The Effects of Dietary Fermented Soybean Meal Supplementation on the Growth, Antioxidation, Immunity, and mTOR Signaling Pathway of Juvenile Coho Salmon (Oncorhynchus kisutch)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diet
2.2. Experimental Fish and Acclimatization
2.3. Sampling
2.4. Growth Performance
2.5. Determination of Muscle Composition
2.6. Determination of Biochemical Indexes and Enzymes
2.7. Determination of Gene Expression
2.8. Data Statistics
3. Results
3.1. Growth Performance
3.2. Muscle Composition
3.3. Serum Biochemical Indexes
3.4. Antioxidant Capacity
3.5. Digestion Capacity
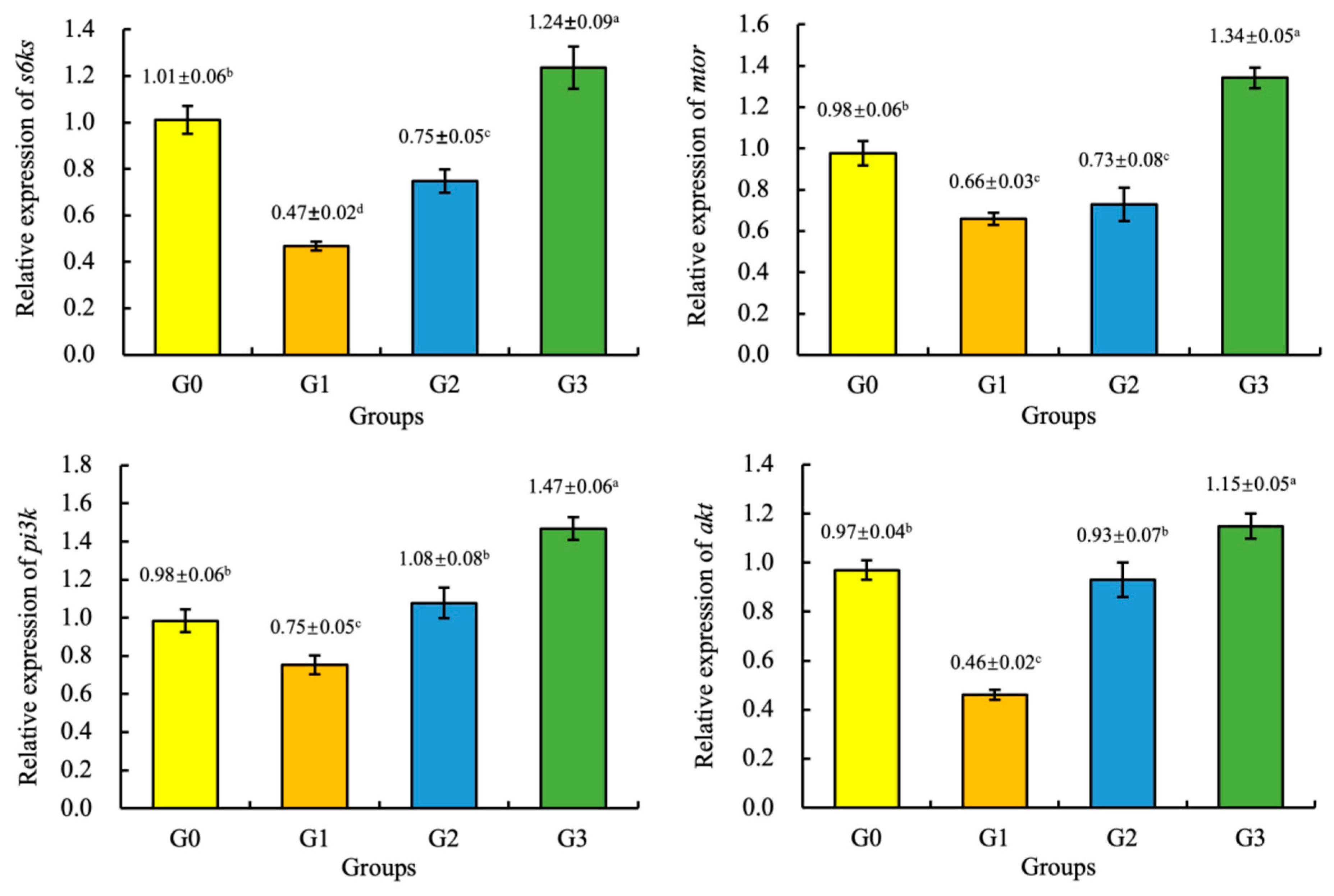
3.6. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janbakhsh, S.; Hosseini Shekarabi, S.P.; Shamsaie Mergan, M. Nutritional value and heavy metal content of fishmeal from the Southwest Caspian Sea. Casp. J. Environ. Sci. 2018, 16, 307–317. [Google Scholar]
- Ramin, G.; Andrea, H. Comparative environmental impact assessment of aquafeed production: Sustainability implications of forage fish meal and oil free diets. Resour. Conserv. Recycl. 2020, 161, 104849. [Google Scholar]
- Fotini, K.; Eleni, F. Aquaculture waste production associated with antinutrient presence in common fish feed plant ingredients. Aquaculture 2018, 495, 295–310. [Google Scholar]
- Zheng, L.; Li, D.; Li, Z.; Kang, L.; Jiang, Y.; Liu, X.; Chi, Y.; Li, Y.; Wang, J. Effects of Bacillus fermentation on the protein microstructure and anti-nutritional factors of soybean meal. Lett. Appl. Microbiol. 2017, 65, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; Zhang, J.J.; Wang, L.; Sun, Y.; Zhang, C. Evaluation of Bacillus pumillus SE5 fermented soybean meal as a fish meal replacer in spotted seabass (Lateolabrax maculatus) feed. Aquaculture 2021, 531, 735975. [Google Scholar] [CrossRef]
- Azarm, H.M.; Lee, S.M. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquacult. Res. 2014, 45, 994–1003. [Google Scholar] [CrossRef]
- Rosario, F.J.; Powell, T.L.; Gupta, M.B.; Cox, L.; Jansson, T. mTORC1 Transcriptional Regulation of Ribosome Subunits, Protein Synthesis, and Molecular Transport in Primary Human Trophoblast Cells. Front. Cell Dev. Biol. 2020, 8, 583801. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Fang, Z.; Guan, Y.; Chen, X.; Loor, J.J.; Jia, H.; Dong, J.; Wang, Y.; Zuo, R.; Liu, G.; et al. High levels of fatty acids inhibit β-casein synthesis through suppression of the JAK2/STAT5 and mTOR signaling pathways in mammary epithelial cells of cows with clinical ketosis. J. Dairy Res. 2020, 87, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lansard, M.; Panserat, S.; Seiliez, I.; Polakof, S.; Plagnes-Juan, E.; Geurden, I.; Médale, F.; Kaushik, S.; Corraze, G.; Skiba-Cassy, S. Hepatic protein kinase B (Akt)–target of rapamycin (TOR)-signalling pathways and intermediary metabolism in rainbow trout (Oncorhynchus mykiss) are not significantly affected by feeding plant-based diets. Br. J. Nutr. 2009, 102, 1564–1573. [Google Scholar] [CrossRef]
