Abstract
A biofloc system is rich in nutrients, which favors the cultivation of macroalgae, but the influence of the system on the performance of macroalgae is unknown. The objective of this study was to analyze the feasibility of introducing the macroalgae Ulva lactuca into the culture of Litopenaeus vannamei in a biofloc system. The first experiment evaluated the influence of 400 mg L−1 and 30 mg L−1 solids concentration of the system in biofloc and von Stosch culture medium on macroalgae growth. In the second experiment, the densities of 1, 2, and 3 g L−1 of U. lactuca were cultivated in an integrated system with shrimp and monoculture treatment. Both experiments had 35 days of cultivation. There was no significant difference in macroalgae growth between the treatments with biofloc and von Stosch culture medium. In the integrated culture, the density of 1 g L−1 showed better nutrient absorption. Shrimp performance was not affected by macroalgae cultivation. In conclusion, the solids did not affect the growth of the macroalgae, and it could be cultivated in a biofloc system for nitrate uptake in integrated culture with shrimp.
1. Introduction
Shrimp production using biofloc technology (BFT), normally carried out without water renewal, results in the accumulation of nutrients in the system [1]. This occurs due to the action of microorganisms that transform shrimp excreta and food remains into protein and inorganic nutrients [2]. Chemoautotrophic and heterotrophic bacteria grow during the culture. The first group of bacteria works in the oxidation of ammonia to nitrite and later to nitrate, which is accumulated in the system, along with phosphorus from the feed [3]. Heterotrophic bacteria participate in the conversion of ammonia into bacterial biomass and, together with the accumulation of feces and feed remains, increase the concentration of total suspended solids in the system. The production of waste is constant during cultivation, and when it is not properly disposed of or treated, it can generate problems in the water quality in the production system and environmental problems in the release of effluents without treatment [4].
The total suspended solids are important in the water quality of the system and must be maintained between 100 to 300 mg L−1 [5]. In addition to water quality, microbial flocs function as a complementary source of natural food within the crop [6]. Azim and Little [7] nutritionally described biofloc containing 38% protein and 3% lipid in dry matter, showing a high nutritional value, which may depend on the carbon source used in the system. Wasielesky et al. [8] showed that it is possible to reduce the feeding frequency of shrimp L. vannamei when cultivated in a biofloc system.
Despite the benefits of microbial flocs in the shrimp culture system, its effect on macroalgae growth is unknown. As macroalgae are photosynthetic organisms, the concentration of solids can interfere with the light capture that is essential for their growth. Brito et al. [9] showed the deposition of solids on macroalgae, which may have a negative effect on its growth. However, the nutritional value of macroalgae can also change when grown in biofloc. Legarda et al. [10] showed an increase in nitrogen, phosphorus, chlorophyll a, and carotenoids when macroalgae were cultivated in an integrated system in biofloc.
One way to take advantage of the nitrogen and phosphorus accumulated in the system is integration with other species of different trophic levels in production, as proposed by Chopin [11] in an integrated multi-trophic aquaculture (IMTA). In the system, residues are reused by different species, increasing the final productivity of the cultivation and sustainability. The IMTA system is composed of a species fed with commercial feed, such as shrimp or fish, and then a species capable of absorbing inorganic compounds dissolved in the water, such as macroalgae, and a species that consumes organic compounds, such as oyster, is inserted into the system, which will feed on suspended particles in the culture [12].
Considering the precepts in the IMTA and associating them with the biofloc, the presence of macroalgae in integrated cultures with shrimp can promote the absorption of nutrients from the culture, such as ammonia, nitrate and phosphate. Thus, the use of cultivation effluents for the cultivation of macroalgae or integrated cultivations can be an alternative with lower financial and environmental costs, bearing in mind that enrichment culture media, such as von Stosch, have a high cost due to expensive chemical compounds including essential vitamins that make their use unfeasible for large-scale production [13].
The choice of species for the composition of the systems can be a limiting factor for the success of the production. Macroalgae have rapid growth due to the efficiency on converting solar energy into biomass, due to their simple cellular structures compared to terrestrial plants [14], generating large biomasses in a short time. Alencar et al. [15] showed that the use of effluents from a shrimp culture provided a relative growth rate of 8.8% day−1 of the macroalgae U. lactuca and an absorption efficiency with an average of 90% for ammonium (NH4+) and orthophosphate (PO4−3). Ramos et al. [16], analyzing integration of another macroalgae, U. fasciata, with the cultivation of Pacific white shrimp (L. vannamei) with sedimentation and filtration systems by oysters, showed that the combination of systems enabled improvements in several aspects of water quality, using macroalgae in the removal of dissolved nutrients in the system.
Another factor to be considered is the commercial importance of the macroalgae cultivated in the system. Seaweed cultivation is economically viable due to the presence of high-value compounds in algae cells, which are extracted and used for the manufacture of cosmetics, pharmaceuticals, and chemical compounds. El-baz et al. [17] showed that among species of red and green algae, U. lactuca had a higher lipid concentration and inhibitory actions on viral and bacterial activities. In industry, macroalgae have wide applicability, with them being a good nutritional alternative and having a good acceptability, as shown by Turan and Tekogul [18].
The use of macroalgae as bioremediators has been widely used and has been shown to be efficient. Copertino et al. [19], carrying out a study with U. chlatrata recirculating water from a L. vannamei culture, showed a maximum relative growth rate of 20% day−1 and an uptake of 90% of total ammonia nitrogen (TAN) of the system, demonstrating the feasibility of this integration. However, depending on the area and dispersion of the effluent, large macroalgae biomasses are necessary and may be unfeasible [20]. There has been an attempt to adjust the proportion of macroalgae in the crop so that the absorption of nutrients is still effective. Alencar et al. [15] found better growth and nutrient absorption results with a density of 3 g L−1; this high density is convenient in small volumes of water. Del Río et al. [21], using U. rigida as a biofilter for fish tanks, found that the results are good at densities between 1.5 and 2.5 g L−1. However, in shrimp farming in a biofloc system with high organic load and nutrients, little is known about the ideal density for the maintenance of the system and on the performance of macroalgae in cultivation in a biofloc system and alternatives to optimize nutrient absorption. Therefore, the objective of this work is to evaluate the influence of the seaweed Ulva lactuca in the cultivation of the shrimp Litopenaeus vannamei in a biofloc system.
2. Materials and Methods
2.1. Study Location and Origin of the Animals and Macroalgae
The experiments were carried out at the Marine Aquaculture Station (EMA), Institute of Oceanography of the Federal University of Rio Grande (IO-FURG), located at Cassino Beach, Rio Grande, Rio Grande do Sul, Brazil. Two experiments were carried out with the macroalgae U. lactuca. Macroalgae were collected at Cassino Beach (32°17′52.30″ S–52°15′59.80″ W), Rio Grande, RS, Brazil. After collection, the algae samples were taken to the laboratory for removal of epiphytes and acclimatized until the beginning of the experiments. The shrimp used in the second experiment came from cultivation in a greenhouse at the Shrimp culture Laboratory, Marine Aquaculture Station (EMA).
2.2. Lab-Scale Experiments
This experiment aimed to evaluate the growth of macroalgae U. lactuca with different concentrations of solids from a cultivation in a biofloc system compared with a specific culture medium.
2.2.1. Experimental Design and Facilities
The experimental design was carried out with three treatments in triplicate, namely: (1) BFT: cultivation of U. lactuca in effluent from shrimp cultivation in a biofloc system, with 400 mg L−1 of TSS; (2) DEC: cultivation of U. lactuca in effluent from shrimp culture in a biofloc system, after a period of decantation of solids, with 30 mg L−1 of TSS; (3) VS: cultivation of U. lactuca in standard von Stosch enrichment solution [22] at a concentration of 10 mL L−1 (Table 1) and sea water, without the presence of TSS.

Table 1.
Composition of von Stosch enrichment medium modified and adapted by Guiry and Cunningham [22].
