Abstract
The Sry-related HMG-box (Sox) family is a group of transcriptional regulators that play a critical role in many important developmental processes in both vertebrates and invertebrates. In aquatic animals, the function of Sox genes on sexual development has attracted particular attention. The present study reported on the molecular characterization of a Sox member (PtSoxE) in the swimming crab, Portunus trituberculatus, and tissue distribution analysis showed it was male-specific. Since the highest expression of PtSoxE was found in the androgenic gland (AG), its relation to the insulin-like androgenic gland hormone (IAG) was further investigated. The PtSoxE siRNA caused a significant decrease in IAG expression in both AG and testis, whereas PtSoxE expression could be induced by treating with AG homogenate and rIAG. The result suggested a transcriptional interaction between PtSoxE and IAG. In addition, PtSoxE expression showed a closely positive correlation with several reported spermatogenesis-related genes, suggesting its involvement in the testicular development of P. trituberculatus.
Keywords:
Portunus trituberculatus; Sox gene; insulin-like androgenic gland hormone; testicular development Key Contribution:
A male-specific SoxE was identified and characterized in the swimming crab, Portunus trituberculatus. PtSoxE showed transcriptional interaction with insulin-like androgenic gland hormone, and it was involved in the testicular development of P. trituberculatus.
1. Introduction
The Sry-related HMG-box (Sox) family is a group of transcriptional regulators defined by the presence of a highly conserved high-mobility group (HMG) domain that mediates DNA binding [1]. This domain was first identified as a male determinant in eutherian mammals, which is called the sex-determining region on the Y chromosome (Sry) [2,3]. Since then, numerous Sox proteins were identified and analyzed, and the presence of Sox genes has now been established in almost all metazoans [4]. Based on homology within the HMG domain and structural features outside the domain, the Sox gene family can be subdivided into groups A to K [5]. It has been shown extensively in vertebrates and invertebrates that the Sox family genes are involved in the regulation of many important developmental processes, such as sex determination and differentiation, cell type specification, neurogenesis, and organogenesis [4,6].
Among the many functions of Sox genes, the regulation of sexual development has been the focus of much attention for aquatic animals, particularly in fish. This is not only because aquatic animals often have diverse reproductive strategies and sex determination systems but also because they exhibit substantial sexual dimorphism, which is closely linked to several economic traits, including growth rate and body size [7]. To date, multiple Sox genes have been implicated in the regulation of sex determination, sex differentiation and gonadal development in fish, although their specific roles may vary between species [6]. Most of these Sox genes showed sexually dimorphic expression in the gonads, and in some cases, sex reversal could be achieved through the genetic manipulation of a single Sox gene [8,9,10].
The most extensively studied Sox genes in fish are probably the members of group E, represented by Sox8, Sox9, and Sox10 [6]. In mammals, Sox9 has been proposed as a gene downstream of Sry because it is capable of determining male sex in the absence of Sry [11]. Sox8 resembles Sox9 in its expression profile and biochemical properties, and it can partly substitute for Sox9 [12]. Sox10 is also involved in male determination with the ability to activate the transcriptional target of Sox9 [13]. Despite the diverse sex-forming mechanisms, numerous studies have shown that Sox8 and Sox9 are also important for male development in fish [6]. Sox9 is specifically expressed in testis in many gonochoristic fish species [14,15,16], and in the sequentially hermaphroditic orange-spotted grouper Epinephelus coioides, the Sox9 mRNA increased during the female-to-male transition, suggesting a role in testis differentiation [17]. So far, reports of Sox10 in fish are very limited, but it seems to be a multifunctional gene that is expressed in the gonads of both sexes [18].
As another important group of aquatic animals, crustaceans also have variable sex determination systems, although the mechanisms involved are poorly understood. Nevertheless, it has been widely accepted that the male differentiation of crustaceans is controlled by an insulin-like androgenic gland hormone (IAG) secreted by the male-specific androgenic gland (AG) [19]. Several transcriptional binding sites for Sox were predicted upstream of IAG genes in the oriental river prawn Macrobrachium nipponense [20,21], implying the involvement of Sox genes in the sexual development of crustaceans. Similar to mammals and fishes, the SoxE members were also proposed as sex-related factors in crustaceans. The mRNA of a SoxE gene was mainly located in oocytes and spermatocytes of oriental river prawn, and its expression in males was significantly higher than in females during post-larval development [22]. In the mud crab, Scylla paramamosain, Sox9 was shown to positively regulate the expression of vitellogenesis-inhibiting hormone (VIH) by directly binding to the promoter region, and its RNA silencing resulted in a significant decrease in VIH as well as an increase in vitellogenin expression in the ovary and hepatopancreas of a mature female [23]. However, the regulatory relationship between SoxE and IAG has not been demonstrated previously.
The swimming crab, Portunus trituberculatus, is an economically important crab species in southeast China, and it has been extensively artificially propagated and cultivated. Elucidating the mechanism of sex development will be beneficial to develop sex control technology, which is essential for the aquaculture industry. In the present study, a male-specific SoxE was characterized in P. trituberculatus, and its transcriptional interaction with IAG was revealed using RNA interference and mock IAG treatment. In addition, the putative role and mechanism of SoxE on testicular development was investigated.
2. Materials and Methods
2.1. Experimental Animals
For tissue sampling, wild male crabs (body weight, 280–350 g) were purchased in February, April, August, October, and December 2021 from the local aquatic market in Guoju District, Ningbo City, Zhejiang Province, China. The timepoints were designed to be consistent with the different stages of testicular development of P. trituberculatus according to previous reports [24]. In addition, female crabs were purchased in December when their ovaries were at the vitellogenic stage. The crabs were anesthetized on ice for 10 min before being sacrificed. Tissues including hepatopancreas, muscle, ovary, testis, androgenic gland, heart, brain, thoracic ganglion, eyestalk, and Y-organ were dissected using sterilized scissors and tweezers, after which they were stored in RNA preservation fluid (Cwbiotech, Taizhou, China) at −80 °C until RNA extraction.
For sampling the specimens from different stages of embryonic and larval development, female crabs with near-mature ovaries were purchased from Sanmen, Ningbo in April 2021, and held in a large tank on the aquaculture base of the Institute of Marine and Fisheries of Ningbo, China. Samples from different developmental stages were collected after ovulation according to previous reports [25]. All samples were stored in RNA preservation fluid (Cwbiotech, Taizhou, China) at −80 °C until RNA extraction.
2.2. Extraction of Total RNA and cDNA Synthesis
According to the manufacturer’s instructions of RNA-Solv® reagent (Omega Bio-tek, Norcross, GA, USA), the total RNA from different samples were isolated and dissolved in RNAfree water. The RNA concentrations were determined using a NanoDrop 2000 UV Spectrophotometer (Thermo Fisher Scientific, Cheshire, UK). After removing the genomic DNA by 10× gDNA Remover Mix (Takara, Kyoto, Japan), the first strand of cDNA was synthesized using a HiFiScript gDNA Removal cDNA SynthesisKit (Cwbiotech, Taizhou, China) according to the manufacturer’s protocol and stored at −80 °C until use.