- Wacyk, J.; Powell, M.; Rodnick, K.; Overturf, K.; Hill, R.A.; Hardy, R. Dietary protein source significantly alters growth performance, plasma variables and hepatic gene expression in rainbow trout (Oncorhynchus mykiss) fed amino acid balanced diets. Aquaculture 2012, 356–357, 223–234. [Google Scholar] [CrossRef]
- Holowaty, M.N.H.; Lees, M.J.; Abou, S.S.; Paulussen, K.J.M.; Jäger, R.; Purpura, M.; Paluska, S.A.; Burd, N.A.; Hodson, N.; Moore, D.R. Leucine ingestion promotes mTOR translocation to the periphery and enhances total and peripheral RPS6 phosphorylation in human skeletal muscle. Amino Acids 2023, 55, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Yuan, Y.; Cui, X.; Bian, H.; Wen, H.; Zhang, X.; Yu, H.; Wu, H. Pinellia ternata lectin induces inflammation through TLR4 receptor and mediates PI3K/Akt/mTOR axis to regulate NF-κB signaling pathway. Toxicology 2023, 486, 153430. [Google Scholar] [CrossRef]
- Sui, Z.; Wei, C.; Wang, X.; Zhou, H.; Liu, C.; Mai, K.; He, G. Nutrient sensing signaling and metabolic responses in shrimp Litopenaeus vannamei under acute ammonia stress. Ecotoxicol. Environ. Saf. 2023, 253, 114672. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, L.; Chen, B.; Shan, L.; Yuan, S.; Yu, H. Dietary copper requirements of postlarval coho salmon (Oncorhynchus kisutch). Aquacult. Nutr. 2021, 27, 2084–2092. [Google Scholar] [CrossRef]
- He, M.; Yu, Y.; Li, X.; Poolsawat, L.; Yang, P.; Bian, Y.; Guo, Z.; Leng, X. An evaluation of replacing fish meal with fermented soybean meal in the diets of largemouth bass (Micropterus salmoides): Growth, nutrition utilization and intestinal histology. Aquacult. Res. 2020, 51, 4302–4314. [Google Scholar] [CrossRef]
- Shiu, Y.; Hsieh, S.; Guei, W.; Tsai, Y.; Chiu, C.; Liu, C. Using bacillus subtilis e20-fermented soybean meal as replacement for fish meal in the diet of orange-spotted grouper (epinephelus coioides, hamilton). Aquacult. Res. 2015, 46, 1403–1416. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, F.; Guo, M.; Qin, M.; Wang, J.; Yu, H.; Xu, J.; Liu, Y.; Tong, T. Growth Performance, Antioxidant and Immunity Capacity Were Significantly Affected by Feeding Fermented Soybean Meal in Juvenile Coho Salmon (Oncorhynchus kisutch). Animals 2023, 13, 945. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Morteza, Y.; Mansour, T.M.; Jasem, G.M.; Omid, S.; Enric, G. Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 2016, 464, 50–59. [Google Scholar]
- Molinari, G.S.; Wojno, M.; McCracken, V.J.; Kwasek, K. The use of dipeptide supplementation as a means of mitigating the negative effects of dietary soybean meal on Zebrafish Danio rerio. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 257, 110958. [Google Scholar] [CrossRef]
- Valdez-González, F.; Gutiérrez-Dorado, R.; Hernández-Llamas, A.; García-Ulloa, M.; Sánchez-Magaña, L.; Cuevas-Rodríguez, B.; Rodríguez-González, H. Bioprocessing of common beans in diets for tilapia: In vivo digestibility and antinutritional factors. J. Sci. Food Agric. 2017, 97, 4087–4093. [Google Scholar] [CrossRef] [PubMed]