Five litter transparent plastic containers (or carboys) with 3 L useful volume and an area of 0.13 m−2 exposed to light were used for lab cultivation of the macroalgae. After algae were transferred into the culture units, the top of the containers was covered with a transparent PVC film to prevent water evaporation and contamination.
The experiment was carried out for 35 days under controlled conditions of a temperature of 26.58 ± 0.05, with 12:12 h light/dark photoperiod, 3013.89 ± 107.64 LUX light intensity, and total light per day of 2.39 ± 0.09 micromole day−1. Constant aeration was provided using a blower (3900 L hour−1) that directed air through a porous airstone of 15 cm in length in each experimental unit. To carry out the experiment in the laboratory, a density of 2 g L−1 [21] of macroalgae was used.
2.2.2. Biofloc Effluent and Culture Medium
For the treatments with biofloc, an effluent from a shrimp culture in a BFT system in a greenhouse was used, which lasted 43 days, at a density of 400 shrimp m3. The water quality of the system was measured with 66 mg L−1 of nitrate, 5.6 mg L−1 of phosphate and 400 mg L−1 of total suspended solids (TSS), indicating that the system was mature [3], and with an acceptable solids concentration for the cultivation of shrimp in biofloc [5].
This effluent was placed in natura in the BFT treatment for the macroalgae culture, with a concentration of 400 mg L−1 of total solids in suspension. For the DEC treatment, the effluent underwent a decantation process for 30 min, so that the denser solids could settle and be removed from the water. This resulted in a concentration of solids lower (30 mg L−1) than that found in the shrimp tanks (400 mg L−1).
For the control treatment, a 10 mL L−1 von Stosch enrichment solution modified by Guiry and Cunningham [22] was used. None of the treatments were renewed in order to maintain a standard. Therefore, the von Stosch solution was only inoculated into the experimental units at the beginning of the experiment. To prepare the von Stosch enrichment solution, 940 mL of filtered seawater was used and 10 mL of each solution made was added (Table 1). To prepare the vitamins, 950 mL of filtered and sterilized water was used, the Thiamine HCl was dissolved, and 1 mL of the other solutions produced was added (Table 1). The initial concentrations of nutrients in the treatment von Stosch solution were 0.0 ± 0.0, 0.0 ± 0.0, 5.0 ± 0.0, and 1.2 ± 0.0 mg·L−1 of total ammonium nitrogen, nitrite, nitrate, and phosphate, respectively.
2.3. Experiments in Greenhouse Conditions
The experiment was carried out in a greenhouse structure unit with the objective of evaluating the optimum density of the macroalgae, U. lactuca, in the integrated culture with the shrimp L. vannamei, to optimize the absorption of nutrients from the system during the 35 days of the experimental period.
2.3.1. Experimental Design and Facilities
Four treatments with three replications were applied as follows: IMTA 1 treatment: integrated shrimp culture with U. lactuca (1 g L−1); IMTA 2 treatment: integrated shrimp culture with U. lactuca (2 g L−1); IMTA 3 treatment: integrated shrimp culture with U. lactuca (3 g L−1); and MONO C treatment: monoculture of shrimp without U. lactuca.
A total of 12 shrimp tanks with 300 L of total capacity with a base diameter of 0.81 m and a height of 0.53 m and 150 L of useful volume for each tank were used for this experiment. Six rectangular floating structures (40 × 30 × 5 cm) were used as U. lactuca cultivation units. Each shrimp tank contained a single floating structure for the macroalgae; they were placed close to the surface, by using a rectangular structure (40 × 30 × 5 cm) made of sponge and a 5 mm polyethylene mesh, covering an area of 0.12 m−2 of the tank surface, and were subject to a daily light intensity of 28.68 ± 8.53 moles day−1 (Figure 1).

Figure 1.
The rectangular floating structure used for U. lactuca cultivation in the shrimp tank.
The water exchange was not applied, and the aeration was maintained by a blower (4 CV) that injected air into three 20 cm-long micro-perforated hoses per tank. The density used in all the experimental treatments was 300 shrimp m3 according to Krummenauer et al. [23].
2.3.2. Biofloc Effluent
To carry out the experiment in a greenhouse, water from shrimp cultivation in a biofloc system in progress (inoculum) was used. The experimental units were filled with 120 L of seawater (80%) and 30 L (20%) of inoculum from a shrimp biofloc culture.
The inoculum had 45 days of culture, with a well-established bacterial community according to Ferreira et al. [3], showing 70 mg L−1 of nitrate, 4 mg L−1 of phosphate, and 650 mg L−1 of total suspend solids (TSS). The concentrations of total ammonia nitrogen, nitrite, nitrate, phosphate, and total suspended solids were measured as 0.8 ± 0.1, 0.1 ± 0.0, 14.0 ± 0.0, 0.7 ± 0.1 and 127.5 ± 23.8 mg L−1, respectively.
2.4. Physical and Chemical Parameters
For water quality, parameters such as temperature (°C), salinity (‰), dissolved oxygen (DO, mg L−1), and pH were measured daily in all of the experimental tanks, with the aid of a multiparameter probe (YSI, model Pro-20, USA) and a benchtop pH meter (Mettler Toledo, FEP20, Brazil). Salinity was measured weekly using a multiparameter (YSI, model Pro-20, USA). Water samples were collected from near the surface and near the aeration points from each experimental tank and the water samples were kept in plastic containers and taken for analysis immediately. The daily total ammonia nitrogen (or TAN, mg L−1) and nitrite (NO2, mg L−1) were analyzed according to the methodology of UNESCO [24] and Bendschneider and Robinson [25]. When the concentration of total ammonia nitrogen (TAN) was greater than 1 mg L−1, molasses was used in ratio 6:1 carbon: nitrogen to control water quality [26]. Nitrate (NO3, mg L−1) and phosphate (PO4, mg L−1) were analyzed using the methodology described by Aminot and Chaussepied [27] and they were monitored three times a week. Turbidity (NTU) was measured by a portable turbidimeter (Hach®, 2100P, Portugal) and total suspended solids (or TSS, mg L−1) were quantified by filtration and gravimetry according to the methodology described by Baumgarten et al. [28]. Turbidity and TSS were determined weekly. The total alkalinity (mg CaCO3 L−1) was monitored according to the methodology presented by APHA [29] and it was measured weekly in the lab-scale experiments and twice a week in the pilot-scale experiments in greenhouse conditions. To maintain CaCO3 above 150 mg L−1, calcium hydroxide was used [30]. Weekly sedimentable solids (or SS, ml L−1) were measured by using the Imhoff cone method [29] in the pilot-scale experiments conducted under greenhouse conditions.
2.5. Growth and Nutrient Absorption by U. lactuca
Initial and final algal biomass yields were measured. In order to determine the wet (or fresh) biomass, Ulva thallus was first collected by hand from the rectangular floating structures. The excess water was removed by using a hand centrifuge, followed by using paper towels. The samples were weighed in a Digital Balance machine (MARTE® BL3200H, SP Labor, São Paulo, Brazil) to determine their wet weight. The following equations were used to calculate the relative growth rate (RGR) [31]:
RGR (% day−1): [in (final weight (g)/initial weight (g))/(final time/initial time) × 100].
The nutrient absorption efficiency [32] of U. lactuca was calculated as follows:
Nutrient removal rate—NRR (%): [(concentration of nutrient in the initial time (mg L−1) − concentration of nutrients in the final time (mg L−1))/(concentration of nutrient in the initial time (mg L−1)) −1] × 100.
2.6. Protein Analysis in U. lactuca
Random algal samples with three replicates for protein analysis were hand-collected from each experimental unit, washed under running tap water, and rinsed with distilled water at the beginning and at the end of the experiments. The excess water was removed by using a hand centrifuge, followed by drying with paper towels. The samples were weighed to determine the wet weight and placed in an oven at 60 °C for 24 h and weighed again to obtain the dry weight. Subsequently, the samples were ground to powder by using a coffee bean grinder machine (Cadance®, Belo Horizonte, Brazil).