2.3. Molecular Cloning and Characterization
The sequence of PtSoxE was obtained using a keyword-based screening of our RNAseq library (SRR13870346) and validated using a pair of specific PCR primers (Table 1) according to the instruction of Es Taq Master Mix (Cwbiotech, Taizhou, China). The PCR products were separated on a 1.5% agarose gel (Vazyme, Nanjing, China) by electrophoresis. The bands corresponding to the expected size were excised and purified, and the amplicon was ligated into the pMD19-T vector (Takara, Kyoto, Japan). The ligated product was transformed into competent Escherichia coli DH5α cells, and five positive clones were selected for sequencing. The open reading frame (ORF) was predicted using the ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) accessed on 15 July 2020, and the conserved domains of PtSoxE were analyzed using SMART (http://smart.embl-heidelberg.de/) accessed on 15 July 2020. The phylogenetic tree was constructed using the Neighbor-Joining (NJ) method by MEGA7.0 (https://www.megasoftware.net/) accessed on 15 July 2020.

Table 1.
PCR Primers used in this study.
2.4. siRNA Synthesis
Three siRNAs for PtSoxE were designed and synthesized by GenePharma (Shanghai, China) with the following sequences: sense and antisense of siRNA-882, 5′-CGGACUCACUAGAAAGUAUTT-3′ and 5′-AUACUUUCUAGUGAGUCCGTT-3′, respectively; sense and antisense of siRNA-905, 5′- CGUGCUGAGAUGAAUAAGUTT-3′ and 5′-ACUUAUUCAUCUCAGCACGTT-3′, respectively; sense and antisense of siRNA-1016, 5′-GCCACCAUGAAUACUGUAATT-3′, and 5′-UUACAGUAUUCAUGGUGGCTT-3′, respectively. A negative control siRNA (sense and antisense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′, respectively), which shares no homology with the sequence of the target PtSoxE, was employed. All the synthetic siRNAs were dissolved in RNAfree water prior to use.
2.5. Preparation of AG Homogenate and Recombinant IAG
The androgenic gland (AG) homogenate was prepared according to Cui [26]. Briefly, 10 AGs were isolated from male crabs purchased in August, and they were homogenized with mortar and pestle on ice in phosphate-buffered saline. After centrifugation for 20 min (16,000× g, 4 °C), the supernatant was collected, and the procedure was repeated one more time. The obtained supernatant was stored at 4 °C until use. For preparation of the recombinant IAG (rIAG), the ORF region of PtIAG (GenBank accession No. KX168425) was amplified, liganded in the pET-28a-sumo (Merck, Rahway, NJ, USA), and expressed in the Escherichia coli Rosetta (DE3). The fusion protein was mainly expressed in soluble form and was further purified by a HisTrap HP column (Cytiva, Grens, Switzerland). The concentration of rIAG was determined using the Bradford method.
2.6. In Vitro Experiments
The testis and androgenic gland (AG) explants were prepared as previously described [25], and they were precultured in M199 medium for 1 h. Two sets of in vitro experiments were conducted. The siRNA-mediated RNA interference of PtSoxE was performed on testis and AG explants, and the experimental siRNA or NC siRNA were mixed with the Lipofectamine® 2000 Reagent (Invitrogen, Carlsbad, CA, USA) in equal volumes and then added into the culture medium for incubation. The treatments with AG homogenate and recombinant IAG were performed only on testis, and 3.5 nM, 35 nM, and 350 nM of recombinant IAG were used. All samples were collected for RNA extraction after co-culture at 26 °C for 8 h.
2.7. Gene Expression Analysis
The tissue distribution of PtSoxE expression was detected by semiquantitative PCR using a pair of specific primers (Table 1) according to the instruction of Es Taq Master Mix (Cwbiotech, Taizhou, China). Tissues sampled in December were used for analysis. β-actin was used as the positive control. Amplification was performed using a PCR amplifier (Eppendorf, Hamburg, Germany) with the following program: denaturation at 94 °C for 5 min, which was followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 90 s, with a final elongation at 72 °C for 10 min. Other gene expression analysis was performed using quantitative real-time PCR (qPCR). The qPCR was carried out using the ABI 7500 qPCR instrument (Thermofisher, Sunnyvale, CA, USA) according to the manufacturer’s instructions of the SYBR® Premix Ex Taq™ II Kit (Takara, Kyoto, Japan). PCR conditions were as follows: 95 °C for 2 min, which was followed by 40 cycles of 95 °C for 15 s and 56 °C for 20 s. Additional melting curve analysis was performed to confirm the product specificity, during which the temperature increased from 55 to 95 °C at a rate of 0.2 °C/s. The amplification efficiencies of qPCR primers (Table 1) were evaluated using the standard curve analysis by preparation of a 5-point 1:10 dilution series of cDNA. For each sample, the reactions were carried out in triplicate for technical replicates. The β-actin was used to normalize the expression of target genes, and the relative mRNA expression levels were calculated using the comparative Ct (2−ΔΔCt) method [27]. The data were subjected to a one-way analysis of variance (ANOVA), which was followed by Student’s t-test or Tukey’s multiple-group comparison test (SPSS 24.0 software, IBM, Armonk, NY, USA). Significant differences were accepted at p < 0.05.