- Skalli, A.; Zambonino-Infante, J.L.; Kotzamanis, Y.; Fabregat, R.; Gisbert, E. Peptide molecular weight distribution of soluble protein fraction affects growth performance and quality in European sea bass (Dicentrarchus labrax) larvae. Aquacult. Nutr. 2014, 20, 118–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Zong, X.; Wang, C.; Shi, C.; Wang, F.; Wang, Y.; Lu, Z. Peptides derived from fermented soybean meal suppresses intestinal inflammation and enhances epithelial barrier function in piglets. Food Agric. Immunol. 2020, 31, 120–135. [Google Scholar] [CrossRef]
- Yao, S.; Agyei, D.; Udenigwe, C.C. Structural Basis of Bioactivity of Food Peptides in Promoting Metabolic Health. Adv. Food Nutr. Res. 2018, 84, 145–181. [Google Scholar]
- Sørensen, S.L.; Park, Y.; Gong, Y.; Vasanth, G.K.; Dahle, D.; Korsnes, K.; Phuong, T.H.; Kiron, V.; Øyen, S.; Pittman, K.; et al. Nutrient Digestibility, Growth, Mucosal Barrier Status, and Activity of Leucocytes From Head Kidney of Atlantic Salmon Fed Marine- or Plant-Derived Protein and Lipid Sources. Front. Immunol. 2021, 11, 623726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, J.; Peng, C.; Yang, Z.; Wang, L.; Lin, J.; Li, L.; Huang, Z.; Gong, B. Co-occurrence network of microbes linking growth and immunity parameters with the gut microbiota in Nile tilapia (Oreochromis niloticus) after feeding with fermented soybean meal. Aquacult. Rep. 2022, 26, 101280. [Google Scholar] [CrossRef]
- Han, F.; Qian, J.; Qu, Y.; Li, Z.; Chen, H.; Xu, C.; Zhang, H.; Qin, J.G.; Chen, L.; Li, E. Partial replacement of soybean meal with fermented cottonseed meal in a low fishmeal diet improves the growth, digestion and intestinal microbiota of juvenile white shrimp Litopenaeus vannamei. Aquacult. Rep. 2022, 27, 101339. [Google Scholar] [CrossRef]
- Wu, P.; Wang, C.; Chen, P.; Hung, J.; Yen, J.; Wu, M. 8-Hydroxydaidzein Downregulates JAK/STAT, MMP, Oxidative Phosphorylation, and PI3K/AKT Pathways in K562 Cells. Biomedicines 2021, 9, 1907. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, J.; Kwak, M.; Go, J.; Son, H.; Kim, D.; Hwang, D. In vitro and in vivo study of effects of fermented soybean product (chungkookjang) on NGF secretion ability and NGF receptor signaling pathway. Lab. Anim. Res. 2013, 29, 113. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, C.; Xu, Y.; Jing, Y.; Yuan, Y.; Wang, L.; Wang, S.; Zhu, X.; Gao, P.; Zhang, Y.; et al. Retraction Note: Alpha-ketoglutarate promotes skeletal muscle hypertrophy and protein synthesis through Akt/mTOR signaling pathways. Sci. Rep. 2020, 10, 18721. [Google Scholar] [CrossRef]
- Da Cruz, T.P.; Michelato, M.; Dal-Pai-Silva, M.; de Paula, T.G.; Macedo, E.A.; Peres, H.; Oliva-Teles, A.; Urbich, A.V.; Furuya, V.R.B.; Furuya, W.M. Growth performance, amino acid retention and mRNA levels of mTORC1 signaling pathway genes in Nile tilapia fingerlings fed protein-bound and crystalline amino acids. Aquaculture 2021, 543, 736953. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, J.; He, S.; Liang, X.; Xie, S.; Xiao, Q. Early leucine programming on protein utilization and mTOR signaling by DNA methylation in zebrafish (Danio rerio). Nutr. Metab. 2020, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Tanaka, M.; Ikeji, T.; Nakanishi, R.; Hirabayashi, T.; Tategaki, A.; Kondo, H.; Ishihara, A.; Fujino, H. Acute effects of lactic acid-fermented and enzyme-digested soybean on protein synthesis via mTOR signaling in the skeletal muscle. Biosci. Biotechnol. Biochem. 2020, 84, 2360–2366. [Google Scholar] [CrossRef]
