Abstract
Coilia nasus are an important fish resource in the Yangtze River, and the Yangtze River Estuary is a crucial migration pathway for them. In this study, we used otolith microchemistry to analyze the strontium/calcium (Sr/Ca) ratios and Sr contents in the sagitta otolith of C. nasus from the south branch (SB) and north branch (NB) of the Yangtze River Estuary and obtained the diversity of migration patterns and spawning ground distribution for C. nasus. The results indicate that C. nasus from both branches include two types of habitat history: freshwater (F)–brackish water (B) (Type I) and F-B seawater (S) (Type II), with Type I being dominant at 62.50% in both branches. The C. nasus from the SB comprise six migration patterns, while that from the NB has seven migration patterns. The C. nasus from both branches hatch in F habitats. At the time of capture, the C. nasus from the SB predominantly remain in F, accounting for 62.5%, while C. nasus from the NB primarily stay in B, accounting for 87.5%. Throughout the migration process, C. nasus from both branches switch between different habitats, with C. nasus from the NB exhibiting more frequent transitions between F and B, showing a greater reliance on the estuarine brackish habitat. The radius of the first blue region near the core (Lf) and freshwater coefficient (Fc) of the otolith for C. nasus from both branches are divided into three groups: long-distance freshwater dependence (LD), medium-distance freshwater dependence (MD), and short-distance freshwater dependence (SD), with the LD only appearing in the SB, while the NB is primarily represented by MD. There is a correlation between the differences in Lf among different groups of C. nasus and the differences in the distance from the spawning grounds of C. nasus in different sections of the Yangtze River to the estuary (DYRE), reflecting the distribution pattern of C. nasus spawning grounds in different sections of the Yangtze River. This study provides theoretical guidance for the protection of migration pathways and maintenance of spawning grounds for C. nasus, which have significant practical value in the precise management of C. nasus resources in the Yangtze River Estuary.
Keywords:
Coilia nasus; otolith microchemistry; migration pattern; spawning ground distribution; Yangtze River Estuary Key Contribution:
Coilia nasus are an important species resource in the Yangtze River and are migratory fish. During the breeding season, C. nasus migrate through the Yangtze River Estuary to different sections of the Yangtze River and connected lakes for reproduction. In order to investigate the migration patterns and spawning ground distribution of C. nasus from the Yangtze River Estuary, this study collected samples from the SB and NB and analyzed the otolith microchemistry to clarify the migration patterns of the C. nasus breeding population that migrated upstream through the Yangtze River Estuary, as well as the distribution of spawning grounds in different sections of the Yangtze River. This study revealed the diversity of migration patterns and spawning ground distribution of C. nasus from the Yangtze River Estuary. The results of this study have important application value in the protection of C. nasus breeding populations, maintenance of key habitats, and precise management of C. nasus resources.
1. Introduction
Coilia nasus belong to the order Clupeiformes, family Engraulidae, genus Coilia. Widely distributed in the Northwest Pacific, including China, Korea, and Japan [1]. In China, C. nasus are an important migratory economic fish in the middle and lower reaches of the Yangtze River. Every spring, the anadromous C. nasus migrate from the coast through estuaries to lakes along the middle and lower reaches of the Yangtze River to breed [2]. Historically, the C. nasus can be traced as far back as the lake of Dongtinghu (DTH), which is 1400 km from the Yangtze River Estuary [3,4,5]. The highest production reached 3945 t (1973), with 390 t caught at the Yangtze River Estuary alone [6,7]. With the increase in fishing intensity, water pollution, and the construction of dams to block the upstream spawning channel, the production of C. nasus has been decreasing since the 1970s, and its population age and individual size have been miniaturized, the age of sexual maturity has advanced, and there is an increase in the number of resident-type C. nasus [8].
Previous studies have generally categorized C. nasus into three different ecotypes: the anadromous migratory type, freshwater resident type, and landlocked type. However, this classification method cannot fully reflect different ecomorphotypes of C. nasus [9]. The habitat history and migration patterns of C. nasus can be clearly determined via otolith microchemistry [10]. Otoliths are a type of calcareous stone carried by osteichthyes fish themselves, which are highly stable and not easily reabsorbed [11,12]. Otoliths are formed during the incubation period of fertilized eggs, after which they grow around a core deposit and record the life pattern and habitat processes experienced by the fish throughout its life [13]. The core corresponds to the early life stage of fish and records their spawning and natal habitats, while the edge corresponds to capture and recent living environment [14]. There is a positive correlation between the trace element content of otoliths and the elemental content of the environment, especially strontium (Sr) and barium (Ba) [15]. Sr is usually much higher in seawater (S) than in freshwater (F), which leads to an increase in the Sr/Ca ratio of otoliths when fishes enter seawater [16]. Using of otolith microchemistry provides a more objective and accurate reflection of fish habitat history and migratory patterns than relying on jaw-to-head ratios [9].
The current research on the habitat history of C. nasus in the Yangtze River and its connected lakes via otolith microchemistry is relatively extensive [2,5,8]. However, studies on the migration patterns and the spawning ground distribution of C. nasus from the Yangtze River Estuary are limited [17]. This study conducts sampling in both the south branch (SB) and north branch (NB) of the Yangtze River Estuary to analyze the habitat history and migration patterns of C. nasus from different sources. By analyzing the correlation between the radius of the first blue region near the core (Lf) of the otolith and the distance from the spawning grounds to the estuary (DYRE), this study aims to clarify the composition of migratory C. nasus in the Yangtze River Estuary and their upstream migration distribution areas in different sections of the Yangtze River to reveal the distribution pattern of their spawning grounds.
2. Materials and Methods
2.1. Sampling Sites, Sample Collection, and Processing
Sixteen fish samples were investigated, which were collected using a trammel gill net from the south branch (SB: S1, S2, S3, S4, S5, S6, S7, and S8) and north branch (NB: N1, N2, N3, N4, N5, N6, N7, and N8) of the Yangtze River Estuary (Figure 1). Before otolith extraction, the fish samples were routinely measured using an electronic vernier caliper and electronic balance to obtain the total length, body length, and wet weight (Table S1). Eight individuals were collected from the SB, with the average total length, body length, and wet weight being 332.25 ± 28.43 mm, 306.00 ± 26.57 mm, and 110.97 ± 28.42 g; another eight individuals were collected from the NB with values of 245.00 ± 12.39 mm, 221.13 ± 13.17 mm and 33.37 ± 7.44 g, respectively.
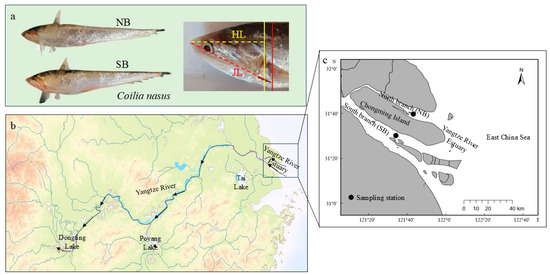
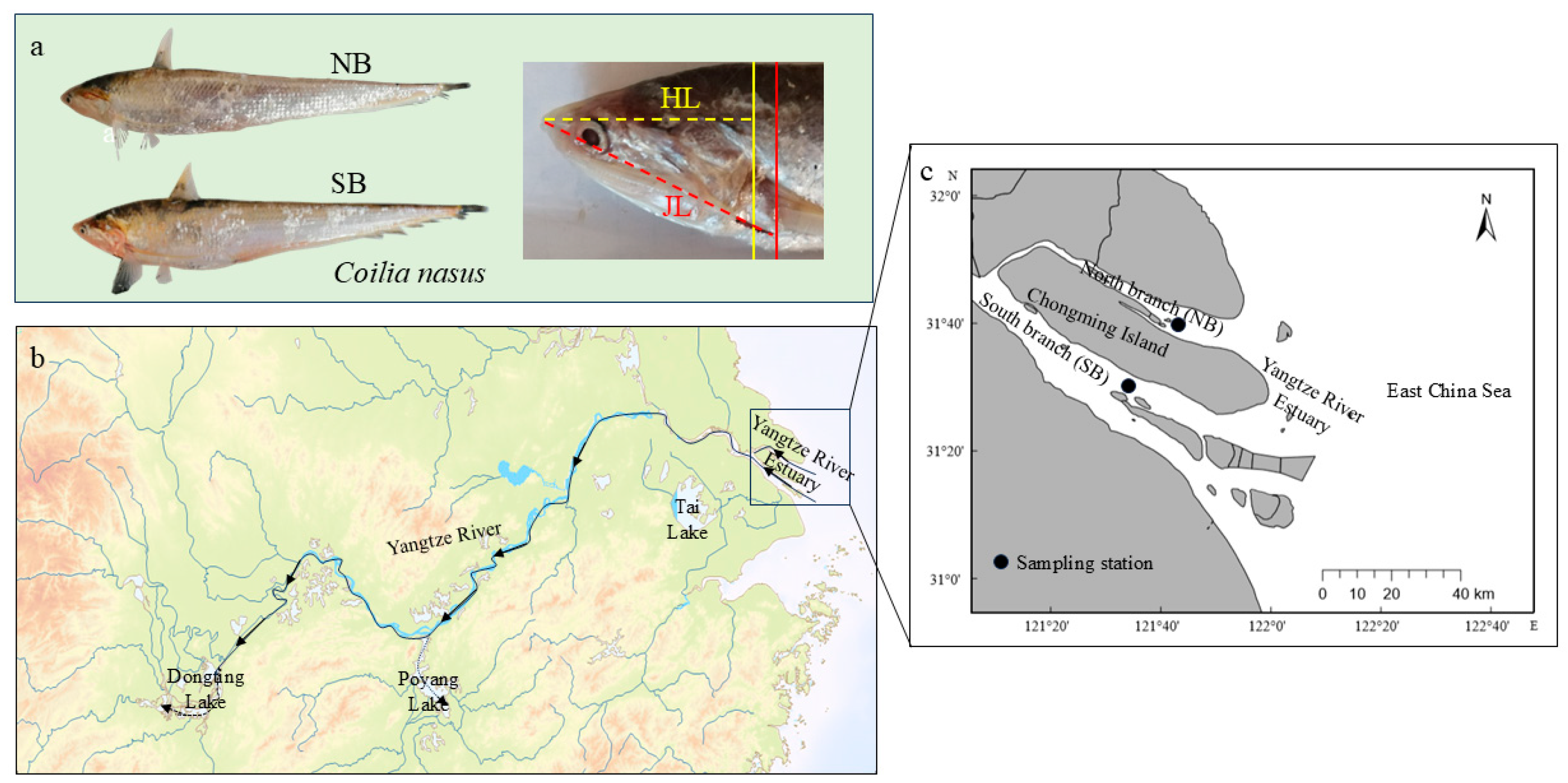
Figure 1.