The nitrogen content of the algae was determined by using the Kjeldahl titration method according to AOAC [33] at the Laboratory of Nutrition of Aquatic Organisms-LANOA (EMA, FURG, Rio Grande, Brazil). The following formula used for converting nitrogen to protein was:
where Vol is the volume spent on titration and Sample is the dry weight of the sample [32].
Protein (% of dry weight) = [(0.1 × Vol × 0.014/Sample) × 5.45] × 100
2.7. Performance of the Shrimp
After shrimp storage, weekly biometrics were made to adjust the amount of feed offered. At the end of the experiment, all of the animals were weighed and counted. The performance variables collected at the end of the experiment were final average weight (g), final biomass yield (g) survival (%), feed conversion rate (FCR), specific growth rate (g week−1), and productivity (kg m−3).
The performance of the shrimp was analyzed from the weekly biometric measurements that included the following equations:
- Final average weight (g): final biomass of live animals (g)/total number of animals;
- Final biomass yield (g): final weight of all live animals (g);
- Survival (%) = (final number of animals/initial number of animals) × 100;
- Feed conversion rate (FCR) = feed offered (g)/(final biomass (g) − initial biomass (g));
- Specific growth rate (g week−1): weight gain (g)/number of weeks;
- Productivity (kg m−3): [(final biomass (kg) − initial biomass (kg)) × 100]/tank volume (L).
2.8. Statistical Analysis
The normality and homoscedasticity of the data were verified using the Shapiro–Wilk and Levene tests, respectively. Once the assumptions were met, ANOVA was performed followed by Tukey’s post-hoc test or t-test. When ANOVA’s assumptions were not satisfied, the Kruskall–Wallis nonparametric test was used. A minimum level of significance of 5% (p ≤ 0.05) was applied in all analyzes.
3. Results
3.1. Lab-Scale Experiments
The final weight of the algae was higher (p ≤ 0.05) then the initial weight of the algae at the beginning of the experiment. However, there was no significant difference (p ≤ 0.05) in the final weight of the algae between the treatment groups, showing only an increase in the relative growth rate (RGR) in all treatments (Table 2).

Table 2.
Initial and final biomass, relative growth rates, and protein contents (% of dry weight) (mean ± standard deviation) of U. lactuca growth in the treatments of BFT (in shrimp culture effluent with 400 mg L−1 of TSS), DEC (in effluent from shrimp culture after the settling period, with 30 mg L−1 of TSS) and VS. (cultivation of U. lactuca in standard von Stosch enrichment solution at a concentration of 10 mL L−1) during the 35 days of the experimental period.
The protein concentrations were evaluated at the end of cultivation and showed a significant difference (p ≤ 0.05) between the treatment groups. The lower protein value was recorded in the experimental group where the algae were grown in von Stosch enrichment solution (Table 2).
The water quality parameters in the treatment groups are summarized in Table 3. The results show that the water temperature, dissolved oxygen, pH, salinity and alkalinity did not show any significant difference between the treatments (p ≤ 0.05) (Table 3). The total suspended solids (TSS) concentrations were significantly lower (p ≤ 0.05) in the DEC treatment where U. lactuca was cultivated in the shrimp effluent water with 30 mg L−1 of TSS. Turbidity, however, showed similar results between the DEC (with 30 mg L−1 of TSS) and BFT groups (with 400 mg L−1 of TSS).

Table 3.
Water quality parameters (mean ± standard deviation) in the treatment groups where U. lactuca was cultivated in different shrimp effluent concentrations (BFT: with 400 mg L−1 of TSS; DEC: with 30 mg L−1 of TSS; and VS: cultivation of U. lactuca in von Stosch enrichment solution at a concentration of 10 mL L−1) during the 35 days of the experimental period.
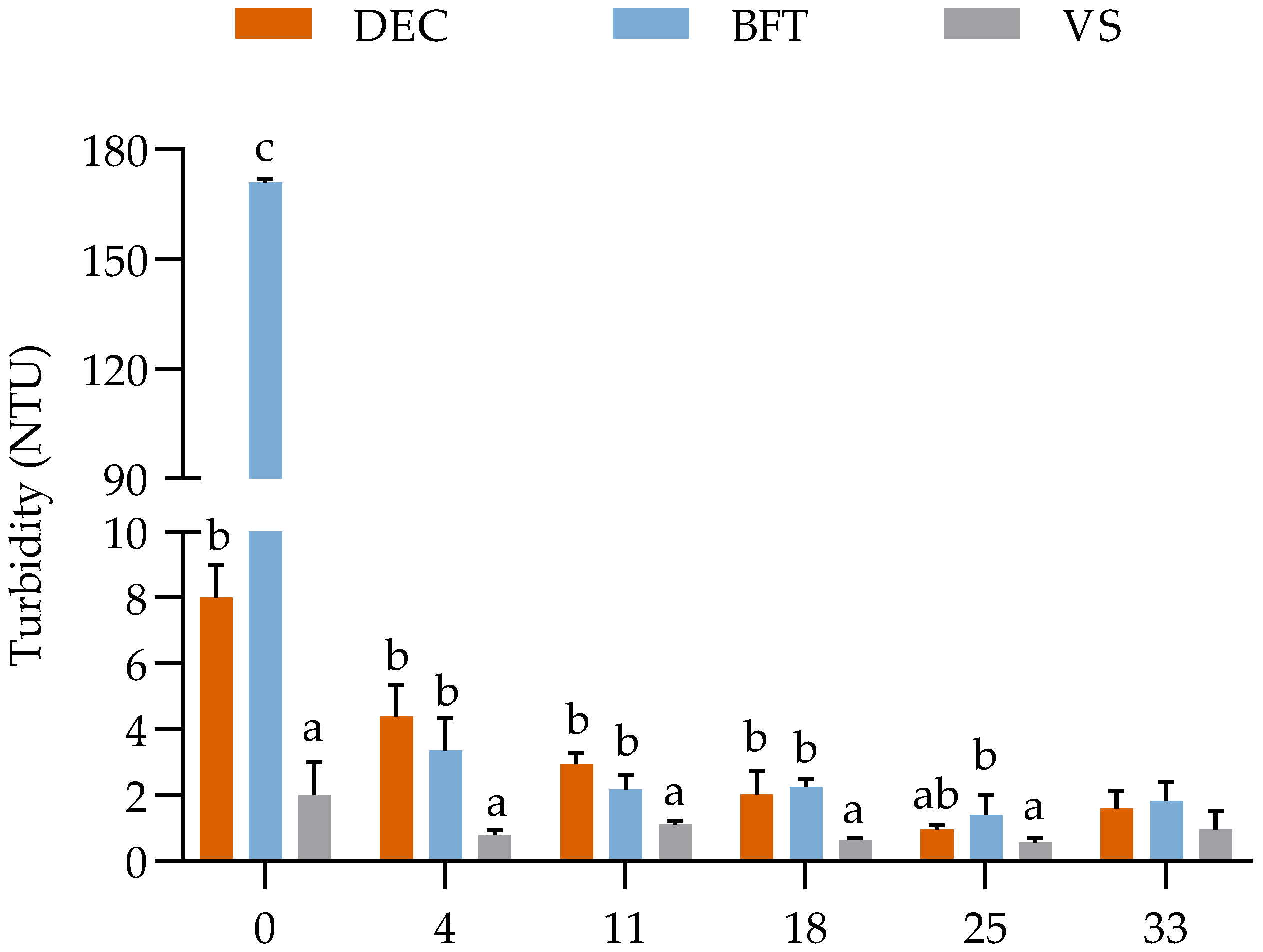
The turbidity values did not differ statistically (p ≤ 0.05) between the initial and final concentrations in the VS treatment where U. lactuca cultivation took place in von Stosch enrichment solution at a concentration of 10 mL L−1. For the biofloc treatments (DEC and BFT), there was a decrease (p ≤ 0.05) in turbidity concentration on the fourth day of sampling and throughout the experiment (Figure 2). There was no significant difference (p ≥ 0.05) with the vs. treatment at the end of cultivation.