- Tian, L.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef] [PubMed]
- Masako, M. Chemical Components, Palatability, Antioxidant Activity and Antimutagenicity of Oncom Miso Using a Mixture of Fermented Soybeans and Okara with Neurospora intermedia. J. Nutr. Sci. Vitaminol. 2006, 52, 216–222. [Google Scholar]
- de Oliveira, N.S.; Ha, N.; Da, C.L.; Cipriani, L.A.; Neto, A.T.; Skoronski, E.; Gisbert, E.; Perez, F.T.E.H. Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen). Animals 2022, 12, 690. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Amiri Moghaddam, J.; Shahhosseini, G.; Aramli, M.S. Effect of Dietary Gamma-irradiated and Fermented Soybean Meal on the Growth Performance, Body Composition, and Digestive Enzymes Activity of Caspian Brown Trout, Salmo truttacaspius, Juvenile. J. World Aquacult. Soc. 2016, 47, 830–842. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lu, K.; Wang, L.; Song, K.; Mai, K.; Davis, D.A.; Zhang, C. Replacement of fish meal with Bacillus pumillus SE5 and Pseudozyma aphidis ZR1 fermented soybean meal in diets for Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol. 2018, 84, 987–997. [Google Scholar] [CrossRef]
- Cunha, L.; Besen, K.P.; Oliveira, N.S.; Delziovo, F.R.; Gomes, R.; Cruz, J.M.; Picoli, F.; Gisbert, E.; Skoronski, E.; El Hadi Perez Fabregat, T. Fermented soybean meal can partially replace fishmeal and improve the intestinal condition of goldfish juveniles reared in a biofloc system. Aquacult. Res. 2022, 53, 6803–6815. [Google Scholar] [CrossRef]
- Bradley, J. Effect of Vitamin E and Memantine on Functional Decline in Alzheimer Disease: The TEAM-AD VA Cooperative Randomized Trial. JAMA 2014, 311, 33–44. [Google Scholar]
- Moreno, G.K.L.; Antunes, R.M.; Martínez, Á.M.; Milán, C.J.; Guajardo, F.D. Evaluation of the antioxidant, anti-inflammatory and antihyperglycemic activities of black bean (Phaseolus vulgaris L.) by-product extracts obtained by supercritical CO2. J. Supercrit. Fluids 2022, 183, 105560. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C. How Fermentation Affects the Antioxidant Properties of Cereals and Legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effects of dietary soybean isoflavones on non-specific immune responses and hepatic antioxidant abilities and mRNA expression of two heat shock proteins (HSPs) in juvenile golden pompano Trachinotus ovatus under pH stress. Fish Shellfish Immunol. 2015, 47, 1043–1053. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H. Enhanced biotransformation of soybean isoflavone from glycosides to aglycones using solid-state fermentation of soybean with effective microorganisms (EM) strains. J. Food Biochem. 2019, 43, 12804. [Google Scholar] [CrossRef] [PubMed]