Coilia nasus (a) sampling sites (b,c): north branch (NB) around 121.814° E, 31.662° N and south branch (SB) around 121.620° E, 31.505° N in the Yangtze River Estuary. The yellow solid line, red solid line, the yellow dashed line, and the red dashed line in (a) indicate the end position of the gill cover and the jawbone, the head length (HL), and the jaw length (JL) (JL > HL), respectively. The arrow in (b) indicates the direction of upstream migration of C. nasus.
2.2. Otolith Treatment and Microchemical Analysis
Right sagittal otoliths were uniformly used for pretreatment and microchemical analysis. The otolith pretreatment and its microchemical analysis process are as follows [17]: The otoliths were embedded in epoxy resin (Epofix, Struers, Copenhagen, Denmark), and then polished with an automated polishing wheel (Discoplan-TS mill, Struers Copenhagen, Denmark) to expose the core. All otoliths were then polished with a LaboPol-35 polishing machine (Struers, Copenhagen, Denmark) to remove scratches from the otolith surface. All the otoliths were washed in a Milli-Q water for 5 min, followed by 6 rinses with Milli-Q water. Afterwards, all the otoliths were dried in an oven at 40 °C and finally coated (36 A for 25 s) using a vacuum coater (EE420, JEOL Ltd., Tokyo, Japan). The quantitative line of Sr/Ca ratios and the X-ray intensity map of Sr content were carried out using an EPMA (JXA-8100, JEOL Ltd., Tokyo, Japan). Calcite (CaCO3) and tausonite (SrTiO3) were used as standards for calibrating the Ca and Sr measurements, respectively [18].
2.3. Data Analysis
The line charts were drawn using Excel 365 MSO, where the X-axis was the radius length of the otolith and the Y-axis was the Sr/Ca ratios, which are customarily calculated and expressed as Sr/Ca × 1000. To reflect the trend of Sr/Ca ratios of the otoliths more directly, the results of the quantitative analysis were trend-shifted using Regime detection 3.2 with a significance level of p = 0.05, a cut-off length of 5, and a Huber’s weight parameter of 1 [19]. To detect the significant differences in the Sr/Ca ratios of the otoliths, IBM SPSS Statistics v.27.0 was used for an independent samples t-test and one-way analysis of variance (ANOVA). Previous studies have confirmed that the otoliths’ Sr/Ca ratios of 3 and 7 for Coilia species are characteristic values for the boundary of different habitats between freshwater (F) and brackish water (B) and between B and saltwater (S), respectively [11,20]. Based on this, the