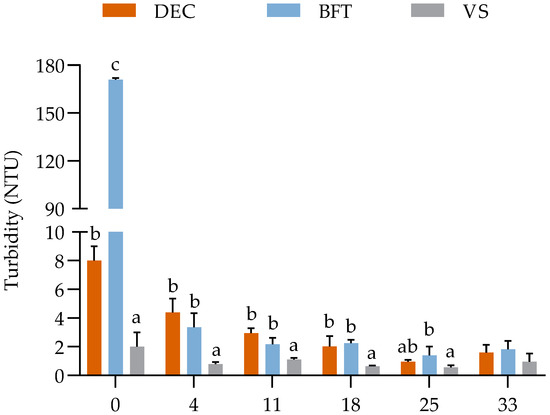
Figure 2.
Weekly turbidity values in the DEC (with 30 mg L−1 of TSS), BFT (with 400 mg L−1 of TSS), and vs. (with von Stosch enrichment solution at a concentration of 10 mL L−1) treatment groups during the 35 days of the experimental period. a, b, c = Different letters on the same day represent a significant difference (p ≤ 0.05) between the treatments after one-way ANOVA with Tukey’s post-hoc test.
3.2. Experiments in Greenhouse Conditions
The macroalgae biomass yields decreased in all the treatment groups compared to the initial biomass values and the difference was significant (p ≤ 0.05). However, at the end of the experiment there was no significant difference (p ≤ 0.05) in fresh biomass values between the treatment groups (Table 4).

Table 4.
Relative growth rates (% day−1) of U. lactuca (mean ± standard deviation) in the IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), and IMTA 3 (3 g Ulva L−1) treatment groups during the 35 days of cultivation.
There was a significant difference (p ≤ 0.05) in the protein content of U. lactuca in the initial samples compared to the final samples taken from different treatment groups after 35 days of culture (Table 5). However, there was no significant differences between the treatments with biofloc in the protein content of U. lactuca.

Table 5.
Initial and final protein concentration (% of dry weight) (mean ± standard deviation) of U. lactuca in the IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), and IMTA 3 (3 g Ulva L−1) treatment groups during the 35 days of cultivation.
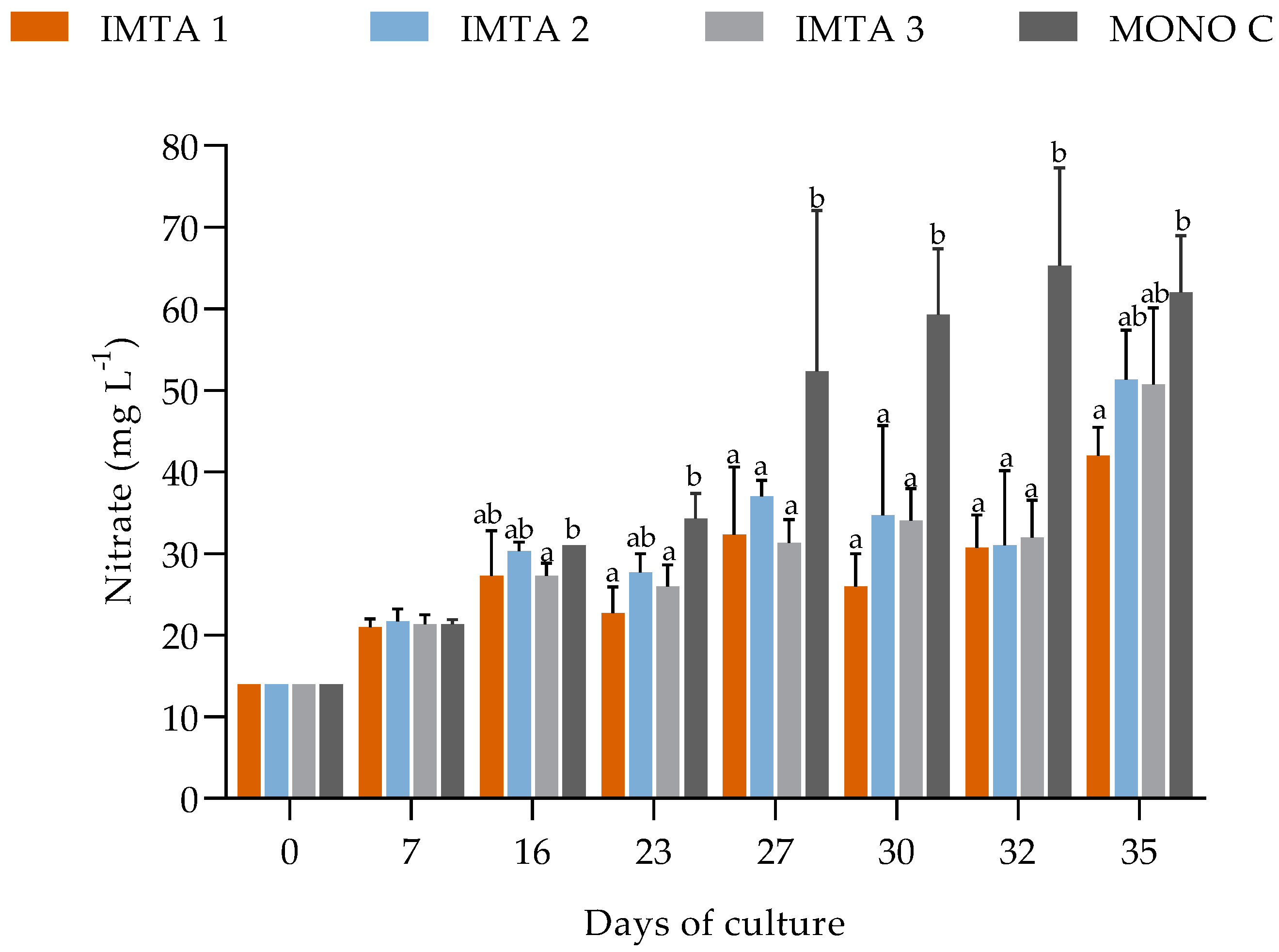
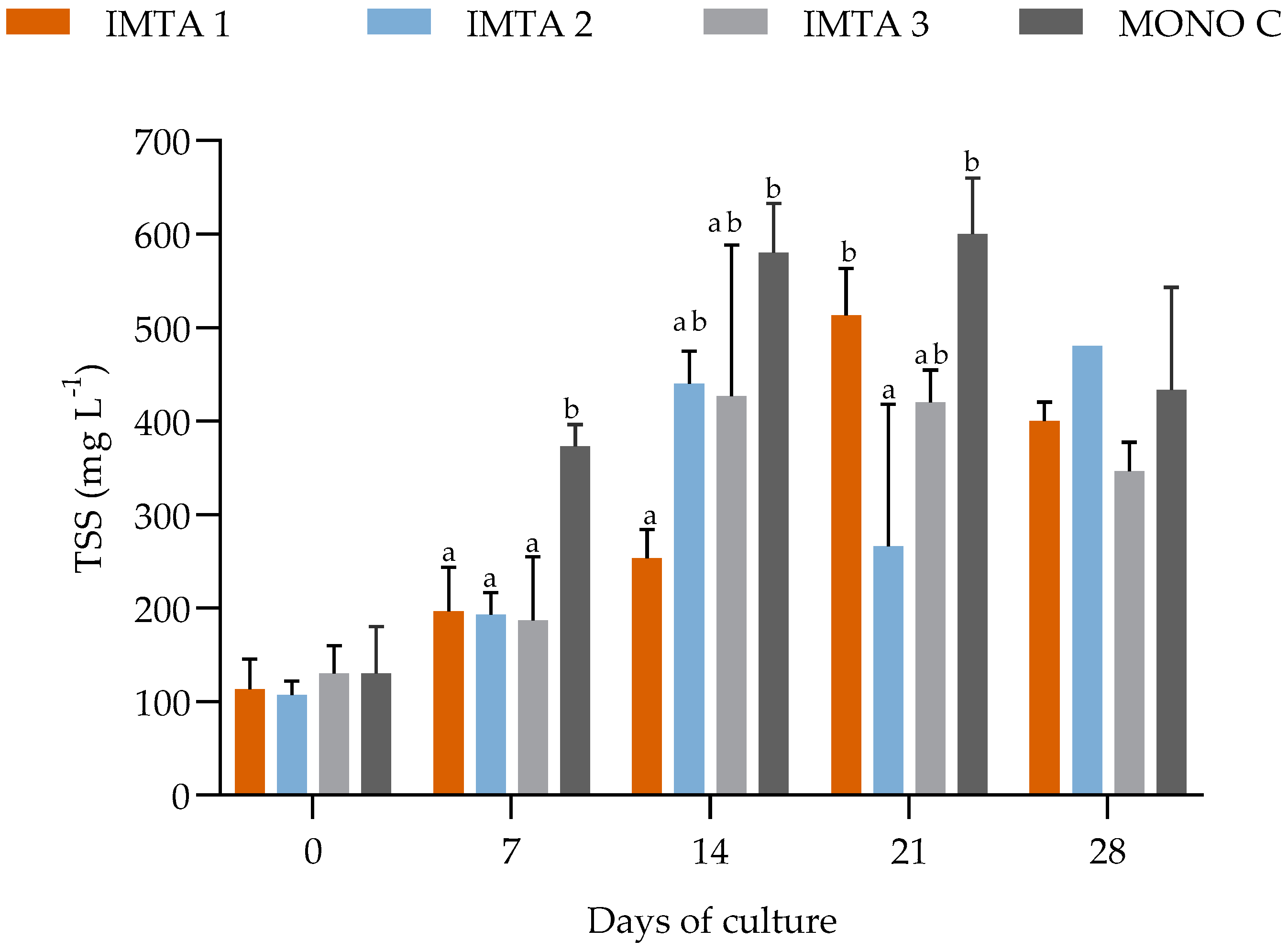
During the 35 days of cultivation in non-climatized greenhouse conditions, the water quality parameters, such as temperature, dissolved oxygen, pH, salinity, phosphate, and nitrite showed no significant difference (p ≥ 0.05) between the treatments (Table 6). However, the introduction of U. lactuca into the shrimp cultivation system resulted in significantly lower mean values of nitrate, turbidity, and settleable solids for treatments IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), and IMTA 3 (3 g Ulva L−1), compared to those found in MONO C (with no Ulva) (Table 6, Figure 3 and Figure 4).

Table 6.
Water quality parameters (mean ± standard deviation) of treatments: the MONO C (with no Ulva), IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), and IMTA 3 (3 g Ulva L−1) treatment groups during the 35 days of the experiment.
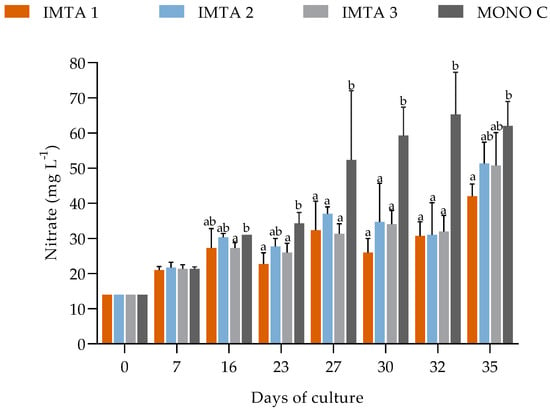
Figure 3.
Variations in the nitrate concentrations in the IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), IMTA 3 (3 g Ulva L−1), and MONO C (with no Ulva) treatment groups during the 35 days of cultivation. a, b = Different lowercase letters represent a significant difference (p ≤ 0.05) on the same days between the treatments after performing one-way ANOVA followed by Tukey’s post-hoc test.
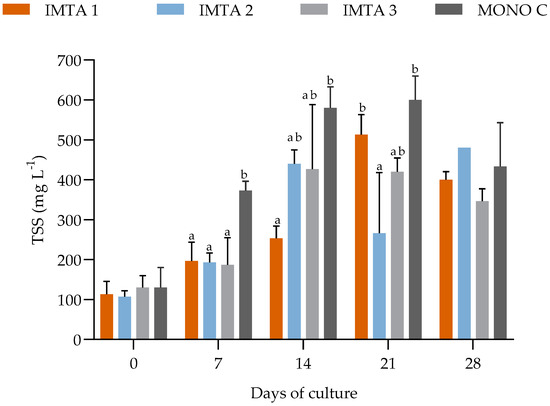
Figure 4.
Variations in the total suspended solids (TSS) in the IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), IMTA 3 (3 g Ulva L−1), and MONO C (with no Ulva) treatment groups during the 35 days of cultivation. a, b = Different lowercase letters on the same day represent a significant difference (p ≤ 0.05) between the treatments after ANOVA followed by Tukey’s post-hoc test.