3. Results
3.1. Molecular Characterization of PtSoxE
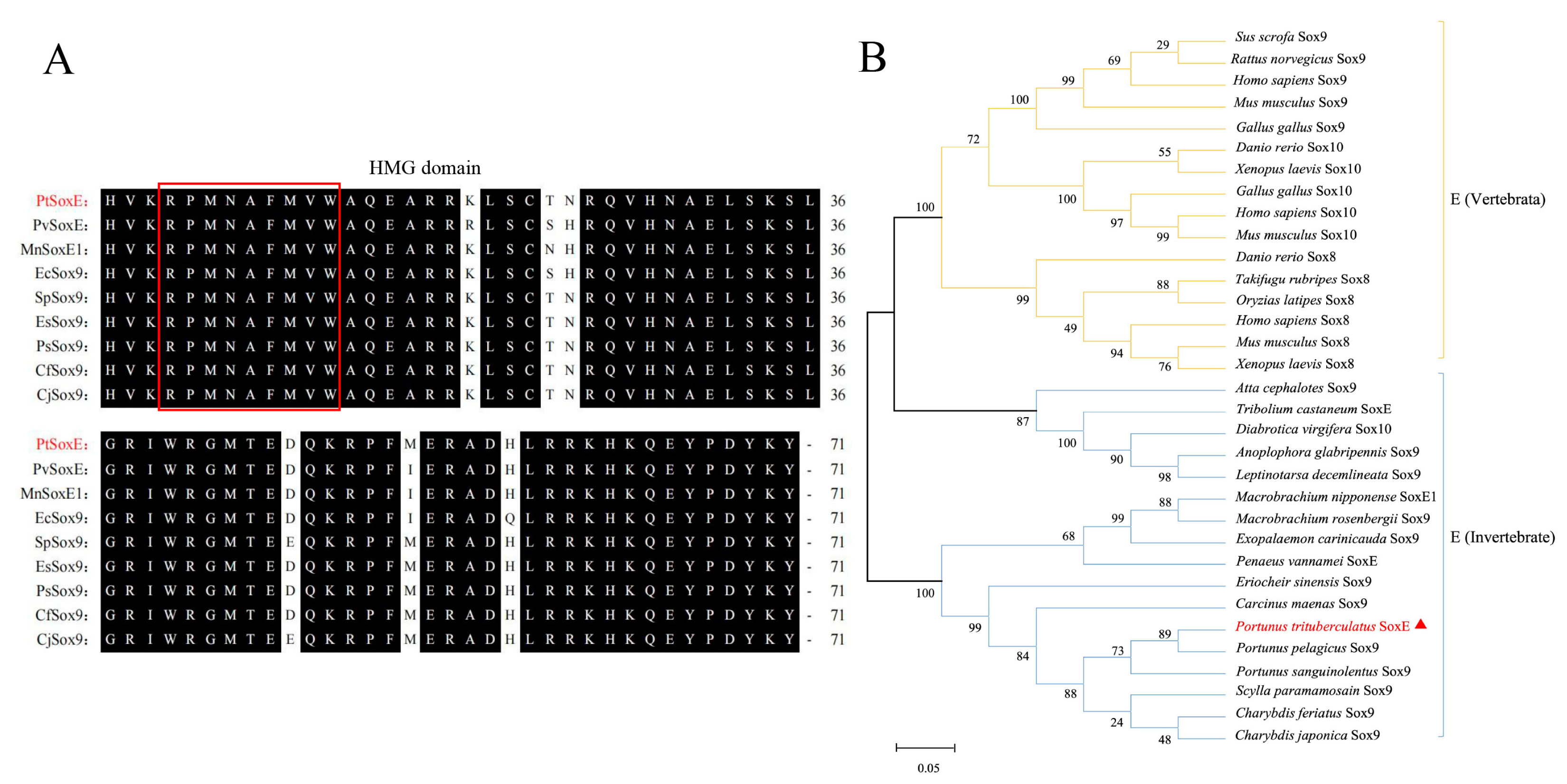
We obtained a 1479 bp PtSoxE cDNA (GenBank accession No. OL9440166) which encodes a protein with 492 amino acids (Supplementary Figure S1). SMART analysis indicated the HMG domain of PtSoxE located from amino acid position 148 to 218, and the multiple sequence alignment with known crustacean SoxE sequences showed that this domain was highly conserved among different species (Figure 1A). It was revealed in phylogenetic analysis that the selected SoxE sequences were divided into three major branches, one for vertebrates, one for insects and one for crustaceans. PtSoxE was clustered within the crustacean branches and showed the closest relation to Sox9 from Portunus pelagicus (Figure 1B).
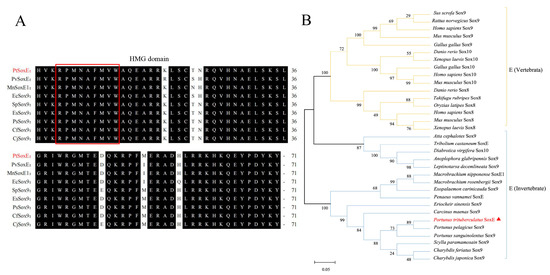
Figure 1.
Multiple sequence alignment of SoxE and phylogenetic analysis. (A) Multiple amino acid sequence alignment based on HMG domains from different crustacean species. The red box indicates the conserved “RPMNAFMVW” motif in Sox proteins. The PtSoxE protein is marked in red. (B) Neighbor-joining phylogenetic tree of representative SoxE proteins from vertebrate and invertebrate. The PtSoxE protein is marked by red triangle. The sequences used in multiple sequence alignment and phylogenetic tree construction are summarized in Table S1.
3.2. Spatial and Temporal Patterns of PtSoxE Expression
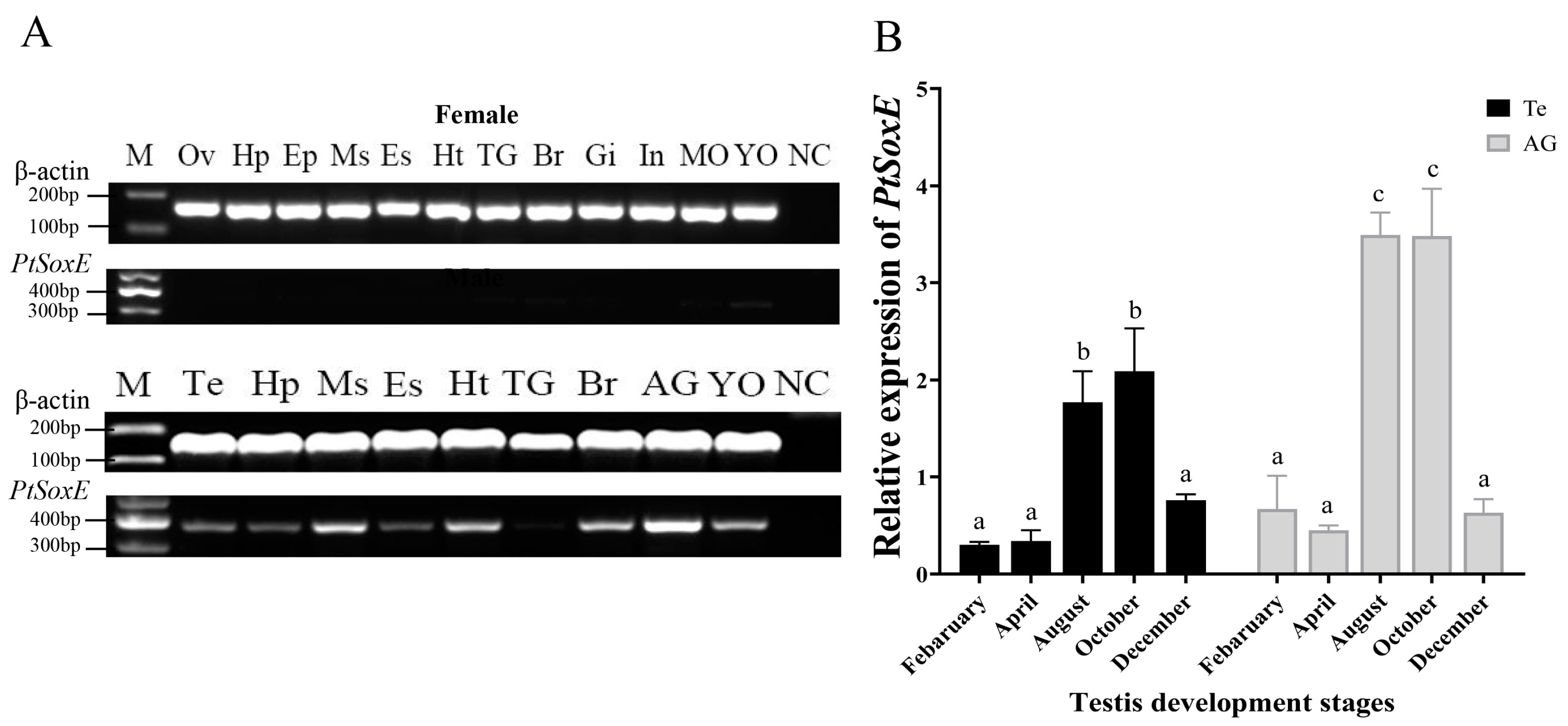
Semiquantitative PCR showed that the PtSoxE gene was specifically expressed in males. PtSoxE exhibited a high level of expression in male tissues, with the highest mRNA levels in AG and followed by the muscle, heart, brain, and Y-organ (Figure 2A). PtSoxE expression was detected in testis but at lower levels when compared with the above tissues.