habitats of C. nasus can be divided into three types as F, B, and S. Moreover, the freshwater coefficient (Fc) refers to the ratio of the time spent in F to the whole life history in the early life history. The formula Fc = Lf/LT was used to calculate the freshwater coefficient, where LT is the analytical radius of the entire otolith and Lf is the radius of the first blue region near the core of the otolith [21].
3. Results
3.1. Sr/Ca Ratio Quantitative Line and Sr Content Analysis
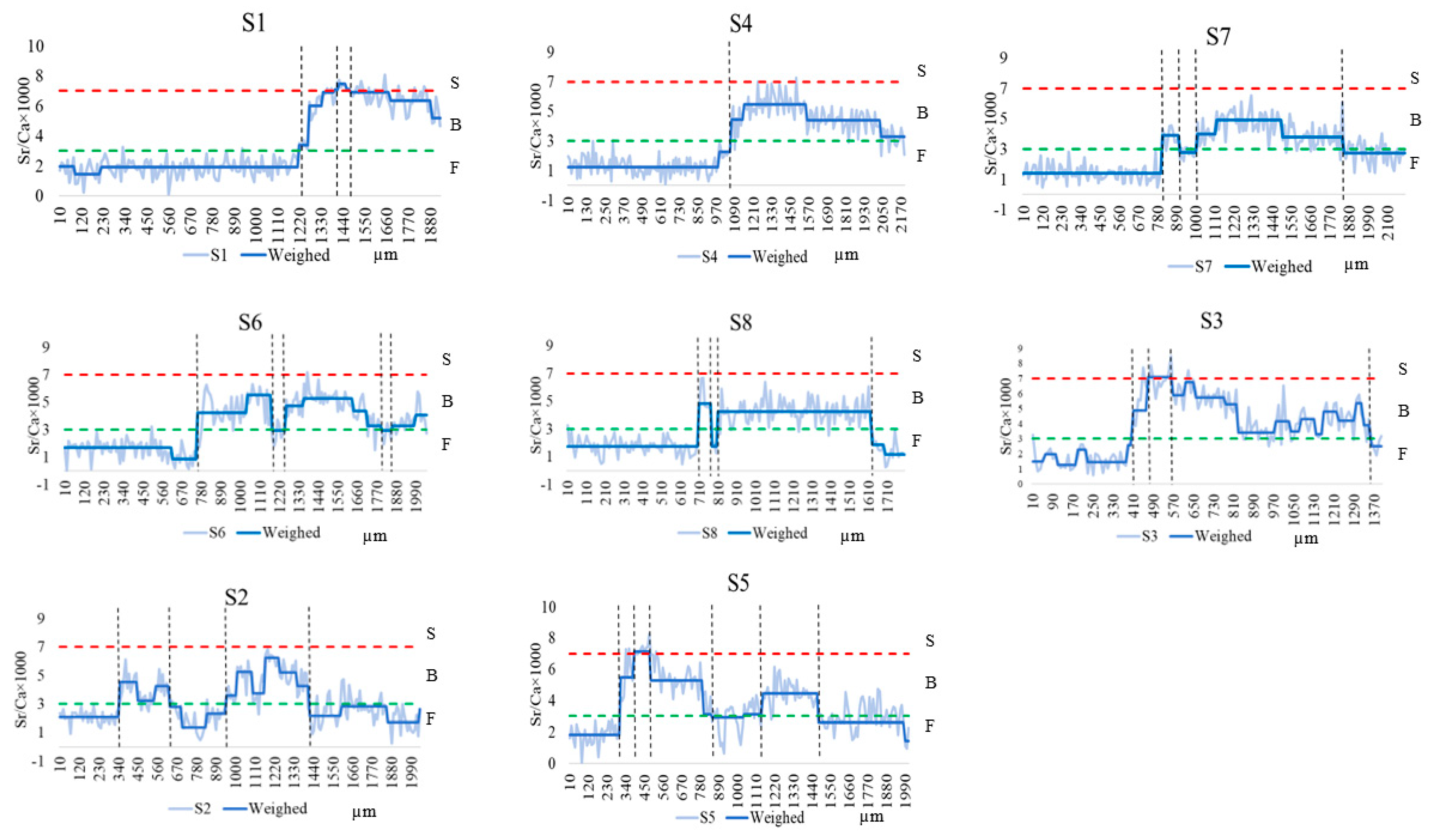
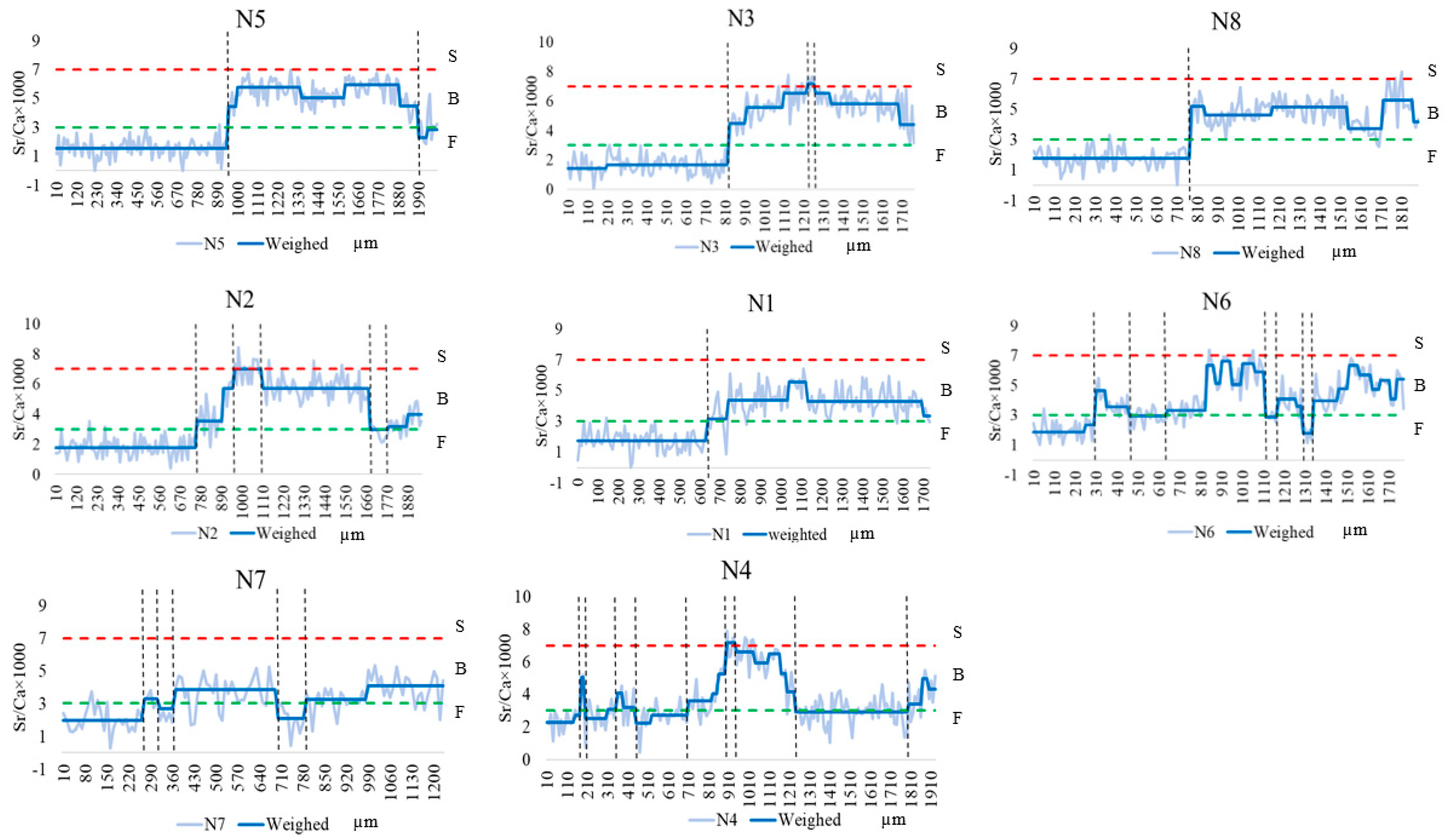
Otoliths of C. nasus from the NB and SB of the Yangtze River Estuary showed various Sr/Ca ratios. As shown in Figure 2, the entire otolith quantification line was divided into three habitats based on the Sr/Ca ratios and their variations, including F, B, and S. In terms of the starting point of the quantification line, C. nasus from both branches with Sr/Ca ratios of less than 3 (below the green dotted line) are distributed in F, all of which are of freshwater origin and the freshwater hatching type. The C. nasus from both branches showed different lengths of the quantitative line in the initial freshwater area (from the start of the quantitative line to the first intersection of the blue solid line with the green dotted line), indicating that their utilization of freshwater habitats varied during the early stages of their life history. From the spanning of the otolith quantification line between different habitats, the presence of individuals spanning two habitats (quantification line values are located below the red dotted line), switching between F and B, and the presence of individuals spanning three habitats (the quantification line intersects with both the green dotted line and the red dotted line), switching between F-B-S, it can be seen that both C. nasus from the SB and NB comprise two types of habitat history types, F-B (Type I) and F-B-S (Type II).
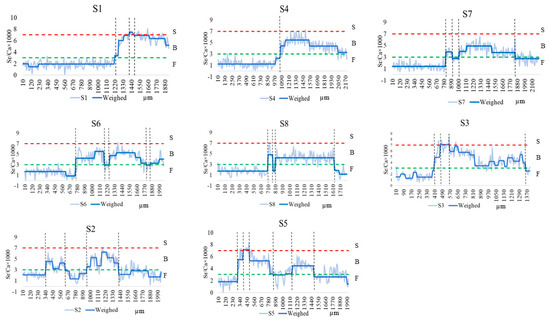
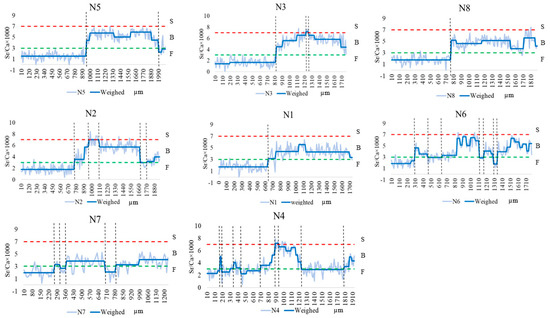
Figure 2.
Fluctuation in the otoliths’ Sr/Ca ratios along line transects from the core (0 µm) to the edge in the otoliths of C. nasus from the SB and NB of the Yangtze River Estuary. The blue solid lines represent the Sr/Ca ratios after trend conversion; the light blue solid lines represent actual Sr/Ca ratios. The red dotted lines represent the border between seawater (S) and brackish water (B). The green dotted lines represent the border between B and freshwater (F).