The alkalinity was higher in treatments with U. lactuca (Table 6), requiring a smaller amount of calcium hydroxide for its maintenance. The IMTA 1 (with 1 g Ulva L−1) treatment required the least amount of calcium hydroxide (90 g in the entire crop), while the IMTA 2 (2 g Ulva L−1), IMTA 3 (3 g Ulva L−1), and MONO C (with no Ulva) treatments required 120, 105, and 157 g of calcium hydroxide to maintain the alkalinity, respectively.
In general, the total ammonium nitrogen (TAN) values showed no significant difference in the treatment groups including algal biomass U. lactuca (p ≤ 0.05) (Table 6). However, TAN in the MONO C (with no Ulva) treatment group showed significantly (p ≤ 0.05) higher values in the first week, with 1.1 ± 0.1 mg L−1 when compared to the treatments including the algae, with 0.7 ± 0.1; 0.7 ± 0.1, and 0.6 ± 0.0 mg L−1 in the treatments IMTA 1, IMTA 2 and IMTA 3, respectively, thus requiring the application of organic carbon, such as molasses, to control of nitrogen in the MONO C treatment.
In the control treatment (MONO C where there was no Ulva), in the first weeks of the experiment, the TSS (total suspended solids) concentrations raised above the established maximum limit of 500 mg L−1 (Figure 4). The clarifiers started to be used to keep optimal shrimp performance as recommended by Gaona et al. [34]. For this reason, during the experiment, the treatments of IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), and IMTA 3 (3 g Ulva L−1) needed 3, 3, and 5 h of clarification, respectively. These three treatment groups had smaller amounts of tailings compared to the treatment MONO C (with no Ulva) which needed 8 h of clarification.
There was no significant difference (p ≤ 0.05) in the shrimp performance parameters between the treatment groups (Table 7).

Table 7.
The performance of shrimp L. vannamei (mean ± standard deviation) in treatments of MONO C (with no Ulva) in the IMTA 1 (with 1 g Ulva L−1), IMTA 2 (2 g Ulva L−1), and IMTA 3 (3 g Ulva L−1) treatment groups during the 35 days of culture.
4. Discussion
The introduction of species in integrated aquaculture is related to their ability to adapt according to system conditions and their interaction with other organisms [35]. In offshore systems, with low water turbidity and water renewal by currents, the integration of the macroalgae into the cultures shows better results. Verdian et al. [4] showed that in these systems the relative growth rate was 14.3 ± 4.3% day−1, probably due to the easy adaptation of the macroalgae to the system because of the similarity with the natural environment. In land based biofloc systems, the environmental characteristics are different. The system is characterized by a high organic load and nutrient accumulation throughout the production cycle. When cultured under unfavorable environmental conditions, such as high solids and nutrient loading in closed systems, such changes may influence the macroalgae’s performance. Different management protocols should be applied for the best adaptation and performance of macroalgae in these systems.