- Dan, Z.; Zhang, W.; Zheng, J.; Gong, Y.; Cui, K.; Mai, K.; Ai, Q. Effects of fishmeal substitution by four fermented soybean meals on growth, antioxidant capacity and inflammation responses of turbot juveniles (Scophthalmus maximus L.). Aquaculture 2022, 560, 738414. [Google Scholar] [CrossRef]
- Maity, A.; Bagchi, D.; De Soumya, K.; Chakraborty, A. Insight into the Lysozyme-Induced Aggregation of Aromatic Amino Acid-Functionalized Gold Nanoparticles: Impact of the Protein Conjugation and Lipid Corona on the Aggregation Phenomena. Langmuir 2023, 2, 3077. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lai, W.; Zhao, Y.; Chen, C. The Exosome-Mediated Cascade Reactions for the Transfer and Inflammatory Responses of Fine Atmospheric Particulate Matter in Macrophages. Environ. Sci. Technol. 2023, 3, 1436. [Google Scholar]
- Li, C.; Tian, Y.; Wang, L.; Zhang, B.; Ma, Q. Effects of Replacing Fishmeal by Raw or Lactobacillus acidophilus-Fermented Soybean Meal on Growth, Intestinal Digestive and Immune-Related Enzyme Activities, Morphology, and Microbiota in Turbot (Scophthalmus maximus L.). Aquacult. Nutr. 2022, 2022, 2643235. [Google Scholar] [CrossRef]
- Choi, D.G.; He, M.; Fang, H.; Wang, X.L.; Li, X.Q.; Leng, X.J. Replacement of fish meal with two fermented soybean meals in diets for rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr. 2019, 26, 37–46. [Google Scholar] [CrossRef]
- Eman, Z.; Engy, R.; Fatma, A.; Hebata, A.M.; Tarek, I. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 38, 149–157. [Google Scholar]
- LópezGarcía, G.; DublanGarcía, O.; ArizmendiCotero, D.; Gómez, O.L.M. Antioxidant and Antimicrobial Peptides Derived from Food Proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef] [PubMed]
- Jahan, H.; Tumpa, I.J.; Qasem, W.A.; Moniruzzaman, M.; Pervin, M.A.; Akter, R.; Omri, A.; Min, T.; Hossain, Z. Evaluation of the Partial Replacement of Dietary Fish Meal With Fermented or Untreated Soybean Meal in Juvenile Silver Barb, Barbonymus gonionotus. Front. Nutr. 2021, 8, 733402. [Google Scholar] [CrossRef]
- Tacchi, L.; Secombes, C.J.; Bickerdike, R.; Adler, M.A.; Venegas, C.; Takle, H.; Martin, S.A.M. Transcriptomic and physiological responses to fishmeal substitution with plant proteins in formulated feed in farmed Atlantic salmon (Salmo salar). BMC Genom. 2012, 13, 363. [Google Scholar] [CrossRef]
- Kongsinkaew, C.; Chittapun, S.; Piyapittayanun, C.; Boonyaratanakornkit, V.; Sooksai, S.; Ajariyakhajorn, K.; Pornpukdeewattana, S.; Krusong, W.; Laemthong, T.; Charoenrat, T. Enhancing Antimicrobial Peptide Productivity in Pichia pastoris (Muts Strain) by Improving the Fermentation Process Based on Increasing the Volumetric Methanol Consumption Rate. Fermentation 2023, 9, 277. [Google Scholar] [CrossRef]
- Fabricio, D.O.S.; Thamires, G.M.; Thaís, J.; Beatriz, D.S.F.; Carlos, A.C.; Mariana, M.; Daniel, P. Soybean meal and fermented soybean meal as functional ingredients for the production of low-carb, high-protein, high-fiber and high isoflavones biscuits. LWT 2018, 90, 224–231. [Google Scholar]