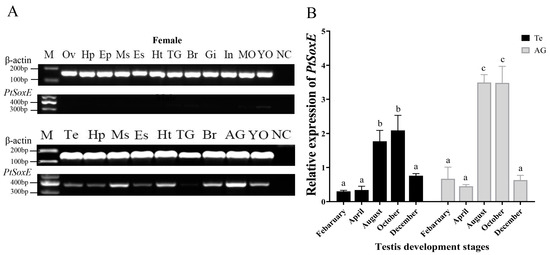
Figure 2.
The tissue distribution and annual expression of PtSoxE. (A) The expression of PtSoxE in different tissues by semiquantitative PCR. Tissues abbreviations are as follows: Ov: Ovary; Hp: Hepatopancreas; Ep: Epidermis; Ms: Muscle; Es: Eyestalks; Ht: Heart; Br: Brain; Gi: Gill; In: Intestines; MO: Mandibular Organ; YO: Y-Organ; Te: Testis; AG: Androgenic Gland; TG: Thoracic Ganglion; Es: Eyestalks; NC: Negative Control. For the full gel picture, see Supplementary Figure S2. (B) The annual expression of PtSoxE in AG and testis by qPCR. Every stage of the collected samples has 4 biological replicates (nr: number of biological replicates, nr = 4). Different letters indicate values with significant difference (p < 0.05).
Given the importance of AG and testis in male sexual development, the annual expression of PtSoxE in these two tissues was further examined. The results showed similar changes in PtSoxE expression in both tissues, which was low in February and May, increased significantly in August and October, and fell back in December (Figure 2B).
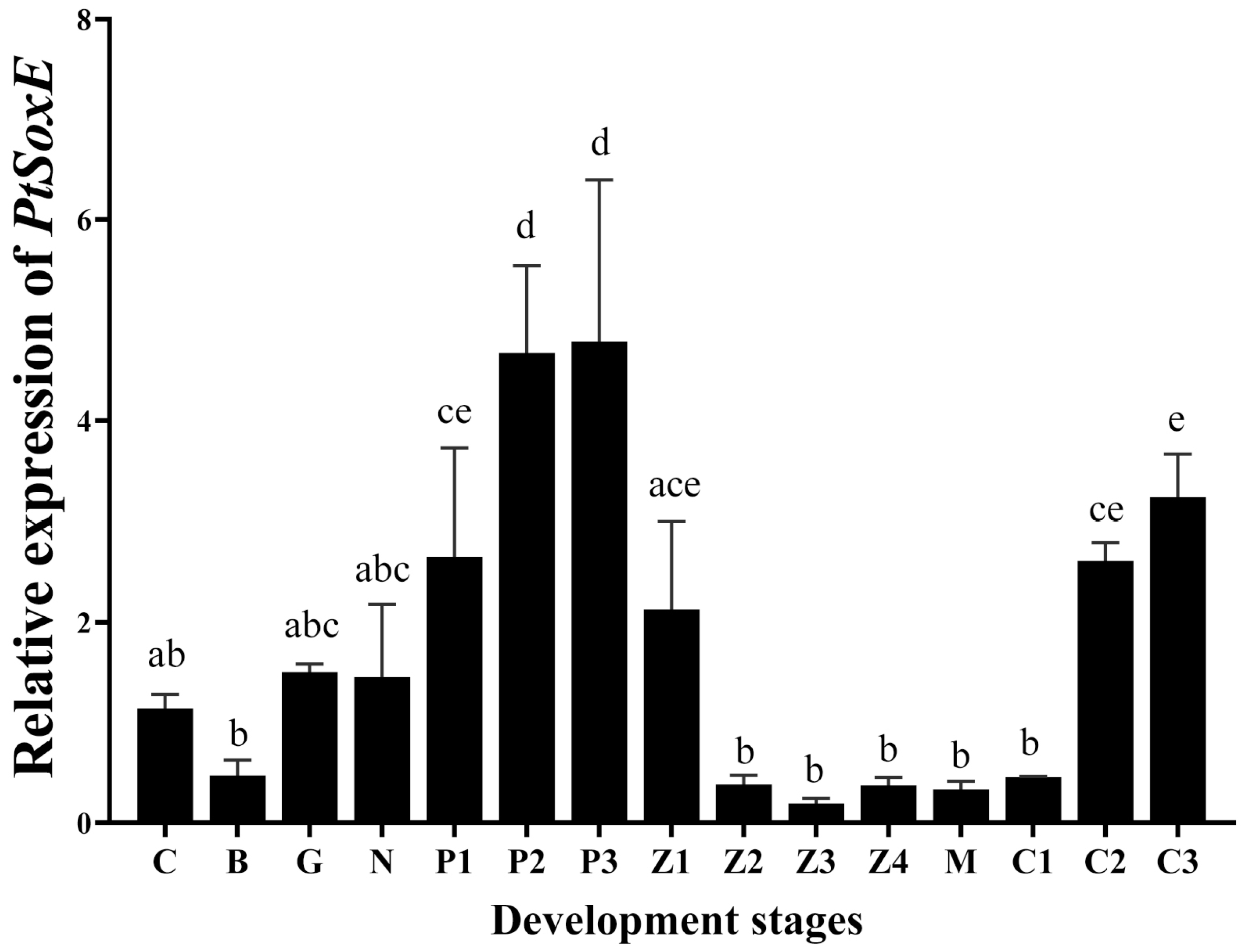
During the development of embryo and larva, PtSoxE gene expression increased from the protozoan I (P1) stage to the maximum level at the P2 and P3 stages. After hatching, PtSoxE mRNA levels decreased rapidly, remaining low during larval development, and returned to high levels at the juvenile crab II stage (C2) (Figure 3).
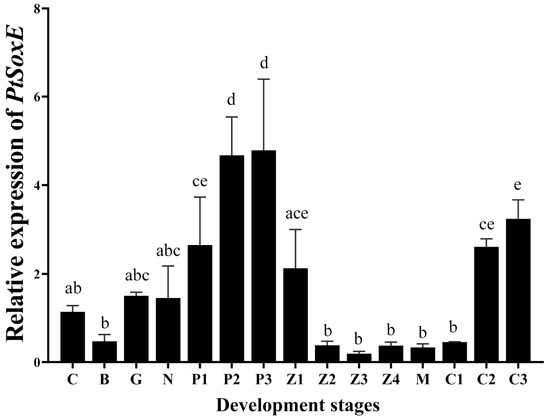
Figure 3.
Temporal expression of PtSoxE during embryonic and larval development. The abbreviations of different developmental stages are as follows: C: cleavage stage; B: blastocyst stage; G: gastrointestinal stage; N: nauplius stage; P1: protozoan stage I; P2: protozoan stage II; P3: protozoan stage III; Z1: zoea stage I; Z2: zoea stage II; Z3: zoea stage III; Z4: zoea stage IV; M: megalopa stage; C1: juvenile crab stage I; C2: juvenile crab stage II; C3: juvenile crab stage III. Every stage sample had 5 biological replicates (nr = 5). Different letters indicate significant difference (p < 0.05).