Our laboratory experiment showed that different concentrations of solids in the biofloc system did not cause biomass loss of macroalgae (see Table 2). However, the system did not provide the best conditions to seaweed growth compared to offshore cultivation. The relative growth rate of the macroalgae was similar between the treatments, demonstrating the feasibility of using shrimp culture water in biofloc systems as culture medium. The laboratory environmental conditions were controlled, with a direct light source on the macroalgae and temperature regulation. Another way to favor macroalgae growth under these conditions was the use of transparent experimental units that allow greater light incidence, which may have influenced the processes of light absorption by the macroalgae even with the deposition of solids.
In the laboratory experimental conditions, there was also no increase in solids throughout the experiment. Since there were no shrimp present, the feces and feed waste were not being produced in the culture, which was characterized as a static system. In controlled and fixed conditions, the macroalgae were able to adapt to the different culture media and grow. In contrast, the conditions of the greenhouse experiment simulated real culture conditions, resulting in biomass loss (see Table 4). The integrated culture of shrimp and macroalgae in an intensive system and with a high feed intake generated an increase in solids. Even with intense aeration, the macroalgae culture structure allowed for greater deposition of organic matter on the macroalgae. This effect was smaller in the laboratory experiment, because the macroalgae were loose in the carboy. Such conditions can be stressful to the macroalgae, impacting their growth throughout the culture, which was verified in this experiment with the loss of biomass. A similar result was also observed by Legarda et al. [10], working in a closed system with integrated culture of the macroalgae U. fasciata, the shrimp L. vannamei, and the fish Mugil liza in a biofloc system, confirming the difficulty of adaptation of the macroalgae in this system.
The availability of nutrients in the biofloc system is advantageous for macroalgae cultures [9]. However, the presence of solids can be an inhibiting factor for macroalgae growth. In both experiments, solids were deposited on the macroalgae, and lower concentrations of suspended solids were found in the water. In the laboratory experiment, a decrease in turbidity was observed in the treatment with the highest concentration of solids (400 mg L−1) on the fourth day of culture (Figure 2), showing that most of the solids were deposited on the macroalgae, even with constant aeration.
In the greenhouse experiment, a similar process was verified, with the aggravating factor that the production of solids was persistent due to the presence of the shrimp in the culture. The settleable solids (ml L−1) showed a significant difference between the integrated culture and monoculture treatments. The lowest concentrations of settleable solids were observed in the U. lactuca treatment. As the individuals of this species are sessile organisms, they probably interfered with the dynamic movement of biofloc particles in the water column, causing the deposition of particles on them, unlike in the monoculture where there was no physical barrier for the particles to decant. Brito et al. [9] also found a decrease in solids levels due to the deposition of flocs on the photosynthetic macroalgae leaves, going from a suspended to a decanted solid. This result also induced the need to use the clarifier to control excess solids in the system. The treatments with the presence of U. lactuca required less clarification time compared to the shrimp monoculture treatments. However, removing the macroalgae from the system will cause solids to become suspended in the water again. Excess solids in the water can cause rapid oxygen depletion [36] and growth performance problems to the shrimp [5]. Some alternatives to control solids presented by Khanjani et al. [35] would be the use of organic consumers in integrated cultivation. Thus, the solids would be controlled without the use of clarifiers and there would not be a large amount of solids deposited on the macroalgae.
The deposition of solids on the macroalgae can be a stressing factor for the species, which may prevent the absorption of light and the performance of photosynthesis (see Figure 4). Such factors can trigger reproduction events such as the release of gametes or spores. These events can be initiated due to several environmental factors, such as high temperatures, the concentration of nutrients, and even the short life cycle of the species, thus resulting in the loss of biomass [19]. During the cultivation period, the presence of “ghost tissues” was also observed in U. lactuca as a sign of sporulation. The loss of macroalgae biomass was also verified by Legarda et al. [10] when cultivating U. fasciata in an integrated system, probably because of the different characteristics of the biofloc system compared to the natural environment where the macroalgae were collected.
The von Stosch nutrient solution used in the laboratory experiment is composed of balanced minerals and nutrients [13]. Despite the biomass gain, the relative growth rate of U. lactuca in this experiment in the laboratory condition was lower compared to the studies with a maximum of 16.9% day−1 for U. prolifera, when cultivated in the laboratory conditions with the nutrient medium F/2 [37]. The von Stosch medium used for the cultivation of U. lactuca and the concentration of the medium used may not be adequate to cause low growth of the algal biomass. In addition, the treatment water was not renewed nor were more nutrients added, which could have limited the growth of the algae. For the production of culture media, specific compounds of high economic value are needed, so alternative culture media and better management practices can facilitate the production of U. lactuca.
The increase in the protein content of U. lactuca cultivated in the biofloc system probably occurred because of the nutritional composition of the macroalgae changes according to the physical and chemical factors of the culture environment. For example, higher concentrations of nitrogen available in the culture system, such as in the biofloc system, provide an increase in tissue nitrogen. According to Duke et al. [38], greater availability of nitrogen in the medium results in its absorption and its transformation into protein, stored in the form of amino acids and pigments [32]. Treatments with biofloc effluent contained higher concentrations of nitrate and phosphate than treatment with von Stosch culture medium. This is due to the origin of the biofloc effluent, which came from a shrimp culture with 43 days of cultivation, with a gradual accumulation of nutrients. This high availability of nitrogen in the water favored the increase in the protein content of the macroalgae. The results obtained were superior to those observed by Fong et al. [39], who obtained protein concentrations ranging from 0.6 to 5.4% in algae grown in nutrient solutions in the laboratory. The high protein value of U. lactuca in the present study shows its importance for human food as a food supplement, for muscle tissue reconstruction, and in vegan foods, similar to the previous study conducted by Bleakley and Hayes [40].
Even with 20% biofloc inoculum in the greenhouse experiment, the ammonia concentrations in the first days of cultivation exceeded the concentration of 1 mg L−1 in the tanks without U. lactuca. Although ammonia concentrations were well controlled in the biofloc system, at times throughout the production cycle it may be necessary to add organic carbon to stimulate the development of heterotrophic bacteria in the system [26]. When using a low percentage of inoculum diluted in seawater, the bacteria can undergo adaptation in the system. Together with the feed supply and continuous excretion of the animals, these bacteria were not able to convert all the ammonia in the system and its concentration increased, with the use of molasses in shrimp monoculture being necessary to increase the number of heterotrophic bacteria, in this experiment. Such instability of the bacteria also occurred in the integrated treatment, but due to the absorption capacity by U. lactuca, there was no increase of ammonia concentration in the system. Castelar et al. [41] observed that the genus Ulva tends to have a preference for ammonia, making its assimilation faster and thus controlling nitrogen in the crop as a consequence.
Nitrate was another nitrogenous compound absorbed by U. lactuca. Its accumulation is constant throughout the production cycle in the biofloc system, with it reaching high concentrations. The U. lactuca absorbed the nitrate, as it is the nitrogenous compound with the highest availability in the system, with the best absorption result occurring in the treatment with the lowest density of U. lactuca (1 g L−1). High densities, above 1 g L−1 are likely to increase intraspecific competition, due to U. lactuca overlap in the structure, and negatively affect nutrient absorption. Alencar et al. [15], testing different densities with U. lactuca, also observed that nutrient removal was impaired when the algae density and growth rate increased. Therefore, unlike the gradual accumulation of nitrate that occurred in the monoculture, the treatment with the density of 1 g L−1 of U. lactuca resulted in a lower concentration of nitrate at the end of the cultivation. Due to absorption by the macroalgae, this compound is used for the production of biomass and pigments.
In addition to nutrient absorption, the integration of U. lactuca into shrimp cultivation also interferes with other components in the system. For the biofloc system, calcium carbonate or calcium hydroxide is required to maintain alkalinity [30]. The integrated cultivation of L. vannamei and U. lactuca required a smaller number of corrections with Ca(OH)2, maintaining a more stable level of alkalinity compared to the monoculture treatment where there was no U. lactuca. This possibly occurred due to the absorption of carbon dioxide from the medium by U. lactuca [36]. Chopin [11] comments on the decrease in water acidification due to the absorption of gases by macroalgae, acting on the greenhouse effect. The same pattern can occur in cropping systems integrated with U. lactuca.