- Paterson, S.; Fernández-Tomé, S.; Galvez, A.; Hernández-Ledesma, B. Evaluation of the Multifunctionality of Soybean Proteins and Peptides in Immune Cell Models. Nutrients 2023, 15, 1220. [Google Scholar] [CrossRef]
- Samurailatpam, S.; Amit, K.R. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar]
- Kühlwein, H.; Merrifield, D.L.; Rawling, M.D.; Foey, A.D.; Davies, S.J. Effects of dietary β-(1,3)(1,6)-D-glucan supplementation on growth performance, intestinal morphology and haemato-immunological profile of mirror carp (Cyprinus carpio L.). J. Anim. Physiol. Anim. Nutr. 2014, 98, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liu, Y.; Ou, W.; Dai, J.; Ai, Q.; Zhang, W.; Mai, K.; Zhang, Y. The protective role of daidzein in intestinal health of turbot (Scophthalmus maximus L.) fed soybean meal-based diets. Sci. Rep. 2021, 11, 3352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, B.; Deng, J.; Yang, Q.; Chi, S.; Pang, A.; Xin, Y.; Liu, Y.; Zhang, H. PRR-Mediated Immune Response and Intestinal Flora Profile in Soybean Meal-Induced Enteritis of Pearl Gentian Groupers, Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂. Front. Immunol. 2022, 13, 814479. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, B.; Zhou, H.; Wang, X.; Pi, X.; Wang, X.; Mai, K.; He, G. Beneficial influences of dietary Aspergillus awamori fermented soybean meal on oxidative homoeostasis and inflammatory response in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2019, 93, 8–16. [Google Scholar] [CrossRef] [PubMed]

| Index | Soybean Meal | Fermented Soybean Meal |
|---|---|---|
| Crude protein (%) | 46.81 ± 0.21 | 55.21 ± 0.33 |
| Crude lipid (%) | 1.84 ± 0.05 | 1.93 ± 0.09 |
| Crude ash (%) | 8.62 ± 0.22 | 7.45 ± 0.19 |
| Moisture (%) | 9.35 ± 0.21 | 9.64 ± 0.15 |
| Polypeptide (%) | 1.39 ± 0.06 | 21.83 ± 0.21 |
| Trypsin inhibitors (mg/g) | 66.13 ± 1.58 | 11.35 ± 0.95 |
| Glycinin (mg/g) | 141.13 ± 3.65 | 24.15 ± 1.75 |
| β-Conglycinin (mg/g) | 105.01 ± 2.74 | 26.39 ± 1.16 |
| Urease (U/g) | 8.01 ± 0.18 | 1.09 ± 0.05 |
| pH | 7.15 ± 0.02 | 6.44 ± 0.04 |
| Ingredients | G0 | G1 | G2 | G3 |
|---|---|---|---|---|
| Fish meal | 40.07 | 25.99 | 25.99 | 25.99 |
| Soybean meal | 0.00 | 21.20 | 10.76 | 0.00 |
| Fermented soybean meal | 0.00 | 0.00 | 7.95 | 15.89 |
| Poultry meal | 10.02 | 10.00 | 10.00 | 10.00 |
| Shrimp meal | 10.02 | 10.00 | 10.00 | 10.00 |
| Wheat middling | 17.88 | 17.84 | 17.84 | 17.84 |
| Starch | 3.02 | 3.01 | 3.01 | 3.01 |
| Cellulose | 8.53 | 0.00 | 2.67 | 5.31 |
| Fish oil | 4.00 | 5.53 | 5.53 | 5.53 |
| Soybean oil | 4.00 | 4.00 | 4.00 | 4.00 |
| Calcium dihydrogen phosphate | 1.01 | 1.01 | 1.01 | 1.01 |
| Mineral premix 1 | 0.52 | 0.52 | 0.52 | 0.52 |
| Vitamin premix 2 | 0.52 | 0.52 | 0.52 | 0.52 |
| Choline | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin C | 0.10 | 0.10 | 0.10 | 0.10 |
| Approximate composition | ||||
| Fish meal protein | 28.00 | 18.00 | 18.00 | 18.00 |
| Poultry meal protein | 6.51 | 6.51 | 6.51 | 6.51 |