3.3. Effects of PtSoxE siRNA on Gene Expression in AG and Testis
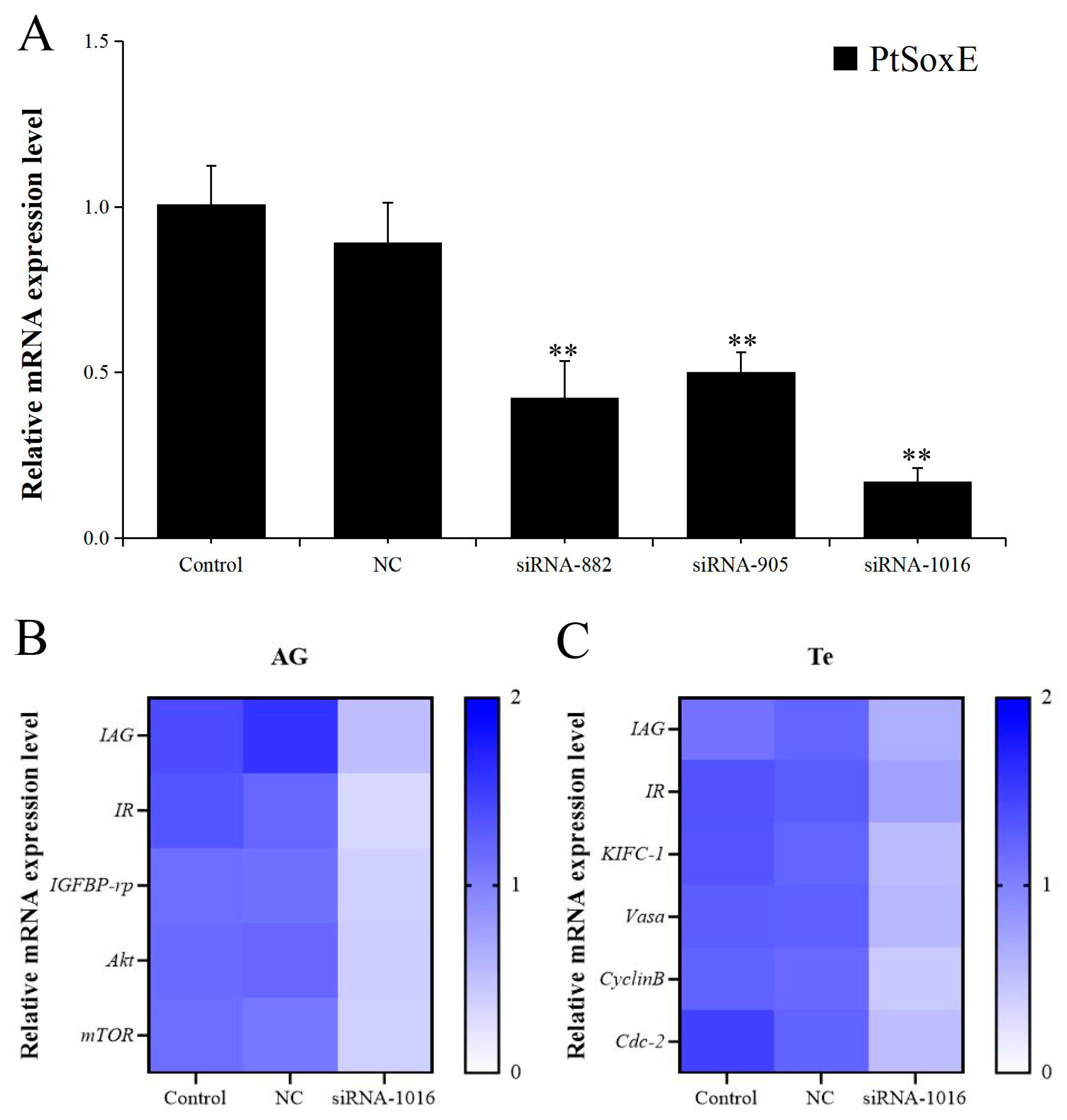
The RNA interference (RNAi) efficiency of the synthetic PtSoxE siRNAs (siRNA-882, siRNA-905, siRNA-1016) was evaluated by examining PtSoxE expression in AG. All three siRNAs caused a significant decrease in PtSoxE expression when compared with the negative control siRNA; the RNAi efficiency was 53% for siRNA-882, 44% for siRNA-905, and 81% for siRNA-1016 (Figure 4A). Therefore, treatment with siRNA-1016 was used for further analysis. In the siRNA-1016-treated AG, the expression of IAG and several insulin pathway genes, including insulin receptor (IR), insulin-like growth factor binding protein-related peptide (IGFBP-rp), protein kinase B (Akt), and mammalian target of rapamycin (mTOR) were also downregulated (Figure 4B). In testis, the siRNA-1016 caused a reduction in the expression of IAG and insulin receptor (IR) as well as the expression of some spermatogenesis-related genes, such as kinesin-like protein (KIFC-1), ATP-dependent RNA helicase (Vasa), CyclinB and cyclin-dependent kinase-2 (Cdc-2) (Figure 4C).
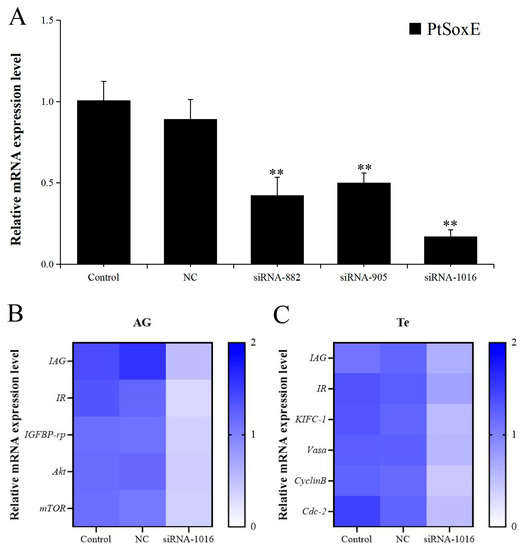
Figure 4.
Effects of PtSoxE siRNA on gene expression in AG and testis. (A) The efficiency of three synthetic PtSoxE siRNAs (siRNA-882, siRNA-905, siRNA-1016). Double asterisk indicates a significant difference from the control group. (B) Heat map of expression levels of insulin pathway genes (IR, IGFBP-rp, Akt, mTOR) in AG after siRNA-1016 treatment. (C) Heat map of expression levels of spermatogenesis-related genes (KIFC-1, Vasa, CyclinB, and Cdc-2) in testis after siRNA-1016 treatment. There were three biological replicates for each treatment experiment (nr = 3). Different colors of the same tested gene between the experimental and control groups indicate significant differences. The darker the color in the heat map, the higher the relative expression of the tested genes and vice versa.
