Young, E. State Intervention and Abuse of the Commons: Fisheries Development in Baja California Sur, Mexico. Ann. Assoc. Am. Geogr. 2001, 91, 283–306. [Google Scholar] [CrossRef]
- Kluger, L.C.; Alff, H.; Alfaro-Córdova, E.; Alfaro-Shigueto, J. On the Move: The Role of Mobility and Migration as a Coping Strategy for Resource Users after Abrupt Environmental Disturbance—The Empirical Example of the Coastal El Niño 2017. Glob. Environ. Chang. 2020, 63, 102095. [Google Scholar] [CrossRef]
- Timmer, M. Financial and Economic Crises in Asia. Rev. Income Wealth. 2001, 47, 125–133. [Google Scholar] [CrossRef]
- Brassett, J.; Rethel, L.; Watson, M. The Political Economy of the Subprime Crisis: The Economics, Politics and Ethics of Response. New Polit. Econ. 2010, 15, 1–7. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations [FAO]: Rome, Italy, 2010; p. 218. [Google Scholar]
- van Senten, J.; Smith, M.A.; Engle, C.R. Impacts of COVID-19 on U.S. Aquaculture, Aquaponics, and Allied Businesses. J. World Aquac. Soc. 2020, 51, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Monirul Alam, G.M.; Islam Sarker, M.N.; Gatto, M.; Bhandari, H.; Naziri, D. Impacts of COVID-19 on the Fisheries and Aquaculture Sector in Developing Countries and Ways Forward. Sustainability 2022, 14, 1071. [Google Scholar] [CrossRef]
- Villasante, S.; Tubío, A.; Ainsworth, G.; Pita, P.; Antelo, M.; Da-Rocha, J.M. Rapid Assessment of the COVID-19 Impacts on the Galician (NW Spain) Seafood Sector. Front. Mar. Sci. 2021, 8, 737395. [Google Scholar] [CrossRef]
- Kobayashi, M. The COVID-19 Impacts and Challenges to Achieving Sustainability in Japan’s Fisheries and Aquaculture. Mar. Policy 2022, 143, 105161. [Google Scholar] [CrossRef] [PubMed]
- Manlosa, A.O.; Hornidge, A.K.; Schlüter, A. Aquaculture-Capture Fisheries Nexus under Covid-19: Impacts, Diversity, and Social-Ecological Resilience. Marit. Stud. 2021, 20, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Nyiawung, R.A.; Ayilu, R.K.; Suh, N.N.; Ngwang, N.N.; Varnie, F.; Loring, P.A. COVID-19 and Small-Scale Fisheries in Africa: Impacts on Livelihoods and the Fish Value Chain in Cameroon and Liberia. Mar. Policy 2022, 141, 105104. [Google Scholar] [CrossRef]
- Amos, H.; Giron-Nava, A.; Nguyen, T.; Cisneros-Montemayor, A.M.; Colléter, M.; González-Espinosa, P.C.; Swartz, W. Collapse and Recovery of Seafood Wholesale Prices in Time of COVID-19. Fish Fish. 2022, 23, 963–976. [Google Scholar] [CrossRef]
- Aqua, Acuicultura + Pesca. Available online: https://www.aqua.cl/2011/05/16/firmas-chilenas-a-la-baja-tras-auge-de-los-ostiones-en-peru/# (accessed on 28 July 2022).