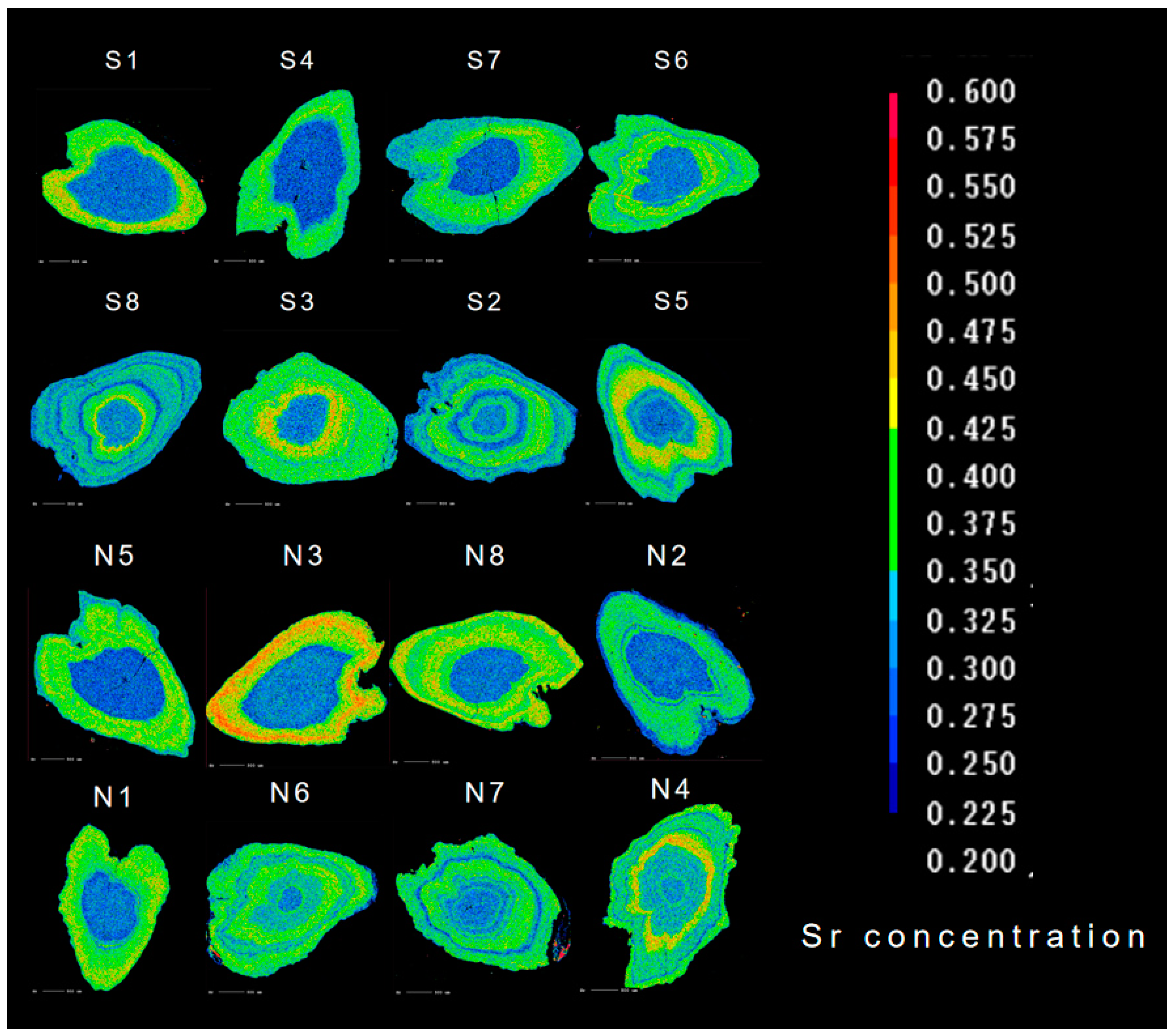
The otoliths of C. nasus from the SB and NB of the Yangtze River Estuary showed various Sr contents. As shown in Figure 3, the otoliths’ Sr content showed different color variations from the core to the edge, and the color variations in the facial distribution of Sr content corresponded to the different habitats shown in the quantitative line analysis (blue corresponds to F, green–yellow corresponds to B, and red corresponds to S) [22]. The core color of the otolith of C. nasus from both branches is blue, indicating that both branches are of freshwater origin. The color distribution in the otolith of some individuals from the SB and NB showed red areas, suggesting that the C. nasus from both branches have seawater habitats. Color changes (alternating between blue and green-yellow) were observed in the otolith of C. nasus from both branches, suggesting that some individuals showed reciprocal movement between F and B, and mainly used brackish water habitats in estuaries.
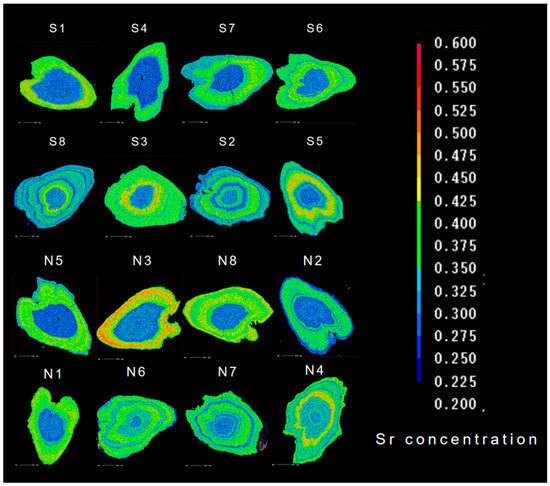
Figure 3.
Two-dimensional imaging using X-ray electron microprobe analysis of the Sr contents in the otoliths of C. nasus from the SB and NB of the Yangtze River Estuary. The values corresponding to Sr contents are represented by 17 colors, from blue (lowest), through green and yellow, to red (highest).
3.2. Habitat History Decomposition and Migration Pattern Analysis
The otoliths of C. nasus from the SB and NB showed various Sr/Ca ratios and Sr contents. The otoliths’ Sr/Ca ratios changed, and the Sr content distribution showed that there were two habitat history types for C. nasus from both branches, but they showed different migration patterns and paths in different types (Figure 2 and Figure 3). In order to explore the diversity of migratory patterns of C. nasus in both branches, the quantitative line was segmented into different stages based on the trend of the otoliths’ Sr/Ca ratios (Figure 2) and the color change in the Sr content distribution (Figure 3). As shown in Table 1, there were significant differences between adjacent stages for the vast majority (92.75%) of individuals, except for N4, suggesting that there was a clear habitat transition between different stages, whereas the difference between stages 2 to 5 (B-F-B-F) for N4 was not significant, suggesting that frequent back-and-forth movements between F and B took place in the early stages of the migratory path for N4. From the changes in Sr/Ca ratios and spectrum colors of different C. nasus in their migratory paths, we can see that there is an S stage (Sr/Ca ratios > 7, red), which is present in S1, S3, and S5 from the SB and in N2, N3, and N4 from the NB. In terms of the number of transitions between different habitats, the frequency of transitions between F and B was higher for S5 (7 times) and S6 (6 times) from the SB and for N4 (10 times), N6 (8 times), N2 (6 times), and N7 (6 times) from the NB, which indicated that some individuals from both branches were highly dependent on the estuary brackish water habitat. The number of highly estuarine-dependent individuals (4) from the NB and the transformation times in the brackish water habitat of the estuary (up to 10 times for N4) were higher than that from the SB (with the number of 2, up to 7 times for S5), which show that individuals from the NB are more dependent on estuarine brackish habitats.

Table 1.
Changes in Sr/Ca ratios in otoliths of C. nasus from SB and NB of Yangtze River Estuary.
Based on the decomposition of the habitat history of C. nasus in Table 1 and the analysis of migration paths, it can be seen that, between the two types of habitat history of C. nasus from both branches, Type I (F-B) was dominant, accounting for 62.5%. There were three migratory patterns in Type I for C. nasus from the SB, with F-B-F-B-F being dominant (37.5%). There were four migratory patterns in Type I for C. nasus from the NB, with F-B being the most preferred (25.0%). In terms of migratory paths, the migratory starting point for C. nasus from both branches was F, and the end point of the habitat history included F and B, with F dominating for C. nasus from the SB, accounting for 62.5%, and B dominating for C. nasus from the NB, accounting for 87.5% (Table S2).
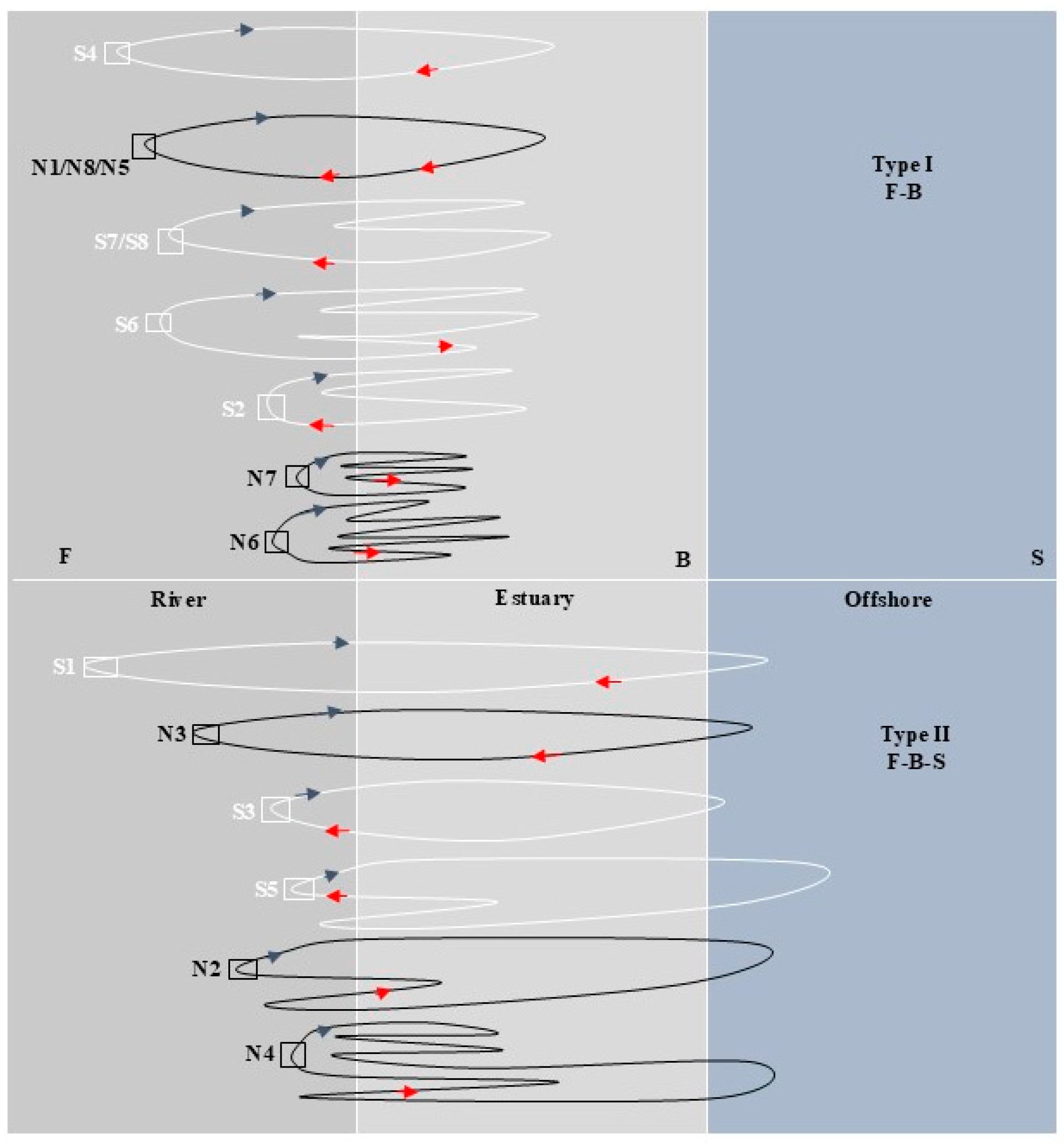
As shown in Figure 4, C. nasus from both branches have similar habitat histories: F-B and F-B-S. In terms of the differences in migration patterns and paths, C. nasus had similar migration patterns in the same type of habitat history, but their migration paths were different. It showed different starting point of migration paths between the individuals from the SB and NB. The starting point of the C. nasus with the same migratory pattern from the SB is far away from the estuary, and the residence time in fresh water is longer in its early life history. From the perspective of the transition between different habitats, the C. nasus from the NB have a more frequent transition between F and B and showed a greater dependence on brackish water in the estuary. In terms of the location and movement direction, C. nasus from the SB were mainly distributed in F when captured, with the movement direction shifting from B to F, showing an upward migratory movement trend, while those from the NB were mainly distributed in B when captured, with the movement direction shifting from F to B, showing a reciprocal movement trend between B and F in the estuary.