3.4. Effects of AG Homogenate and rIAG on PtSoxE Expression in Testis
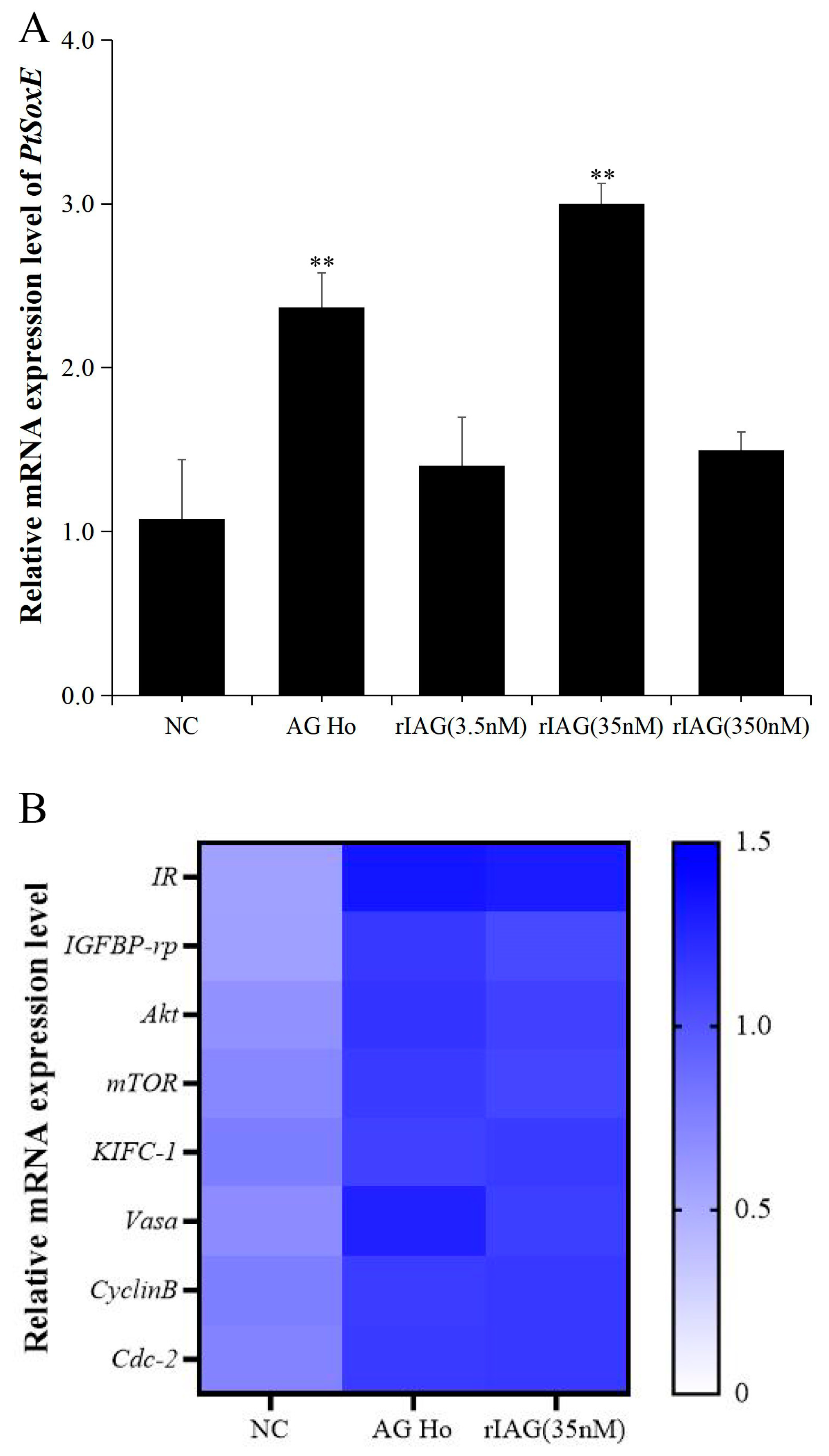
To investigate whether IAG may have a role on PtSoxE expression, the testis explants were treated with AG homogenate and recombinant IAG, and the changes in PtSoxE transcript levels were determined. Significant increases in PtSoxE expression were found in treatments with AG homogenate and 35 nm of rIAG when compared with the control group, while there was no obvious change in PtSoxE expression when treated with 3.5 and 350 nm of rIAG (Figure 5A). In the AG homogenate and 35 nm rIAG groups, genes involved in the insulin pathway (IR, IGFBP-rp, Akt, mTOR) and spermatogenesis (KIFC-1, Vasa, CyclinB, and Cdc-2) were also up-regulated (Figure 5B).
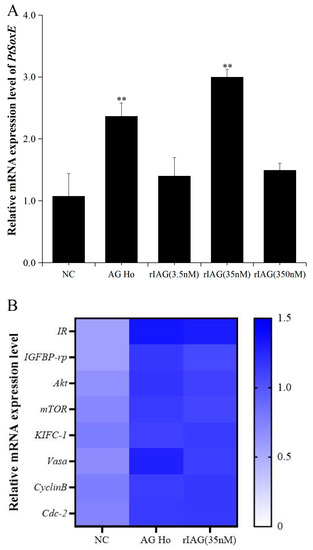
Figure 5.
Effects of AG homogenate and recombinant IAG on gene expression in testis. (A) Effects of AG homogenate (AG Ho) and different concentrations (3.5 nM, 35 nM, 350 nM) of recombinant IAG (rIAG) on expression of PtSoxE in testis. Double asterisk indicates significant difference from the control group. (B) Heat map of expression levels of genes involved in the IIS (insulin/insulin-like growth factor signaling pathway) pathway (IR, IGFBP-rp, Akt, mTOR) and spermatogenesis (KIFC-1, Vasa, CyclinB, Cdc-2) after treatments with AG homogenate and rIAG (35 nM). There were three biological replicates for each treatment experiment (nr = 3). Different colors of the same tested gene between the experimental and control groups indicated significant differences. The darker the color in the heat map, the higher the relative expression of the tested genes and vice versa.
4. Discussion
In this study, a Sox family gene sequence hallmarked with an HMG domain was identified in the swimming crab, P. trituberculatus. Multiple sequence alignment showed that the obtained DNA sequence shares high identities with the known SoxE family sequences of crustaceans and that their HMG domains are highly conserved, thus designating the focal gene as PtSoxE. We noted that many SoxE sequences of crustaceans were designated as Sox9, but this may not be rigorous. In vertebrates, the SoxE subfamily mainly consists of Sox8, Sox9, and Sox10, whereas in invertebrates, only one or two SoxE sequences were identified within a species. In an early report, the Drosophila Sox100B gene was clustered into the SoxE branch but separated from mouse Sox8, Sox9, and Sox10 [6,8,28]. Our phylogenetic analysis supports the separation of vertebrate and invertebrate SoxE proteins, and apparently, invertebrate SoxE genes can be referred to as orthologues of vertebrate Sox8, 9, and 10, but they cannot be specifically classified into any one of these groups.