- Montoya, M. Análisis de las Inversiones Realizadas en el Sector Pesquero Artesanal Entre 1992 Y 1999; SUBPESCA: Valparaíso, Chile, 2002; p. 21. [Google Scholar]
- Badiola, M.; Basurko, O.C.; Piedrahita, R.; Hundley, P.; Mendiola, D. Energy Use in Recirculating Aquaculture Systems (RAS): A Review. Aquac. Eng. 2018, 81, 57–70. [Google Scholar] [CrossRef]
- You, W.; Hedgecock, D. Boom-and-Bust Production Cycles in Animal Seafood Aquaculture. Rev. Aquac. 2019, 11, 1045–1060. [Google Scholar] [CrossRef]
- Bavestrello-Riquelme, C.; Rios, R.S.; Farías, W.J.; Cárcamo, C.B.; Pérez, H.; Brokordt, K. The Effect of Hybridization between Natural and Cultivated Peruvian Scallop Argopecten purpuratus Populations on Growth and Tolerance to Abiotic Stress. J. Shellfish Res. 2021, 40, 9–18. [Google Scholar] [CrossRef]
- Ahmed, A.S. Wind Energy as a Potential Generation Source at Ras Benas, Egypt. Renew. Sustain. Energy Rev. 2010, 14, 2167–2173. [Google Scholar] [CrossRef]
- Peceño, B.; Bakit, J.; Cortes, N.; Alonso-Fariñas, B.; Bonilla, E.; Leiva, C. Assessing Durability Properties and Economic Potential of Shellfish Aquaculture Waste in the Construction Industry: A Circular Economy Perspective. Sustainability 2022, 14, 8383. [Google Scholar] [CrossRef]
- Campling, L.; Havice, E.; Mccall Howard, P. The Political Economy and Ecology of Capture Fisheries: Market Dynamics, Resource Access and Relations of Exploitation and Resistance. J. Agrar. Chang. 2012, 12, 177–203. [Google Scholar] [CrossRef]
- Jentoft, S. Institutions in Fisheries: What They Are, What They Do, and How They Change. Mar. Policy 2004, 28, 137–149. [Google Scholar] [CrossRef]
- Kumar, G.; Engle, C.; Tucker, C. Factors Driving Aquaculture Technology Adoption. J. World Aquac. Soc. 2018, 49, 447–476. [Google Scholar] [CrossRef]
- Eisenberg, R.S. Public Research and Private Development: Patents and Technology Transfer in Government-Sponsored Research. Va. Law Rev. 1996, 82, 1663. [Google Scholar] [CrossRef] [Green Version]
- Pinto, P.E.; Vallone, A.; Honores, G.; González, H. The Dynamics of Patentability and Collaborativeness in Chile: An Analysis of Patenting Activity between 1989 and 2013. World Pat. Inf. 2017, 49, 52–65. [Google Scholar] [CrossRef]
- Crespi, G.; Zuniga, P. Innovation and Productivity: Evidence from Six Latin American Countries. World Dev. 2012, 40, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.A. Patents, Trade Secrets and International Technology Transfer. Econ. Lett. 2022, 210, 110180. [Google Scholar] [CrossRef]
- Bas, T.G.; Kunc, M.H. National Systems of Innovations and Natural Resources Clusters: Evidence from Copper Mining Industry Patents. Eur. Plan. Stud. 2009, 17, 1861–1879. [Google Scholar] [CrossRef]
- Clapp, R.A. Creating Competitive Advantage: Forest Policy as Industrial Policy in Chile. Econ. Geogr. 1995, 71, 273–296. [Google Scholar] [CrossRef]
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernández Polanco, J.M.; et al. The Decline of Mussel Aquaculture in the European Union: Causes, Economic Impacts and Opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Nakajima, T.; Matsui, T.; Sakai, Y.; Yagi, N. Structural Changes and Imperfect Competition in the Supply Chain of Japanese Fisheries Product Markets. Fish. Sci. 2014, 80, 1337–1345. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kumar, A.; Joshi, P.K.; Dsouza, A. Monopsonists, Disruptive Innovation and Food Security: The Case of High-Value Commodity. Appl. Econ. Perspect. Policy. 2022, 44, 460–476. [Google Scholar] [CrossRef]
- Colán-Ramos, C.; Gómez-Sánchez, M.; Alcazar-Zamora, J.A.; Aguirre-Velarde, A. Use of Soft Waste of the Peruvian Scallops Argopecten purpuratus (Lamarck, 1819) to Produce High Protein Meal. Rev. Investig. Vet. del Peru 2019, 30, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Mullon, C.; Fréon, P.; Cury, P. The Dynamics of Collapse in World Fisheries. Fish Fish. 2005, 6, 111–120. [Google Scholar] [CrossRef]