Figure 4.
Migration path simulation of C. nasus from the SB and NB of the Yangtze River Estuary. The white closed-loop line represents the migratory path of the SB individuals, the black closed-loop line represents the migratory path of the NB individuals, the blue arrow indicates the direction at the start of the migratory path, and the red arrow indicates the direction at the end of the migratory path.
3.3. Freshwater Dependence Analysis and Spawning Ground Distribution Inference
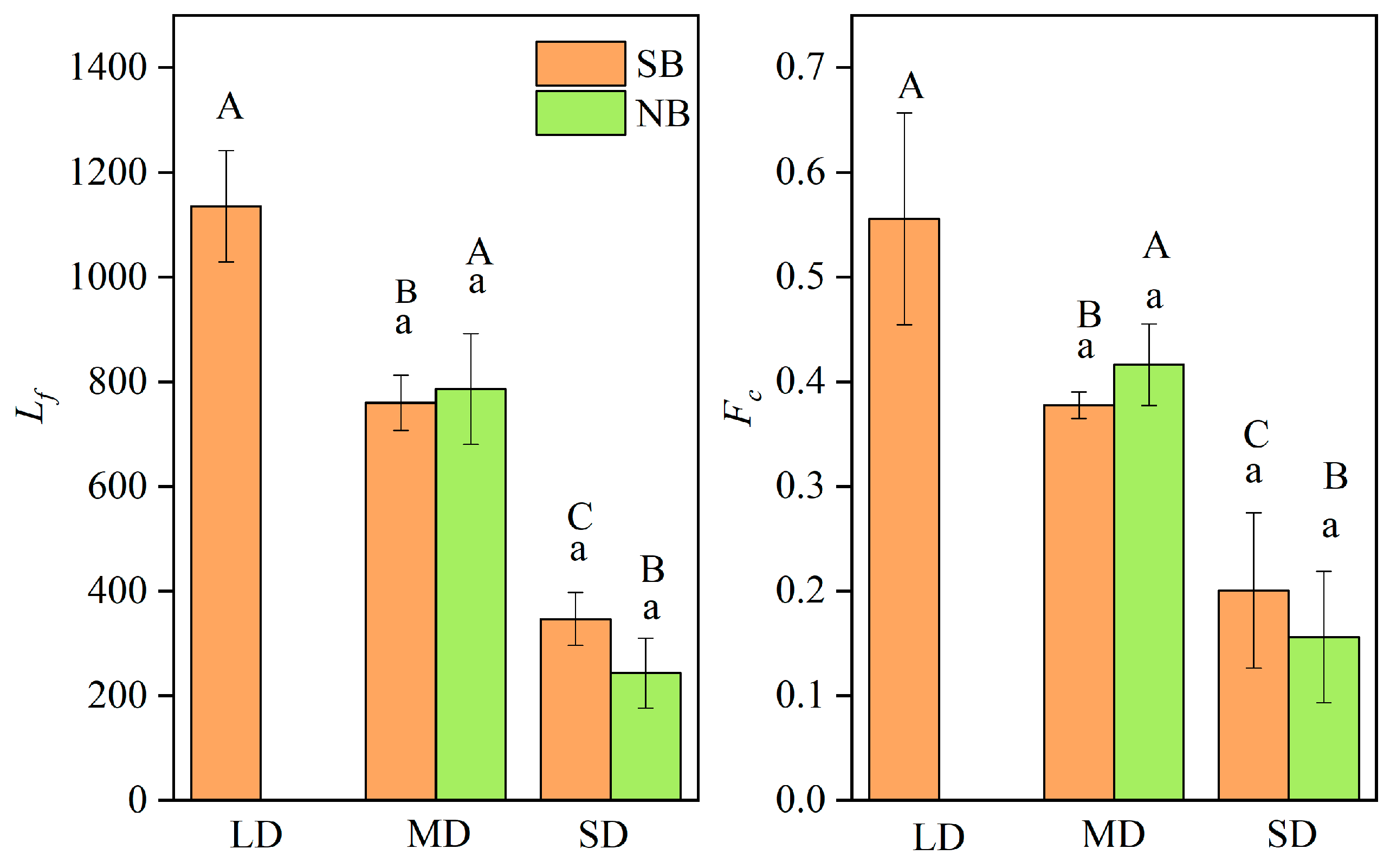
The Lf and Fc can reflect the time spent utilizing freshwater habitats for the C. nasus in their early life history, from hatching to entering the high-salinity estuary, and reflect the distance between the spawning grounds in different sections of the Yangtze River to the estuary [5,18]. As shown in Table S3, the Lf were different for individuals from different branches, among those from the SB ranged from 300 to 1210 μm, while those from the NB ranged from 260 to 940 μm. The individuals with the longest Lf were distributed in the SB, while those with the shortest Lf were distributed in the NB. The average Fc of C. nasus from the SB is 0.36 and that from the NB is 0.32, and the maximum Fc of the SB individuals is 0.63 and that of the NB individuals is 0.45. The analysis of the Lf and Fc of C. nasus from both branches showed that they can be clearly divided into different gradient groups of the long-distance freshwater dependence type (LD), the medium-distance freshwater-dependent type (MD) and the short-distance freshwater dependence type (SD) (Table S3), indicating that different individuals have different utilization times of F in the early stages of their life history. This not only reflects the different times from hatching to migration to the estuary, but it also reflects the different distances from the spawning ground in different sections of the Yangtze River to the estuary [2,5,18]. As shown in Figure 5, there is a significant difference in Lf and Fc between different groups (p < 0.05), even though the difference between the same group is not significant (p > 0.05), indicating that it is scientific and reliable to classify different freshwater dependence groups based on Lf and Fc. In terms of the composition of individuals with different freshwater-dependent types, there were three types of LD, MD, and SD for C. nasus from the SB, and two types of MD and SD for those from the NB. For the MD individuals, the number, proportion, Lf and Fc of C. nasus from the SB were 3, 37.50%, 760.00 ± 52.92 μm and 0.38 ± 0.01, respectively, which were lower than those of C. nasus from the NB, with that of 5, 62.50%, 786.00 ± 105.97 μm and 0.42 ± 0.04, respectively. For the SD, the number of C. nasus individuals in both branches were three, and both accounted for 37.50%. In summary, the individuals of LD were distributed in the SB, those of MD dominated in the NB, and both branches contained the same proportion of SD.