Tissue distribution analysis showed that PtSoxE was expressed exclusively in male tissues of P. trituberculatus, suggesting that it may play an important role in male sexual development. To the best of our knowledge, this is the first report of male-specific expression of the SoxE gene in crustaceans. In two other reports, the SoxE genes from the mud crab S. paramamosain and the oriental freshwater prawn M. nipponense were expressed in both sexes, and both ovarian- and testicular-related roles were proposed [22,29]. This difference in expression and function seems difficult to explain given that the highly conserved DNA-binding domains (HMG domains) may lead to similar transcriptional regulatory mechanisms. However, it is the case that the expression patterns and physiological functions of fish SoxE genes also vary considerably across species. For instance, in the gonochoristic fishes, many studies have shown that Sox9 is expressed specifically in testis, but its biased expression in ovaries is not uncommon [6].
The PtSoxE transcripts were found to be most abundant in AG, which gives rise to the possibility that it may be related to the IAG. Although potential transcription factor binding sites for Sox proteins had been predicted in the 5′-flanking region of M. nipponense IAG [20,21], the regulatory effect of Sox members on IAG was not previously reported. The siRNA treatments in the present study showed that PtSoxE silencing led to a reduction in IAG expression in the AG and testis, which suggested that PtSoxE might be an upstream regulator of IAG. As an insulin-like peptide, it has been widely accepted that the molecular action of IAG was achieved through the classical IIS pathway [30,31,32]. In our previous report, treatment with IAG dsRNA caused a significant decrease in the expression of several IIS pathway genes, such as IR, IGFBP-rp, Akt, and mTOR [24]. In AG explants, these IIS pathway genes were also down-regulated by PtSoxE silencing. One explanation for this might be the reduction in IAG signaling induced by siRNA treatment, but drawing a firm conclusion requires the demonstration of whether PtSoxE has a direct regulatory role on these IIS pathway genes.
In its annual pattern of expression, PtSoxE in AG was highly expressed in August and October, and the AG was in the secretory phase during this period [33]; at the same time, August and October also are the peak periods of testis development [24], which suggests PtSoxE may have an important regulatory role in male reproductive development. However, according to a previous study by our group, the highest expression of PtIAG occurs during the synthesis phase of AG, which is the time period from May to July [34]. This inconsistency suggested that other mechanisms may be involved in the regulation of IAG expression, but it also raised the question about whether IAG affects PtSoxE expression. Treatments with AG homogenate and rIAG (35 nM) showed a stimulatory regulation of IAG on PtSoxE, and the induced expression of IIS pathway genes inferred a putative activation mechanism. Interestingly, high concentrations of IAG (350 nM) exhibited no effect on PtSoxE expression. Since the hemolymph titer of IAG in P. trituberculatus has not been reported, the physiological significance of this result is unclear.
Although tissue distribution analysis did not show high levels of PtSoxE in the testis, the annual pattern of expression suggested that this may be related to the period in which the samples were collected. A high expression of PtSoxE was observed in August and October, which is a period of rapid spermatogenesis and testicular development. The result was similar to that for Sox9 from S. paramamosain, whose mRNA level was most abundant at the spermatid stage during testicular development [29]. In mammals and fishes, the SoxE family genes have shown their involvement in the differentiation and development of testis [6,12], and in crustaceans, this function seems to be conserved. Our results showed that the silencing of PtSoxE led to the downregulation of several reported spermatogenesis-related genes, including KIFC-1 [35], Vasa [36], CyclinB [37] and Cdc-2 [38], and vice versa when PtSoxE expression was activated, providing molecular evidence of the testicular development role of PtSoxE.
5. Conclusions
To conclude, a male-specific SoxE from P. trituberculatus was identified and characterized in the present study. It was shown by siRNA-mediated gene silencing that PtSoxE positively regulates IAG expression in the AG and testis. On the other hand, PtSoxE could be induced by treating with AG homogenate and rIAG, suggesting a transcriptional interaction between PtSoxE and IAG. PtSoxE expression showed a closely positive correlation with several reported spermatogenesis-related genes, suggesting its involvement in the testicular development of P. trituberculatus. It should be noted that the functional studies of PtSoxE in this study mainly involved its effect on the expression of related genes, but the underlying mechanisms are still largely uncertain. This may require further investigations on the upstream regulatory regions of PtSoxE, IAG, and related genes. In addition, in vivo experiments will also be required to validate the phenotypical effects of PtSoxE on the processes including sex differentiation and spermatogenesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8070351/s1, Figure S1: The cDNA and amino acid sequences of the gene encoding SoxE in P. trituberculatus. The initiation codon (ATG) and the stop codon (TAA) are characterized in bold; the HMG-box is in gray; the Sox family of landmark motifs are all characterized in red font; the glycosylation sites are presented with a box; the phosphorylation sites are indicated with double underline; Figure S2: The full gel picture of semiquantitative PCR; Table S1: Information on other Sox genes.
Author Contributions
D.Z. and X.X. designed the study, Q.J. conducted the research and investigation work, including the design of methodology, then wrote the manuscript, Q.J., D.X. and M.W. performed the experiments, Q.J. and X.X. analyzed the data, X.X. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National natural Science Foundation of China (Grant Nos. 41776165 and 31802265), Natural Science Foundation of Zhejiang province (LY20C190004), and the K. C. Wong Magna Fund of Ningbo University.
Institutional Review Board Statement
In China, ethical approval is not required for experiments on crabs. All the experiments comply with the requirements of the governing regulation for the use of experimental animals in Zhejiang Province (Zhejiang provincial government order No. 263, released on 17 August 2009, effective from 1 October 2010) and the Animal Care and Use Committee of Ningbo University.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamachi, Y.; Kondoh, H. Sox proteins: Regulators of cell fate specification and differentiation. Development 2013, 140, 4129–4144. [Google Scholar] [CrossRef] [PubMed]
- Gubbay, J.; Collignon, J.; Koopman, P.; Capel, B.; Economou, A.; Münsterberg, A.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990, 346, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.-M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Paese, C.L.B.; Leite, D.J.; Schönauer, A.; McGregor, A.P.; Russell, S. Duplication and expression of Sox genes in spiders. BMC Evol. Biol. 2018, 18, 205. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Wang, M.; Zhang, W.; Pan, J.; Qin, Q.; Zhong, L.; Shao, J.; Sun, M.; Jiang, H.; et al. Genome-wide identification, phylogeny and expressional profile of the Sox gene family in channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 28, 17–26. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, B.; Du, H. A review on Sox genes in fish. Rev. Aquac. 2021, 13, 1986–2003. [Google Scholar] [CrossRef]