| Data Variable | Description |
|---|---|
| Type of document | Article|Book chapter|Conference paper|Proceeding paper|Communication |
| Type of paper | Conceptual|Conceptual review|Review|Empiric|Data paper |
| Type of data | Survey|Survey–Official data|Official data|Experimental|Modelling|N.D. * |
| Type of analysis | Qualitative|Qualitative–Quantitative|Quantitative |
| Scale | Global|National|Regional|Local |
| Dimensions | Sub-Dimensions |
|---|---|
| Reproduction | Gonadal|Spawning|Condition|Others |
| Larval culture | Larvae|Survival|Growth|Others |
| Environment | Recruitment|Foods|Toxins|Pathogens|Pollutants|Carrying capacity|Others |
| Technology culture | Culture systems|Biomass production|Hatchery|Recirculating aquaculture systems (RAS)|Others |
| Products | Nutritional|Subproducts|Others |
| Governance | Statistical|Management|Others |
| Commerce | Markets|Supply chain|Costs production|Exports|Others |
| Species | Toxin | Syndrome | Geographical Location | Date | Duration | Source |
|---|---|---|---|---|---|---|
| Pseudo-nitzschia australis | Domoic Acid | Amnesic shellfish poisoning (ASP) | Bahía Inglesa (27°7′ S; 70°52′ W) | Sept. 2000 | 5–6 weeks | [73] |
| Pseudo-nitzschia sp. | Domoic Acid | Amnesic shellfish poisoning (ASP) | Bahía Inglesa (27°7′ S; 70°52′ W) | Oct. 2000 | 5–6 weeks | [73] |
| Dinophysis acuminata | Pectenotoxin | Diarrheic shellfish poisoning (DSP) | N.D. | 2005–2006 | Several episodes | [71,72] |
| Alexandrium sp. | Saxitoxin | Paralytic shellfish poisoning (PSP) | Bahía Mejillones (23°30′ S; 70°27′ W) | May 2006 | 2–3 weeks | [74] |
| Alexandrium sp. | Saxitoxin | Paralytic shellfish poisoning (PSP) | Bahía Tongoy (30°15′ S, 71°20′ W) and Bahía Guanaqueros (30°11′ S, 71°25′ W) | June 2006 | 1–2 weeks | [74] |
| Pseudo-nitzschia australis | Domoic Acid | Amnesic shellfish poisoning (ASP) | Bahía Inglesa (27°7′ S; 70°52′ W) | Nov. 2006 | 1–2 weeks | [71] |
| Protoceratium reticulatum | Yessotoxin | Diarrheic shellfish poisoning (DSP) | Bahía Mejillones (23°30′ S; 70°27′ W) | March 2007 | N.D. | [75] |
| Country | Dickey–Fuller Statistic Value | p-Value |
|---|---|---|
| Chile | −4.38764 | 0.010 |
| Peru | −3.05993 | 0.167 |
| Rank | Eigenvalue | Trace Test | 10% Critical Value | 5% Critical Value |
|---|---|---|---|---|
| 0 | 0.44522 | 22.81 | 17.85 | 19.96 |
| 1 | 0.25048 | 7.50 | 7.52 | 9.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakit, J.; Álvarez, G.; Díaz, P.A.; Uribe, E.; Sfeir, R.; Villasante, S.; Bas, T.G.; Lira, G.; Pérez, H.; Hurtado, A.; et al. Disentangling Environmental, Economic, and Technological Factors Driving Scallop (Argopecten purpuratus) Aquaculture in Chile. Fishes 2022, 7, 380. https://doi.org/10.3390/fishes7060380
Bakit J, Álvarez G, Díaz PA, Uribe E, Sfeir R, Villasante S, Bas TG, Lira G, Pérez H, Hurtado A, et al. Disentangling Environmental, Economic, and Technological Factors Driving Scallop (Argopecten purpuratus) Aquaculture in Chile. Fishes. 2022; 7(6):380. https://doi.org/10.3390/fishes7060380
Chicago/Turabian StyleBakit, José, Gonzalo Álvarez, Patricio A. Díaz, Eduardo Uribe, Rodrigo Sfeir, Sebastian Villasante, Tomas Gabriel Bas, Germán Lira, Hernán Pérez, Andrés Hurtado, and et al. 2022. "Disentangling Environmental, Economic, and Technological Factors Driving Scallop (Argopecten purpuratus) Aquaculture in Chile" Fishes 7, no. 6: 380. https://doi.org/10.3390/fishes7060380
APA StyleBakit, J., Álvarez, G., Díaz, P. A., Uribe, E., Sfeir, R., Villasante, S., Bas, T. G., Lira, G., Pérez, H., Hurtado, A., González-Ávalos, R., & Castillo-Venenciano, J. (2022). Disentangling Environmental, Economic, and Technological Factors Driving Scallop (Argopecten purpuratus) Aquaculture in Chile. Fishes, 7(6), 380. https://doi.org/10.3390/fishes7060380