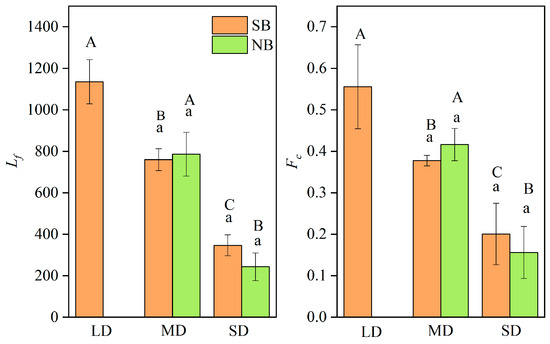
Figure 5.
Comparison of Lf and Fc of different freshwater-dependent types of LD, MD, and SD for C. nasus from the SB and NB of the Yangtze River Estuary. Lf: the radius of the first blue region near the core, Fc: freshwater coefficient; LD: long-distance freshwater-dependent type, MD: medium-distance freshwater-dependent type, SD: short-distance freshwater-dependent type. Different large letters (A, B, C) indicate that there are significant differences among different groups of LD, MD, and SD (p < 0.05), while the same small letters (a) indicate that there are no significant differences for the same group between the individuals from the SB and NB (p > 0.05).
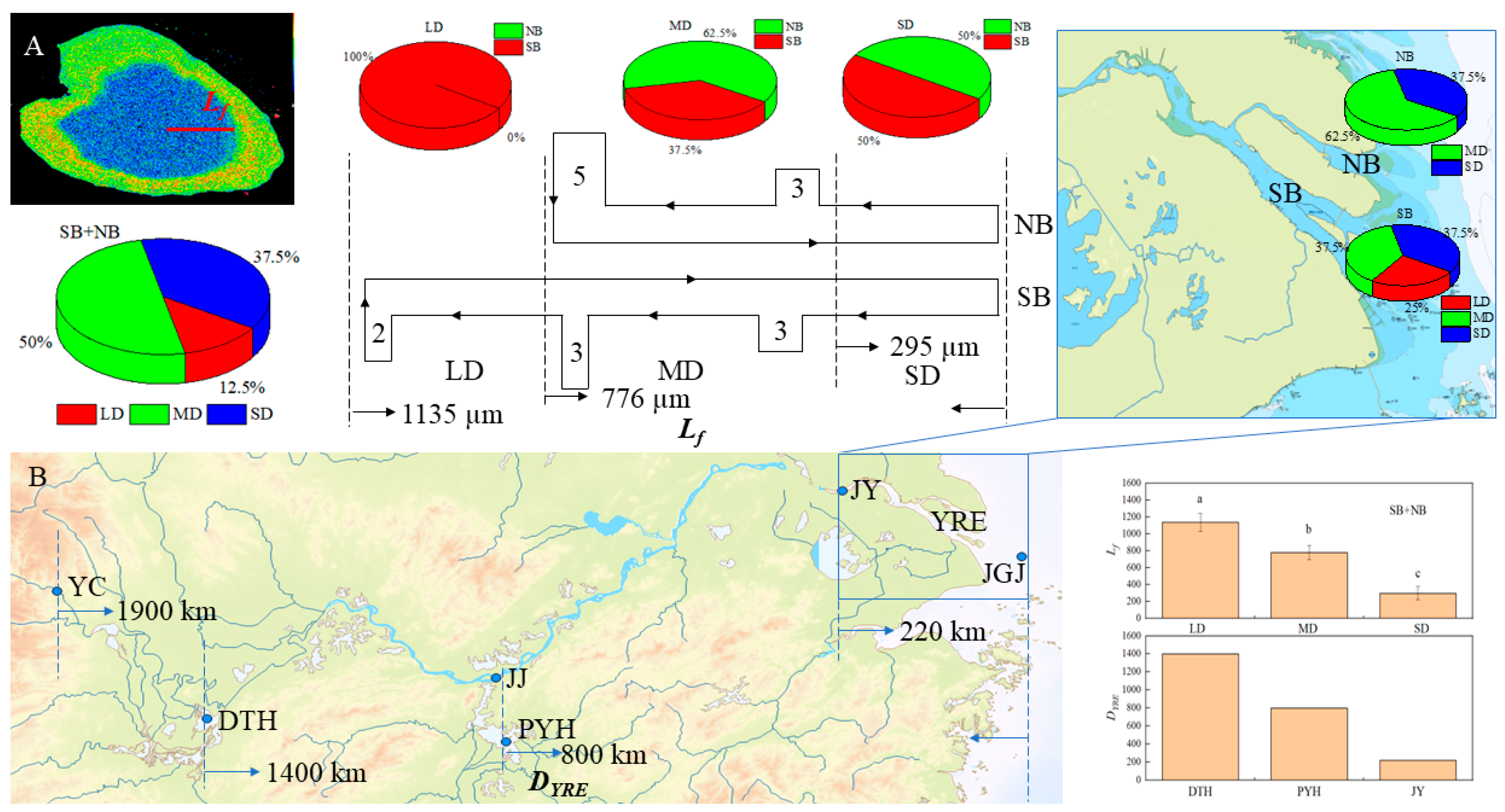
Based on the analysis of the Lf of C. nasus otoliths from the Yangtze River Estuary, it was found that they could be clearly divided into three groups, with an average length of 1135 µm in the longest group of LD, accounting for 12.5%, followed by MD, with an average length of 776 µm, accounting for 50.0%, and the shortest group of SD, with an average length of 295 µm, accounting for 37.5% (Figure 6A). In terms of the distribution of the different groups in the SB and NB, the LD group was all distributed in the SB, the MD group was mainly distributed in the NB, and the SD group had the same proportion of distribution in both branches (Figure 6A). In terms of the composition of the different groups, the individuals from the SB included three types, with LD accounting for 25.00%, while that from the NB included two types of MD and SD and was dominated by MD with a predominance of 62.50% (Figure 6A). Since the Lf can visually reflect the distance of spawning grounds to the estuary [5], a corresponding analysis was conducted on Lf and DYRE. It was found that there was an obvious correlation between Lf and DYRE (r = 0.996). The Lf of the LD group corresponds to the DYRE of the spawning grounds near DTH (about 1400 km away from the estuary [5]), the Lf of the MD group corresponds to the DYRE of the spawning grounds near PYH (about 800 km away from the estuary [8]), and the Lf of the SD group corresponds to the DYRE of the spawning ground in the upper reaches of JY (>220 km from the estuary [1]) adjacent to the Yangtze River Estuary waters (Figure 6B).
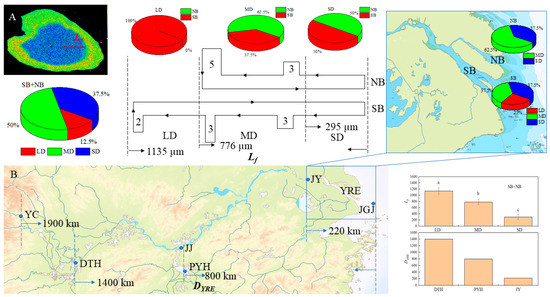
Figure 6.
Correspondence between Lf and DYRE. (A) shows the Lf and its spatial distribution, (B) shows the corresponding relationship between Lf and DYRE and the location of the spawning ground. Lf, LD, MD, and SD are the same with those in Figure 5. DYRE: the distance from the spawning grounds of C. nasus in different sections of the Yangtze River to the estuary; YC, JJ, and JY show the Yichang (YC), Jiujiang (JJ), and Jiangyin (JY) sections in the middle and lower reaches of the Yangtze River; JGJ shows the reef of Jigujiang (JGJ) in the Yangtze River Estuary (YRE). DTH and PYH show the lakes of Dongtinghu (DTH) and Poyanghu (PYH); Different letters (a, b, c) indicate that there are significant differences among different groups of LD, MD, and SD (p < 0.05).