The physical and chemical parameters of water quality were kept in the ideal ranges for L. vannamei cultivation, such as temperature [42], salinity [43], dissolved oxygen [44], pH [45], alkalinity [30], and TSS [5]. The shrimp cultivated in this study had a similar performance to that found in the literature for monoculture systems [26]. Therefore, the integration of U. lactuca in the system did not interfere in the performance of the shrimp, with it being environmentally advantageous. In the study of Brito et al. [9], a higher average final weight of the shrimp was observed when cultivated with the macroalgae, possibly due to nutritional advantages provided by the macroalgae to the shrimp. Although the macroalgae are grown in a separate floating structure from the shrimp, they are both grown in the same tank. Therefore, the reduction in the production of biomass served by Ulva can also be explained by its consumption by shrimp. In future studies, it is recommended to observe and monitor if the shrimp consume algae when they are integrated in the same tank as well as when they are in a separated cultivation structure.
In a conventional farming system, there is a significant loss of nitrogen that is not incorporated by the animals and becomes available in the water as a residue that can be toxic and the main source of environmental pollution [46]. The use of macroalgae for the absorption of nutrients from the system has been widely used due to greater sustainability and productivity gain [4]. The use of macroalgae U. lactuca at a density of 1 g L−1 in an integrated system with shrimp L. vannamei in biofloc was also viable due to the incorporation of nitrogen by the algae, resulting in a biomass with higher protein content, in addition to increasing the system productivity and sustainability.
5. Conclusions
The cultivation of macroalgae in biofloc promoted changes in water quality in both experiments. The concentration of total suspended solids decreased in both experiments with macroalgae integration.
When cultivated in an integrated system with shrimp, the addition of macroalgae at a density of 1 g L−1 in the system promoted the absorption of nitrogen, nitrate, and ammonia, generating lower final concentrations of these nutrients at the end of cultivation compared to shrimp monoculture conditions. In addition, the integration of the macroalgae into the system resulted in less use of inputs, such as molasses and calcium hydroxide.
Finally, the use of U. lactuca did not negatively affect the performance of the shrimp. Despite the loss of biomass under the conditions tested in the integrated system, the macroalgae U. lactuca showed potential for the consumption of nutrients available in the system.
Author Contributions
Conceptualization, A.C., L.H.P. and M.H.; methodology, A.C., G.T. and L.H.P.; software, A.C. and L.C.d.O.C.; validation, A.C., L.C.d.O.C., M.H., L.H.P. and G.T.; formal analysis, A.C. and L.C.d.O.C.; investigation, A.C., L.C.d.O.C., M.H., L.H.P. and G.T.; resources, G.T. and L.H.P.; data curation, A.C., L.C.d.O.C. and M.H.; writing—original draft preparation, A.C. and L.C.d.O.C.; writing—review and editing, L.C.d.O.C., M.H., L.H.P. and G.T.; visualization, A.C., L.C.d.O.C., M.H., L.H.P. and G.T.; supervision, G.T. and L.H.P.; project administration, L.C.d.O.C., M.H., L.H.P. and G.T.; funding acquisition, G.T. and L.H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ASTRAL Project—H2020 grant Agreement 863034.
Institutional Review Board Statement
The experiment was approved by the Ethics and Animal Welfare Committee of FURG (Case number 23116.005895/2016-42).
Acknowledgments
The Authors are grateful to the ASTRAL project that has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 863034. The Authors are also thankful to John Bolton of Biological Sciences at the University of Cape Town, South Africa for identification of the species and Amir Neori of National Center for Mariculture at Israel Oceanographic & Limnological Research Ltd, Eilat, Israel for valuable information and suggestions in the preparation and editing of this manuscript. Special thanks to GUABI Nutrition for donating the commercial diets.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Da Silva, K.R.; Wasielesky, W.; Abreu, P.C. Nitrogen and Phosphorus Dynamics in the Biofloc Production of the Pacific White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- Krummenauer, D.; Abreu, P.C.; Poersch, L.; Reis, P.A.C.P.; Suita, S.M.; dos Reis, W.G.; Wasielesky, W. The Relationship between Shrimp (Litopenaeus vannamei) Size and Biofloc Consumption Determined by the Stable Isotope Technique. Aquaculture 2020, 529, 735635. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Santos, D.; Schmachtl, F.; Machado, C.; Fernandes, V.; Bögner, M.; Schleder, D.D.; Seiffert, W.Q.; Vieira, F.N. Heterotrophic, Chemoautotrophic and Mature Approaches in Biofloc System for Pacific White Shrimp. Aquaculture 2021, 533, 736099. [Google Scholar] [CrossRef]
- Verdian, A.H.; Effendi, I.; Budidardi, T.; Diatin, I. Production Performance Improvement of White Shrimp (Litopenaeus vannamei) Culture with Integrated Multi Trophic Aquaculture System in Seribu Islands, Jakarta, Indonesia. Iran. J. Fish. Sci. 2020, 19, 1415–1427. [Google Scholar] [CrossRef]
- Gaona, C.A.P.; de Almeida, M.S.; Viau, V.; Poersch, L.H.; Wasielesky, W. Effect of Different Total Suspended Solids Levels on a Litopenaeus vannamei (Boone, 1931) BFT Culture System during Biofloc Formation. Aquac. Res. 2017, 48, 1070–1079. [Google Scholar] [CrossRef]
- Ahmad, I.; Babitha Rani, A.M.; Verma, A.K.; Maqsood, M. Biofloc Technology: An Emerging Avenue in Aquatic Animal Healthcare and Nutrition. Aquac. Int. 2017, 25, 1215–1226. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The Biofloc Technology (BFT) in Indoor Tanks: Water Quality, Biofloc Composition, and Growth and Welfare of Nile Tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Wasielesky, W.; Bezerra, A.; Poersch, L.; Hoffling, F.B.; Krummenauer, D. Effect of Feeding Frequency on the White Shrimp Litopenaeus vannamei during the Pilot-Scale Nursery Phase of a Superintensive Culture in a Biofloc System. J. World Aquac. Soc. 2020, 51, 1175–1191. [Google Scholar] [CrossRef]
- Brito, L.O.; Arantes, R.; Magnotti, C.; Derner, R.; Pchara, F.; Olivera, A.; Vinatea, L. Water Quality and Growth of Pacific White Shrimp Litopenaeus vannamei (Boone) in Co-Culture with Green Seaweed Ulva lactuca (Linaeus) in Intensive System. Aquac. Int. 2014, 22, 497–508. [Google Scholar] [CrossRef]
- Legarda, E.C.; da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; de Lorenzo, M.A.; Hayashi, L.; do Nascimento Vieira, F. Sea Lettuce Integrated with Pacific White Shrimp and Mullet Cultivation in Biofloc Impact System Performance and the Sea Lettuce Nutritional Composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- Chopin, T. Marine Aquaculture in Canada: Well-Established Monocultures of Finfish and Shellfish and an Emerging Integrated Multi-Trophic Aquaculture (IMTA) Approach Including Seaweeds, Other Invertebrates, and Microbial Communities. Fisheries 2015, 40, 28–31. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Mansilla, A.; Rodriguez, J.P.; Souza, J.M.C.; Rosenfeld, S.; Ojeda, J.; Yokoya, N.S. Growth Responses to Temperature, Salinity and Nutrient Variations, and Biomass Variation and Phenology of Ahnfeltia plicata (Rhodophyta, Ahnfeltiales): A Commercially Interesting Agarophyte from the Magellanic Region, Chile. J. Appl. Phycol. 2014, 26, 1133–1139. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G. Seaweeds for Food and Industrial Applications. In Food Industry; IntechOpen: London, UK, 2013; pp. 735–748. [Google Scholar]