- Mei, J.; Gui, J.F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef]
- Gonen, N.; Futtner, C.R.; Wood, S.; Garcia-Moreno, S.A.; Salamone, I.M.; Samson, S.C.; Sekido, R.; Poulat, F.; Maatouk, D.M.; Lovell-Badge, R. Sex reversal following deletion of a single distal enhancer of Sox9. Science 2018, 360, 1469–1473. [Google Scholar] [CrossRef]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin, I.T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A.; et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 2014, 5, 4157. [Google Scholar] [CrossRef]
- Schartl, M.; Schories, S.; Wakamatsu, Y.; Nagao, Y.; Hashimoto, H.; Bertin, C.; Mourot, B.; Schmidt, C.; Wilhelm, D.; Centanin, L.; et al. Sox5 is involved in germ-cell regulation and sex determination in medaka following co-option of nested transposable elements. BMC Biol. 2018, 16, 16. [Google Scholar] [CrossRef]
- Canning, C.A.; Lovell-Badge, R. Sry and sex determination: How lazy can it be? Trends Genet. 2002, 18, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Koopman, P. Sex determination: A tale of two Sox genes. Trends Genet. 2005, 21, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Wilhelm, D.; Davidson, T.L.; Knight, D.; Koopman, P. Sox10 gain-of-function causes XX sex reversal in mice: Implications for human 22q-linked disorders of sex development. Hum. Mol. Genet. 2010, 19, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Adolfi, M.C.; Carreira, A.C.O.; Jesus, L.W.O.; Bogerd, J.; Funes, R.M.; Schartl, M.; Sogayar, M.C.; Borella, M.I. Molecular cloning and expression analysis of dmrt1 and Sox9 during gonad development and male reproductive cycle in the lambari fish, Astyanax altiparanae. Reprod. Biol. Endocrinol. 2015, 13, 2. [Google Scholar] [CrossRef]
- Johnsen, H.; Tveiten, H.; Torgersen, J.S.; Andersen, Ø. Divergent and sex-dimorphic expression of the paralogs of the Sox9-Amh-Cyp19a1 regulatory cascade in developing and adult atlantic cod (Gadus morhua L.). Mol. Reprod. Dev. 2013, 80, 358–370. [Google Scholar] [CrossRef]
- Zheng, J.; Jia, Y.; Liu, S.; Chi, M.; Cheng, S.; Gu, Z. Molecular characterization and expression profiles of transcription factor Sox gene family in Culter alburnus. Gene Expr. Patterns 2020, 36, 119112. [Google Scholar] [CrossRef]
- Luo, Y.S.; Hu, W.; Liu, X.C.; Lin, H.R.; Zhu, Z.Y. Molecular cloning and mRNA expression pattern of Sox9 during sex reversal in orange-spotted grouper (Epinephelus coioides). Aquaculture 2010, 306, 322–328. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Zhang, L.; Du, Q.; Sun, J.; Chang, Z. Molecular cloning and mRNA expression pattern of Sox10 in Paramisgurnus dabryanus. Mol. Biol. Rep. 2013, 40, 3123–3134. [Google Scholar] [CrossRef]
- Ventura, T.; Sagi, A. The insulin-like androgenic gland hormone in crustaceans: From a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol. Adv. 2012, 30, 1543–1550. [Google Scholar] [CrossRef]
- Li, F.J.; Jiang, F.W.; Bai, H.K.; Fu, H.T.; Jin, S.B.; Sun, S.M.; Qiao, H.; Zhang, W.Y. Genomic cloning, expression, and single nucleotide polymorphism association analysis of the insulin-like androgenic gland hormone gene in the oriental river prawn (Macrobrachium nipponense). Genet. Mol. Res. 2015, 14, 5910–5921. [Google Scholar] [CrossRef]
- Ma, K.Y.; Li, J.L.; Qiu, G.F. Identification of putative regulatory region of insulin-like androgenic gland hormone gene (IAG) in the prawn Macrobrachium nipponense and proteins that interact with IAG by using yeast two-hybrid system. Gen. Comp. Endocrinol. 2016, 229, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jin, S.; Fu, H.; Qiao, H.; Zhang, W.; Jiang, S.; Gong, Y.; Xiong, Y.; Wu, Y. Functional analysis of a SoxE gene in the oriental freshwater prawn, Macrobrachium nipponense by molecular cloning, expression pattern analysis, and in situ hybridization (de Haan, 1849). 3 Biotech 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Z.; Jia, X.; Zou, Z.; Liang, K.; Wang, Y. Transcriptional Regulation of Vih by Oct4 and Sox9 in Scylla paramamosain. Front. Endocrinol. 2020, 11, 650. [Google Scholar] [CrossRef]
- Wang, M.; Xu, R.; Tu, S.; Yu, Q.; Xie, X.; Zhu, D. Putative Role of CFSH in the eyestalk-AG-testicular endocrine axis of the swimming crab Portunus trituberculatus. Animals 2023, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, X.; Wang, M.; Zheng, H.; Zheng, L.; Zhu, D. Molecular characterization and expression analysis of the inverbrate Dmrt1 homologs in the swimming crab, Portunus trituberculatus (Miers, 1876) (Decapoda, Portunidae). Crustaceana 2020, 93, 851–866. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, H.; Lo, T.S.; Chu, K.H. Inhibitory effects of the androgenic gland on ovarian development in the mud crab Scylla paramamosain. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the Sox family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef]
- Wan, H.; Liao, J.; Zhang, Z.; Zeng, X.; Liang, K.; Wang, Y. Molecular cloning, characterization, and expression analysis of a sex-biased transcriptional factor Sox9 gene of mud crab Scylla paramamosain. Gene 2021, 774, 145423. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, Y.M.; Xu, H.J.; Li, J.W.; Luo, J.Y.; Wang, M.-R.; Ma, W.-M. The characterization and knockdown of a male gonad-specific insulin-like receptor gene in the white shrimp Penaeus vannamei. Aquac. Rep. 2022, 27, 101345. [Google Scholar] [CrossRef]
- Herran, B.; Bertaux, J.; Grève, P. Divergent evolution and clade-specific duplications of the Insulin-like Receptor in malacostracan crustaceans. Gen. Comp. Endocrinol. 2018, 268, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Li, Y.; Zhou, M.; Wang, W. siRNA knockdown of MrIR induces sex reversal in Macrobrachium rosenbergii. Aquaculture 2020, 523, 735172. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, D.F.; Yang, J.F.; Qi, Y. Microstructure and ultrastructure of androgenic gland in wwimming crab Portunus trituberculatus. Fish. Sci. 2010, 29, 193–197. (In Chinese) [Google Scholar] [CrossRef]
- Wang, M.E.; Zheng, H.; Xie, X.; Xu, R.; Zhu, D. Molecular identification and putative role of insulin growth factor binding protein-related protein (IGFBP-rp) in the swimming crab Portunus trituberculatus. Gene 2022, 833, 146551. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.C.; Yang, W.X. Acroframosome-Dependent KIFC1 facilitates acrosome formation during spermatogenesis in the caridean shrimp Exopalaemon modestus. PLoS ONE 2013, 8, e76065. [Google Scholar] [CrossRef]
- He, X.Y.; Fang, X.; Luo, B.Y.; Qiu, G.F. Identification and characterization of a new germline-specific marker vasa gene and its promoter in the giant freshwater prawn Macrobrachium rosenbergii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 259, 110716. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Xiong, Y.; Chen, T.; Jiang, S.; Qiao, H.; Gong, Y.; Wu, Y.; Jin, S.; Fu, H. RNA Interference analysis of the functions of cyclin B in male reproductive development of the oriental river prawn (Macrobrachium nipponense). Genes 2022, 13, 2079. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Xiong, Y.; Chen, T.; Jiang, S.; Qiao, H.; Gong, Y.; Wu, Y.; Jin, S.; Fu, H. RNA interference analysis of the potential functions of cyclin-dependent kinase 2 in sexual reproduction of male oriental river prawns (Macrobrachium nipponense). Aquac. Int. 2023, in press. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).