4. Discussion
Earlier studies have focused on morphological features and geographical distribution to determine the ecological type of C. nasus [23]. Some studies have also used helminth marking, stable isotopes, and molecular biology methods to distinguish the anadromous C. nasus from the non-anadromous C. nasus [24,25,26]. In recent years, otolith microchemistry has been used to distinguish freshwater sedentary C. nasus from anadromous C. nasus more objectively, and Sr content distribution can clearly distinguish these two ecotypes [27,28]. In this study, all samples of C. nasus belonged to the long-jawed type (jaw length/head length ratio > 1) (Figure 1a). Both the quantitative line of the otoliths’ Sr/Ca ratios (Figure 2) and the two-dimensional imaging of Sr contents (Figure 3) conformed that all C. nasus belong to the migratory type. There have been many studies on the migration habits of C. nasus in the Yangtze River. The C. nasus from Anqing section of the Yangtze River showed two types of migration, F-B and F-B-S [29], while the short-jawed C. nasus from Hexian section in Anhui Province showed two types of F and F-B, and the long-jawed C. nasus belonged to F-B-S [30]. The C. nasus from the Nanjing section were anadromous and freshwater sedentary [31], while the C. nasus from the Taizhou section in Jiangsu Province were all migratory [32]. The C. nasus from Jingjiang section were anadromous migratory long-jawed C. nasus as well as freshwater sedentary short-jawed C. nasus [33]. The results of the above study were highly compatible with the results of the present study, which showed that the habitat history of migratory C. nasus in the Yangtze River are classified into two types: F-B and F-B-S (Table S2 and Figure 4). In the aforementioned study, the C. nasus primarily originate from freshwater habitats in different sections of the Yangtze River, with habitat history types mainly including freshwater resident and upstream migratory types. However, for the C. nasus from the Yangtze River Estuary, their sources and distribution are relatively complex, involving various habitats such as F, B, and S. Currently, research on the habitat history and migratory patterns of C. nasus from the Yangtze River Estuary is relatively limited [17]. To better elucidate the migration patterns and upstream migration distances of C. nasus from different sources, this study conducted a detailed decomposition and quantitative analysis of the habitat history of C. nasus from the SB and NB. This analysis identifies the composition of migration patterns and differences in migration paths of C. nasus from different branches of the Yangtze River Estuary (Table S2, Figure 4).
In the life history of C. nasus, newly hatched fry leave freshwater to enter estuaries or even the sea when they are less than one year old. After reaching maturity, most C. nasus anadromously migrate in February-April, or as late as October, in search of suitable spawning grounds in the middle and lower reaches of the Yangtze River [1]. Previous studies have shown that most C. nasus have a freshwater origin, while a very few have a green core and originate from brackish waters [34]. Based on the starting values of the Sr/Ca ratios, as well as the color of the otolith cores, all C. nasus in this research belonged to the freshwater origin and freshwater hatching type. Specifically, the early development of the C. nasus from the Yangtze River Estuary took place in freshwater, with spawning grounds potentially distributed in different freshwater areas along the Yangtze River. To further clarify the distribution pattern of spawning grounds for C. nasus from the Yangtze River Estuary, this study grouped C. nasus into three groups—LD, MD, and SD—based on the length of Lf to reflect the distance of DYRE [5]. A correspondence analysis was conducted between the length of Lf and the distance from traditional spawning grounds of C. nasus in various sections of the Yangtze River to the estuary [3,4], revealing a clear correlation between these two (Figure 6). An analysis Lf of C. nasus from DTH showed an average length of 1065 µm [5], consistent with that of the LD group in this study (average of 1135 µm). Therefore, it is speculated that the spawning grounds for the LD group of C. nasus are distributed in DTH and nearby river sections (about 1400 km from the estuary in the middle reaches of the Yangtze River) [5]. For C. nasus from PYH and its connected river sections of Ganjiang, the shortest length of Lf is 650 µm [2], consistent with that in the MD group from this study. Hence, it is speculated that the spawning grounds for the MD group are located in PYH and adjacent river sections (about 800 km from the estuary in the lower reaches of the Yangtze River) [2,8,35]. The average Lf of the SD group is 295 µm, suggesting that its spawning grounds are in the upstream of the JY section (about 220 km from the estuary) and near the Yangtze River Estuary [1] (Figure 6).
This study confirms the existence of spawning grounds for C. nasus in different sections of the Yangtze River, highlighting the diversity of their spawning ground distribution. C. nasus hatching from various spawning grounds may be at different developmental stages when they migrate to the estuary, resulting in a simultaneous presence of individuals with different life history stages such as larvae, juveniles, and adults in the estuarine waters. Each stage utilizes the habitat differently, and the Yangtze River Estuary can provide nursery, feeding, and migration pathways for C. nasus at different growth stages, reflecting the functional diversity of their habitat. The composition analysis of C. nasus from the two branches show that the C. nasus from the SB has the LD type, while that from the NB is primarily composed of the MD type, with the same number of SD-type individuals present in both branches. The differences in Lf among the various groups of C. nasus indicate that different early life history stages utilize freshwater habitats for varying durations, revealing different spatial distribution patterns of spawning grounds in different sections of the Yangtze River and highlighting the diversity of spawning ground distribution for C. nasus from the Yangtze River Estuary. By analyzing the composition of different freshwater-dependent types of C. nasus, it is found that the individuals that migrated upstream to the middle reaches of the Yangtze River are distributed in the SB, while individuals from both branches migrate to the lower reaches of the Yangtze River. Through this study, the migration pattern of C. nasus from the Yangtze River Estuary and the distribution pattern of their spawning grounds in different sections of the Yangtze River were found. In future research, the composition and resource status of long-distance migration C. nasus can be further studied, and the supplementary effect of migratory C. nasus from the Yangtze River Estuary on the reproductive population in different sections of the Yangtze River deserves further study. Through future research, the protection of C. nasus species will be more scientific, and the management of their resources will be more accurate and effective.
5. Conclusions
This study used otolith microchemistry to analyze Sr/Ca ratios and Sr content distribution, obtaining habitat histories and migration patterns. Based on differences in Lf, it analyzed the upstream migration distances of C. nasus from the Yangtze River Estuary and the distribution patterns of their spawning grounds in different sections of the Yangtze River. The results indicate that C. nasus from both branches contain two types of habitat histories, both originating from freshwater. The C. nasus from the south branch exhibit six migration patterns, predominantly F-B-F-B-F, while that from the north branch has seven migration patterns, mainly F-B. In the migration paths of C. nasus from both branches, there are transitions between F and B, with the C. nasus from the north branch exhibiting more frequent back-and-forth transitions between these habitats. The C. nasus from the south branch show a migration direction from B to F when captured, indicating an upstream migration trend, while those from the north branch show a migration direction from F to B, reflecting a back-and-forth migratory state within the estuarine brackish waters. The individuals from the south branch include the LD type, while those from the north branch are primarily composed of the MD type, with the SD group equally distributed in both branches. The C. nasus from the south branch can migrate upstream to the spawning grounds near the Dongtinghu water area, while the individuals from the north branch mainly migrate to the spawning grounds near the Poyanghu water area. This reflects the supporting role of C. nasus migrating upstream through the Yangtze River Estuary in replenishing the reproductive population in different sections of the Yangtze River, revealing the diversity of migration patterns and spawning ground distribution for C. nasus from different branches of the Yangtze River Estuary.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes9100402/s1, Table S1: Basic information of C. nasus in different water areas; Table S2: Habitat history and migration patterns of C. nasus from the SB and NB of the Yangtze River Estuary; Table S3: Lf and Fc for C. nasus from the SB and NB of the Yangtze River Estuary.
Author Contributions
Conceptualization and writing—original draft, C.S. and W.Y.; writing—review and editing, C.S., W.Y., F.Z. and F.L.; investigation and methodology, J.X. and R.L.; resources and funding acquisition, C.S., F.Z. and P.Z.; validation and supervision, F.L. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2022YFF0608203) and Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD14).
Institutional Review Board Statement
The experiments comply with current laws in China. All the samples in this study were obtained from legal commercial fisheries, and the samples were dead when they were obtained.
Data Availability Statement
The data presented in this study are available in this article. Further information is available upon request from the corresponding author.