| Shrimp meal protein | 6.01 | 6.01 | 6.01 | 6.01 |
| Soybean meal protein | 0.00 | 10.00 | 5.00 | 0.00 |
| Fermented soybean meal protein | 0.00 | 0.00 | 5.00 | 10.00 |
| Flour protein | 2.41 | 2.41 | 2.41 | 2.41 |
| Crude protein | 42.93 | 42.93 | 42.93 | 42.93 |
| Fish meal fat | 4.94 | 3.41 | 3.41 | 3.41 |
| Poultry meal fat | 1.22 | 1.22 | 1.22 | 1.22 |
| Shrimp meal fat | 1.03 | 1.03 | 1.03 | 1.03 |
| Fish oil fat | 4.00 | 5.53 | 5.53 | 5.53 |
| Soybean oil fat | 4.00 | 4.00 | 4.00 | 4.00 |
| Crude fat | 15.21 | 15.21 | 15.21 | 15.21 |
| Ash (%) | 7.26 | 7.64 | 7.54 | 7.33 |
| Moisture (%) | 9.52 | 9.57 | 9.53 | 9.49 |
| Primer Name | Primer Sequence | Product Size (bp) | TM (°C) | GenBank |
|---|---|---|---|---|
| β-actin 1 | F: CCAAAGCCAACAGGGAGAA R: AGGGACAACACTGCCTGGAT | 91 | 60.0 | XM_031822094.1 |
| Mtor 2 | F: CTTCGCCAACTACCTCCG R: TGCCCTCTTCACCTCAAACT | 139 | 60.0 | XM_020506200.2 |
| Akt 3 | F: GCAGCCATCCTACAAATC R: TGAAACAGGGTCCACAAG | 178 | 60.0 | XM_031831237.1 |
| pi3k 4 | F: CCAGTGGCTCAAGGACAAGAACAG R: GGATGAAGGTGGCTACGCAGTATC | 98 | 60.0 | XM_020466892.2 |
| s6ks 5 | F: CAGCACCTGAGCAGCAGTTAGC R: CTCGGATCGGCAGTGGAAAGTTC | 131 | 60.0 | XM_020465833.2 |
| Lyz 6 | F: GCTGTTGTTGTTCTCCTGCTTGTG R: TGTTTCCAGCGTAGCCATCCATTC | 109 | 60.0 | XM_020457770.2 |
| tnf-α 7 | F: GGCGAGCATACCACTCCTCT R: TCGGACTCAGCATCACCGTA | 125 | 60.0 | XM_020497470.2 |
| il-1β 8 | F: GCGACATGGTGCGTTTCCTTTT R: TGTCTACCGGTTTGGTGTAGTCCT | 129 | 60.0 | XM_020475860.2 |
| il-6 9 | F: GAGCTACGTAACTTCCTGGTTGAC R: GCAAGTTTCTACTCCAGGCCTGAT | 134 | 60.0 | XM_020472300.2 |
| Index | G0 | G1 | G2 | G3 | F-Values | p-Values |
|---|---|---|---|---|---|---|
| Initial weight | 152.25 ± 2.96 | 152.25 ± 2.96 | 152.25 ± 2.96 | 152.25 ± 2.96 | 0.000 | 1.000 |
| Final weight | 512.35 ± 10.20 a | 416.26 ± 9.84 c | 472.18 ± 10.61 b | 524.28 ± 8.92 a | 72.549 | 0.000 |
| WGR 1 (%) | 336.52 ± 6.70 a | 273.41 ± 6.46 c | 310.13 ± 6.97 b | 344.35 ± 5.86 a | 72.552 | 0.000 |
| SGR 2 (%/day) | 1.73 ± 0.03 a | 1.44 ± 0.04 c | 1.62 ± 0.04 b | 1.77 ± 0.03 a | 66.083 | 0.000 |
| HIS 3 (%) | 1.42 ± 0.02 c | 1.50 ± 0.02 a | 1.46 ± 0.02 b | 1.44 ± 0.02 bc | 13.176 | 0.002 |
| CF 4 (%) | 1.24 ± 0.03 a | 1.07 ± 0.04 b | 1.11 ± 0.03 b | 1.22 ± 0.02 a | 23.229 | 0.000 |
| FCR 5 | 1.53 ± 0.05 c | 2.09 ± 0.08 a | 1.72 ± 0.06 b | 1.48 ± 0.04 c | 71.489 | 0.000 |
| PER 6 (%) | 2.92 ± 0.07 a | 2.13 ± 0.06 c | 2.56 ± 0.012 b | 2.97 ± 0.07 a | 66.551 | 0.000 |
| SR 7 (%) | 93.33 ± 2.89 | 91.67 ± 2.89 | 93.33 ± 2.89 | 95.00 ± 0.00 | 0.889 | 0.487 |
| Index | G0 | G1 | G2 | G3 | F-Values | p-Values |
|---|---|---|---|---|---|---|
| Moisture (%) | 72.17 ± 0.16 | 72.79 ± 0.88 | 71.74 ± 0.30 | 72.48 ± 0.74 | 1.678 | 0.248 |
| Crude protein (%) | 20.73 ± 0.24 b | 19.67 ± 0.25 c | 20.10 ± 0.33 c | 21.22 ± 0.21 a | 18.181 | 0.001 |
| Crude fat (%) | 5.73 ± 0.22 | 5.59 ± 0.12 | 5.68 ± 0.14 | 5.87 ± 0.18 | 1.431 | 0.304 |
| Ash (%) | 1.48 ± 0.04 | 1.53 ± 0.04 | 1.51 ± 0.04 | 1.46 ± 0.05 | 1.589 | 0.267 |
| Index | G0 | G1 | G2 | G3 | F-Values | p-Values |
|---|---|---|---|---|---|---|