- de Alencar, J.R.; Junior, P.A.H.; Celino, J.J. Cultivo de Camarão Branco Litopenaeus vannamei (Boone, 1931) Com a Macroalga Ulva lacuata Linneaus ( Chlorophyta ) No Tratamento de Efluentes Em Sistema Fechado de Recirculação. Rev. Biol. Ciênc. Terra 2010, 10, 117–137. [Google Scholar]
- Ramos, R.; Vinatea, L.; Santos, J.; Da Costa, R. Tratamiento de Efluentes Del Cultivo de Litopenaeus vannamei Mediante Procesos de Sedimentación, Filtración y Absorción. Lat. Am. J. Aquat. Res. 2010, 38, 188–200. [Google Scholar] [CrossRef]
- El Baz, F.K.; El-Baroty, G.S.; Ibrahim, A.E.; Abd El Baky, H.H. Cytotoxicity, Antioxidants and Antimicrobial Activities of Lipids Extracted from Some Marine Algae. J. Aquac. Res. Dev. 2014, 5, 5–9. [Google Scholar] [CrossRef]
- Turan, G.; Tekogul, H. The Turkish Mezzes Formulated with Protein-Rich Green Sea Vegetable (Chlorophyta), Ulva rigida, Cultured in Onshore Tank System. J. Aquat. Food Prod. Technol. 2014, 23, 447–452. [Google Scholar] [CrossRef]
- Copertino, M.D.S.; Tormena, T.; Seeliger, U. Biofiltering Efficiency, Uptake and Assimilation Rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) Cultivated in Shrimp Aquaculture Waste Water. J. Appl. Phycol. 2009, 21, 31–45. [Google Scholar] [CrossRef]
- Wei, Z.; You, J.; Wu, H.; Yang, F.; Long, L.; Liu, Q.; Huo, Y.; He, P. Bioremediation Using Gracilaria lemaneiformis to Manage the Nitrogen and Phosphorous Balance in an Integrated Multi-Trophic Aquaculture System in Yantian Bay, China. Mar. Pollut. Bull. 2017, 121, 313–319. [Google Scholar] [CrossRef]
- del Río, M.J.; Ramazanov, Z.; García-Reina, G. Ulva rigida (Ulvales, Chlorophyta) Tank Culture as Biofilters for Dissolved Inorganic Nitrogen from Fishpond Effluents. Hydrobiologia 1996, 326, 61–66. [Google Scholar] [CrossRef]
- Guiry, M.D.; Cunningham, E.M. Photoperiodic and Temperature Responses in the Reproduction of North-Eastern Atlantic Gigartina acicularis (Rhodophyta: Gigartinales). Phycologia 1984, 23, 357–367. [Google Scholar] [CrossRef]
- Krummenauer, D.; Peixoto, S.; Cavalli, R.O.; Poersch, L.H.; Wasielesky, W. Superintensive Culture of White Shrimp, Litopenaeus vannamei, in a Biofloc Technology System in Southern Brazil at Different Stocking Densities. J. World Aquac. Soc. 2011, 42, 726–733. [Google Scholar] [CrossRef]
- Unesco. Chemical Methods for Use in Marine Environmental Monitoring; Intergovernmental Oceanographic Commission: Paris, France, 1983. [Google Scholar]
- Bendschneider, K.; Robinson, R.J. A New Spectrophotometric Method for the Determination of Nitrite in Water; Technical Report No. 8; Office of Naval Research: Seattle, WA, USA, 1952. [Google Scholar]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.M.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of Molasses as Carbon Source in Limited Discharge Nursery and Grow out Systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques en Milieu Marin; Centre National Pour L’Exploitation des Océans: Paris, France, 1983. [Google Scholar]
- Baumgarten, M.D.G.Z.; De Barros Rocha, J.M.; Niencheski, L.F.H. Manual de Análises em Oceanografia Química; Universidade Federal do Rio Grande: Rio Grande, Brazil, 1996. [Google Scholar]
- American Public Health Association APHA; American Water Works Association; Water Pollution Control Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Effect of Calcium Hydroxide, Carbonate and Sodium Bicarbonate on Water Quality and Zootechnical Performance of Shrimp Litopenaeus vannamei Reared in Biofloc Technology (BFT) Systems. Aquaculture 2011, 321, 130–135. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Reis, R.P.; Critchley, A.T. In Vitro Cultivation of Three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) Variants (Green, Red and Brown) Exposed to a Commercial Extract of the Brown Alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J. Appl. Phycol. 2010, 22, 101–104. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Alley, M. A Manual Colorimetric Procedure for Measuring Ammonium Nitrogen in Soil and Plant Kjeldahl Digests. Commun. Soil Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 2005; p. 245. [Google Scholar]
- Gaona, C.A.P.; Poersch, L.H.; Krummenauer, D.; Foes, G.K.; Wasielesky, W.J. Effect of Solids Removal on Production of Shrimp. Int. J. Recirc. Aquac. 2011, 12, 54–73. [Google Scholar]
- Khanjani, M.H.; Zahedi, S.; Mohammadi, A. Integrated Multitrophic Aquaculture (IMTA) as an Environmentally Friendly System for Sustainable Aquaculture: Functionality, Species, and Application of Biofloc Technology (BFT). Environ. Sci. Pollut. Res. 2022, 29, 67513–67531. [Google Scholar] [CrossRef]
- Granada, L.; Lopes, S.; Novais, S.C.; Lemos, M.F.L. Modelling Integrated Multi-Trophic Aquaculture: Optimizing a Three Trophic Level System. Aquaculture 2018, 495, 90–97. [Google Scholar] [CrossRef]
- Luo, M.B.; Liu, F.; Xu, Z.L. Growth and Nutrient Uptake Capacity of Two Co-Occurring Species, Ulva prolifera and Ulva linza. Aquat. Bot. 2012, 100, 18–24. [Google Scholar] [CrossRef]
- Duke, C.S.; Litaker, W.; Ramus, J. Effects of the Temperature, Nitrogen Supply and Tissue Nitrogen on Ammonium Uptake Rates of the Chlorophyte Seaweeds Ulva Curvata and Codium decorticatum. J. Phycol. 1989, 25, 113–120. [Google Scholar] [CrossRef]
- Fong, P.; Boyer, K.E.; Desmond, J.S.; Zedler, J.B. Salinity Stress, Nitrogen Competition, and Facilitation: What Controls Seasonal Succession of Two Opportunistic Green Macroalgae? J. Exp. Mar. Biol. Ecol. 1996, 206, 203–221. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Castelar, B.; Reis, R.P.; dos Santos Calheiros, A.C. Ulva lactuca and U. flexuosa (Chlorophyta, Ulvophyceae) Cultivation in Brazilian Tropical Waters: Recruitment, Growth, and Ulvan Yield. J. Appl. Phycol. 2014, 26, 1989–1999. [Google Scholar] [CrossRef]
- Wyban, J.; Walsh, W.A.; Godin, D.M. Temperature Effects on Growth, Feeding Rate and Feed Conversion of the Pacific White Shrimp (Penaeus vannamei). Aquaculture 1995, 138, 267–279. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Effects of Different Salinity Levels on Water Quality, Growth Performance and Body Composition of Pacific White Shrimp (Litopenaeus vannamei Boone, 1931) Cultured in a Zero Water Exchange Heterotrophic System. Ann. Anim. Sci. 2020, 20, 1471–1486. [Google Scholar] [CrossRef]
- Schveitzer, R.; Arantes, R.; Costódio, P.F.S.; do Espírito Santo, C.M.; Arana, L.V.; Seiffert, W.Q.; Andreatta, E.R. Effect of Different Biofloc Levels on Microbial Activity, Water Quality and Performance of Litopenaeus vannamei in a Tank System Operated with No Water Exchange. Aquac. Eng. 2013, 56, 59–70. [Google Scholar] [CrossRef]
- Wasielesky, W.; Krummenauer, D.; Lara, G.; Fóes, G. Cultivo de Camarões Em Sistema de Bioflocos: Realidade e perspectivas. Rev. ABCC 2013, 15, 16–26. [Google Scholar]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated Multitrophic Aquaculture Applied to Shrimp Rearing in a Biofloc System. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).