Acknowledgments
The authors acknowledge researcher Yang, J. and associate researcher Jiang, T. from the Key Laboratory of Fishery Ecological Environment Assessment and Resource Conservation in the Middle and Lower Reaches of the Yangtze River, Chinese Academy of Fishery Sciences, for providing the experimental instruments and processing the experimental materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhuang, P.; Zhang, T.; Li, S.F.; Ni, Y.; Wang, Y.H.; Deng, S.M.; Zhang, L.Z.; Ling, J.Z.; Hu, F.; Yang, G.; et al. Fishes of the Yangtze Estuary; China Agriculture Press: Beijing, China, 2018; pp. 124–127. [Google Scholar]
- Yang, Y.F.; Jiang, T.; Gao, X.P.; Xuan, Z.Y.; Chen, X.B.; Li, L.K.; Liu, H.B.; Yang, J. Discovery of anadromous Coilia nasus in the Ganjiang River, Lake Poyang Basin, China. J. Lake Sci. 2021, 33, 1595–1606. [Google Scholar]
- Yuan, C.M. Spawning migration of Coilia nasus. Bull. Biol. 1987, 12, 1–3. [Google Scholar]
- Zhu, D.L. Natural reproduction and observation of embryonic development of Coilia nasus from the Yangtze River. Fish. Sci. Technol. Inf. 1992, 19, 49–51. [Google Scholar]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Qiu, C.; Chen, X.B.; Yang, J. Are there still anadromous the estuarine tapertail anchovies Coilia nasus in Dongting lake? Acta Hydrob. Sin. 2020, 44, 838–843. [Google Scholar]
- Zhang, M.Y.; Xu, D.P.; Liu, K.; Shi, W.G. Studies on biological characteristics and change of resource of Coilia nasus schlegel in the lower research of the Yangtze River. Resour. Environ. Yangtze Basin 2005, 14, 694–698. [Google Scholar]
- Li, Y.X.; He, W.P.; Liu, J.S.; Li, Z.J.; Xie, S.G. Annulus validation and age and growth estimation of anadromous Coilia ectenes in the Yangtze Estuary. Acta Hydrob. Sin. 2010, 34, 787–793. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, X.Q.; Liu, H.B.; Liu, H.Z.; Yang, J. Two microchemistry patterns in otoliths of Coilia nasus from Poyang Lake, China. J. Fish. China 2013, 37, 239–244. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Xuan, Z.Y.; Chen, X.B.; Yang, J. Classification of ecomorphotypes of Coilia nasus from the middle and lower reaches of the Yangtze River Basin. J. Lake Sci. 2020, 32, 518–527. [Google Scholar]
- Hu, Y.H.; Jiang, T.; Liu, H.B.; Chen, X.B.; Yang, J. Habitat histories of different ecomorphotypes of Coilia nasus from the Yalu River in Dandong City of Liaoning Province based on otolith microchemical analysis. Mar. Fish. 2023, 45, 278–290. [Google Scholar]
- Xu, Q.; Ren, Q.Q.; Jiang, T.; Lin, B.A.; Jiang, X.B.; Yang, J.; Liu, M. Otolith microchemistry reveals diverse habitat uses and migratory patterns of two Coilia species (Engraulidae) in the Min River Estuary, southern China. Mar. Environ. Res. 2024, 193, 106296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Slypko, I.; Demianenko, K.; Zhu, G.P. Otolith chemistry reveals ontogenetic movement of the Antarctic toothfish (Dissostichus Mawsoni) in the Amundsen Sea Polynya, Antarctica. Fish. Res. 2024, 276, 107046. [Google Scholar] [CrossRef]
- Doerr, L.R.; Houghton, C.J.; Hansen, S.P.; Pangle, K.L.; Ransom, A.L.; Forsythe, P.S. Can otolith microchemistry identify the natal origin of larval lake whitefish Coregonus clupeaformis in the waters of Green Bay? J. Great Lakes Res. 2021, 47, 1771–1780. [Google Scholar] [CrossRef]
- Xu, Q.; Ren, Q.Q.; Jiang, T.; Jiang, C.R.; Fang, L.P.; Zhang, M.Z.; Yang, J.; Liu, M. Otolith microchemistry reveals various habitat uses and life histories of Chinese gizzard shad Clupanodon thrissa in the Min River and the estuary, Fujian Province, China. Fish. Res. 2023, 264, 106723. [Google Scholar] [CrossRef]
- Teichert, N.; Lizé, A.; Tabouret, H.; Roussel, J.M.; Bareille, G.; Trancart, T.; Acou, A.; Virag, L.S.; Pécheyran, C.; Carpentier, A.; et al. European flounder foraging movements in an estuarine nursery seascape inferred from otolith microchemistry and stable isotopes. Mar. Environ. Res. 2022, 182, 105797. [Google Scholar] [CrossRef]
- Zhong, L.; Guo, H.; Shen, H.; Li, X.; Tang, W.; Liu, J.; Jin, J.; Mi, Y. Preliminary results of Sr:Ca ratios of Coilia nasus in otoliths by Micro-PIXE. Nucl. Instrum. Methods B 2007, 260, 349–352. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.H.; Feng, G.P.; Yang, J.; Zhao, F.; Shen, C.C.; Song, C.; Jiang, T. Otolith microchemistry assessment: Evidence of migratory Coilia nasus of Yangtze River living in the Shengsi sea area. Fishes 2022, 7, 172. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Shen, X.Q.; Shimasaki, Y.; Oshima, Y.; Yang, J. Life history variations among different population of Coilia nasus along the Chinese coast inferred from otolith microchemistry. J. Fac. Agric. Kyushu Univ. 2014, 59, 383–389. [Google Scholar] [CrossRef]
- Stirnimann, L.; Conversi, A.; Marini, S. Detection of regime shifts in the environment: Testing “STARS” using synthetic and observed time series. ICES J. Mar. Sci. 2019, 76, 2286–2296. [Google Scholar] [CrossRef]
- Yang, J.; Arai, T.; Liu, H.; Miyazaki, N.; Tsukamoto, K. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. J. Fish. Biol. 2006, 69, 1120–1135. [Google Scholar] [CrossRef]
- Kotake, A.; Arai, T.; Ozawa, T.; Nojima, S.; Miller, M.J.; Tsukamoto, K. Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa Islands, Japan, inferred from otolith Sr/Ca ratios. Mar. Biol. 2003, 142, 849–854. [Google Scholar] [CrossRef]
- Sokta, L.; Jiang, T.; Liu, H.B.; Xuan, Z.Y.; Qiu, C.; Chen, X.B.; Yang, J. Loss of Coilia nasus habitats in Chinese freshwater lakes: An otolith microchemistry assessment. Heliyon 2020, 6, e04571. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.M.; Lin, J.B.; Qin, A.Z.; Liu, R.H. On the history and present situation of the taxonomy of Coilia in China—Some experiences on reforming the old taxonomy of fishes. J. Nanjing Univ. 1976, 2, 1–13. [Google Scholar]
- Li, W.X.; Wang, G.T. Helminth communities in Coilia nasus from anadromous freshwater and landlocked stocks. Chin. J. Zool. 2014, 49, 233–243. [Google Scholar]
- Wang, L.; Tang, W.Q.; Dong, W.X. The signatures of stable isotopes δ15N and δ13C in anadromous and non-anadromous Coilia nasus living in the Yangtze River, and the adjacent sea waters. J. Ocean Univ. China 2015, 14, 1053–1058. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Wang, Q.; Delser, P.M.; Li, C.H. Multiple freshwater invasions of the tapertail anchovy (Clupeiformes: Engraulidae) of the Yangtze River. Ecol. Evol. 2019, 9, 12202–12215. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Yang, J. Otolith microchemistry and microsatellite DNA provide evidence for divergence between estuarine tapertail anchovy (Coilia nasus) populations from the Poyang Lake and the Yangtze River Estuary of China. Reg. Stud. Mar. Sci. 2022, 56, 102649. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Chen, X.B.; Yang, J. Otolith microchemical evidence revealing multiple spawning site origination of the anadromous tapertail anchovy (Coilia nasus) in the Changjiang (Yangtze) River Estuary. Acta Oceanol. Sin. 2023, 42, 120–130. [Google Scholar] [CrossRef]
- Li, M.M.; Jiang, T.; Chen, T.T.; Liu, H.B.; Yang, J. Otolith microchemistry of the estuarine tapertail anchovy Coilia nasus from the Anqing section of the Yangtze River and its significance for migration ecology. Acta Ecol. Sin. 2017, 37, 2788–2795. [Google Scholar]
- Li, M.M.; Jiang, T.; Khumbanyiwa, D.D.; Liu, H.B.; Yang, J. Reconstructing habitat history of Coilia nasus from the Hexian section of the Yangtze River in Anhui province by otolith microchemistry. Acta Hydrob. Sin. 2017, 41, 1054–1061. [Google Scholar]
- Chen, T.T.; Jiang, T.; Li, M.M.; Liu, H.B.; Yang, J. Inversion of habitat history for the long-jaw ecotype Coilia nasus collected from Nanjing section of the Yangtze River. J. Fish. China 2016, 40, 882–892. [Google Scholar]
- Hu, Y.H.; Jiang, T.; Liu, H.B.; Chen, X.B.; Yang, J. Otolith microchemical fingerprints of Coilia nasus from the Taizhou section of Changjiang River in Jiangsu Province. Chin. J. Ecol. 2024, 43, 967–974. [Google Scholar]
- Chen, T.T.; Jiang, T.; Lu, M.J.; Liu, H.B.; Yang, J. Microchemistry analysis of otoliths of Coilia nasus and Coilia brachygnathus from the Jingjiang section of the Yangtze River. J. Lake Sci. 2016, 28, 149–155. [Google Scholar]
- Liu, H.B.; Jiang, T.; Xuan, Z.Y.; Qiu, C.; Yang, J. Otolith microchemical analysis of Tapertail Anchovy Coilia nasus from Ariake sea and its adjacent tributaries in Japan. Fish. Sci. 2020, 39, 500–508. [Google Scholar]
- Jiang, T.; Yang, J.; Lu, M.J.; Liu, H.B.; Chen, T.T.; Gao, Y.W. Discovery of a spawning area for anadromous Coilia nasus Temminck et Schlegel, 1846 in Poyang Lake, China. J. Appl. Ichthyol. 2017, 33, 189–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).