| GLU 1 (mmol/L) | 6.60 ± 0.28 a | 4.20 ± 0.10 c | 4.75 ± 0.26 b | 6.20 ± 0.18 a | 83.585 | 0.000 |
| T-CHO 2 (mmol/L) | 8.02 ± 0.25 a | 3.62 ± 0.22 c | 6.79 ± 0.23 b | 8.15 ± 0.28 a | 220.104 | 0.000 |
| TP 3 (μg/mL) | 62.37 ± 6.03 a | 40.07 ± 2.30 b | 44.00 ± 4.81 b | 63.60 ± 5.29 a | 94.329 | 0.000 |
| ALB 4 (μg/mL) | 31.12 ± 1.07 a | 18.15 ± 2.94 b | 18.35 ± 2.44 b | 33.81 ± 3.05 a | 32.854 | 0.000 |
| AKP 5 (U/L) | 184.71 ± 13.23 a | 73.69 ± 5.50 c | 124.66 ± 16.32 b | 183.21 ± 10.07 a | 377.06 | 0.000 |
| GOT 6 (U/L) | 3.93 ± 0.32 | 3.43 ± 0.35 | 3.55 ± 0.29 | 3.63 ± 0.37 | 1.223 | 0.363 |
| GPT 7 (U/L) | 3.63 ± 0.29 | 3.60 ± 0.40 | 3.99 ± 0.40 | 3.37 ± 0.53 | 1.151 | 0.387 |
| Index | G0 | G1 | G2 | G3 | F-Values | p-Values |
|---|---|---|---|---|---|---|
| T-AOC 1 (mmol/gprot) | 0.70 ± 0.03 a | 0.16 ± 0.01 b | 0.18 ± 0.02 b | 0.72 ± 0.07 a | 212.364 | 0.000 |
| CAT 2 (U/mgprot) | 103.22 ± 15.27 b | 42.46 ± 2.37 d | 68.82 ± 3.74 c | 311.69 ± 23.33 a | 305.071 | 0.000 |
| SOD 3 (U/mgprot) | 266.22 ± 23.26 b | 93.26 ± 2.47 d | 174.91 ± 14.97 c | 387.75 ± 32.57 a | 104.646 | 0.000 |
| MDA 4 (nmol/mgprot) | 2.09 ± 0.23 b | 3.60 ± 0.09 a | 2.21 ± 0.14 b | 2.07 ± 0.08 b | 75.718 | 0.000 |
| Index | G0 | G1 | G2 | G3 | F-Values | p-Values |
|---|---|---|---|---|---|---|
| Pepsin (U/mgprot) | 12.02 ± 0.63 b | 4.18 ± 0.40 d | 8.62 ± 0.82 c | 17.32 ± 1.25 a | 124.414 | 0.000 |
| Trypsin (U/mgprot) | 2268.31 ± 117.36 b | 1028.04 ± 117.85 d | 1566.38 ± 134.81 c | 2586.07 ± 111.89 a | 100.913 | 0.000 |
| α-amylase (U/mgprot) | 0.40 ± 0.04 b | 0.31 ± 0.02 c | 0.41 ± 0.03 b | 0.61 ± 0.07 a | 24.654 | 0.000 |
| Lipase (U/mgprot) | 26.18 ± 1.10 b | 24.71 ± 0.50 c | 26.88 ± 0.69 b | 32.24 ± 1.50 a | 30.939 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Yang, Q.; Guo, M.; Li, F.; Qin, M.; Xie, Y.; Xu, J.; Liu, Y.; Tong, T. The Effects of Dietary Fermented Soybean Meal Supplementation on the Growth, Antioxidation, Immunity, and mTOR Signaling Pathway of Juvenile Coho Salmon (Oncorhynchus kisutch). Fishes 2023, 8, 448. https://doi.org/10.3390/fishes8090448
Zhang Q, Yang Q, Guo M, Li F, Qin M, Xie Y, Xu J, Liu Y, Tong T. The Effects of Dietary Fermented Soybean Meal Supplementation on the Growth, Antioxidation, Immunity, and mTOR Signaling Pathway of Juvenile Coho Salmon (Oncorhynchus kisutch). Fishes. 2023; 8(9):448. https://doi.org/10.3390/fishes8090448
Chicago/Turabian StyleZhang, Qin, Qiuyue Yang, Mengjie Guo, Fanghui Li, Meilan Qin, Yi Xie, Jian Xu, Yongqiang Liu, and Tong Tong. 2023. "The Effects of Dietary Fermented Soybean Meal Supplementation on the Growth, Antioxidation, Immunity, and mTOR Signaling Pathway of Juvenile Coho Salmon (Oncorhynchus kisutch)" Fishes 8, no. 9: 448. https://doi.org/10.3390/fishes8090448
APA StyleZhang, Q., Yang, Q., Guo, M., Li, F., Qin, M., Xie, Y., Xu, J., Liu, Y., & Tong, T. (2023). The Effects of Dietary Fermented Soybean Meal Supplementation on the Growth, Antioxidation, Immunity, and mTOR Signaling Pathway of Juvenile Coho Salmon (Oncorhynchus kisutch). Fishes, 8(9), 448. https://doi.org/10.3390/fishes8090448







