Living Along Distribution Margins: Differences in the Body and Biochemistry of Red Squat Lobster Morphotypes (Grimothea monodon) from the Humboldt Current System
Abstract
:1. Introduction
2. Materials and Methods
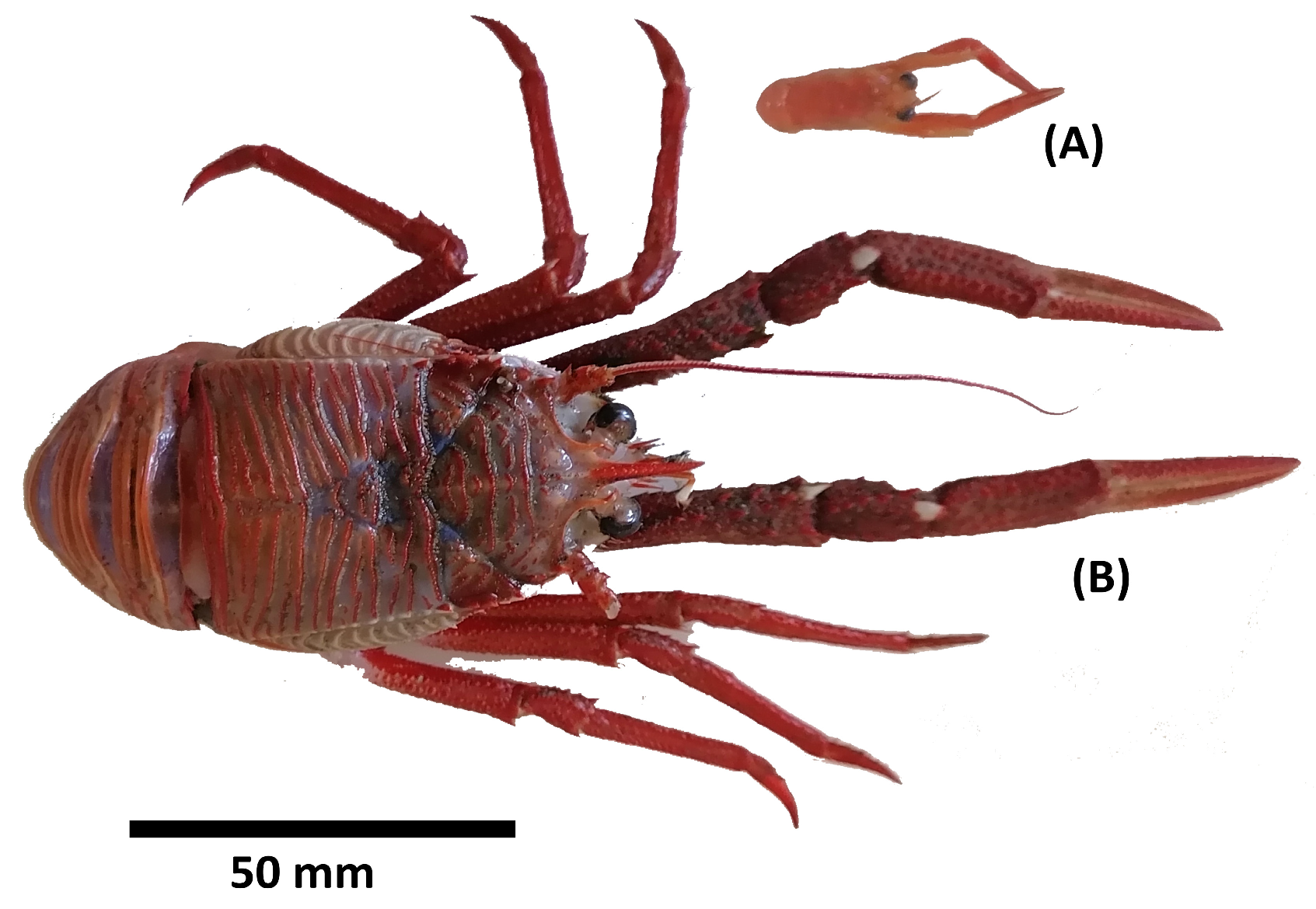
2.1. Collection of Samples
2.2. Body Condition (Length, Weight, Relative Condition-Kn)
2.3. Biochemical Condition (Glucose, Protein, Lipids, Fatty Acid Profile)
2.3.1. Glucose Content
2.3.2. Protein Content
2.3.3. Lipid Content
2.3.4. Fatty Acids Profile
2.4. Statistical Analysis
3. Results
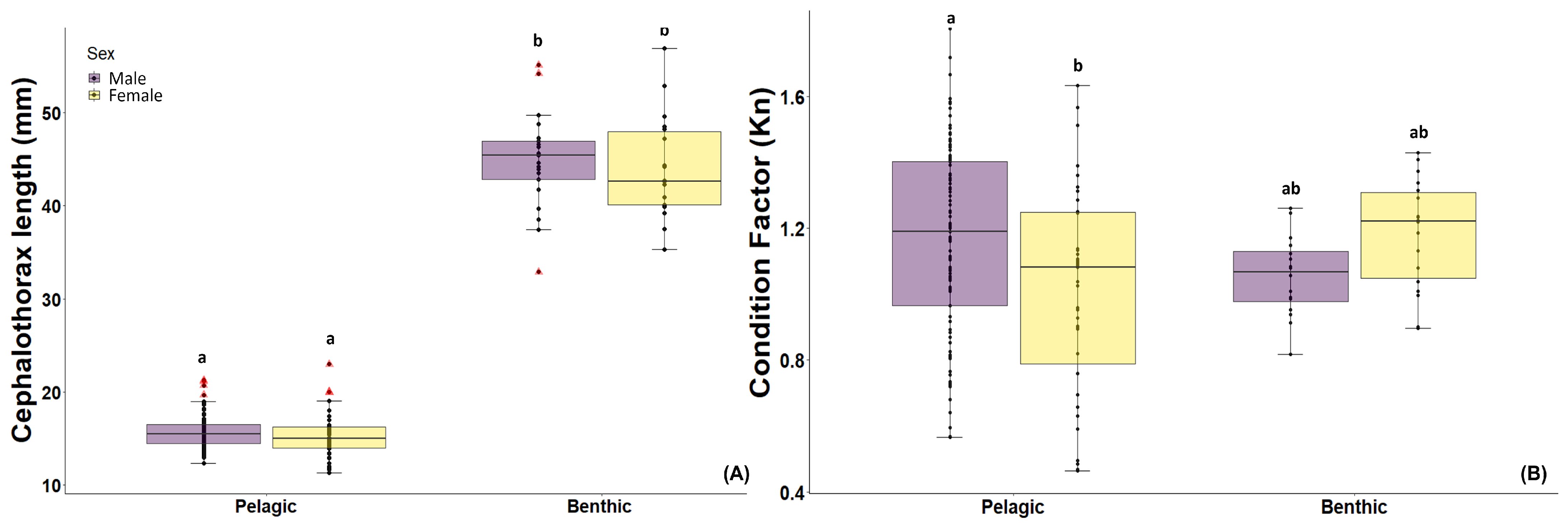
3.1. Body Condition
3.2. Biochemical Condition
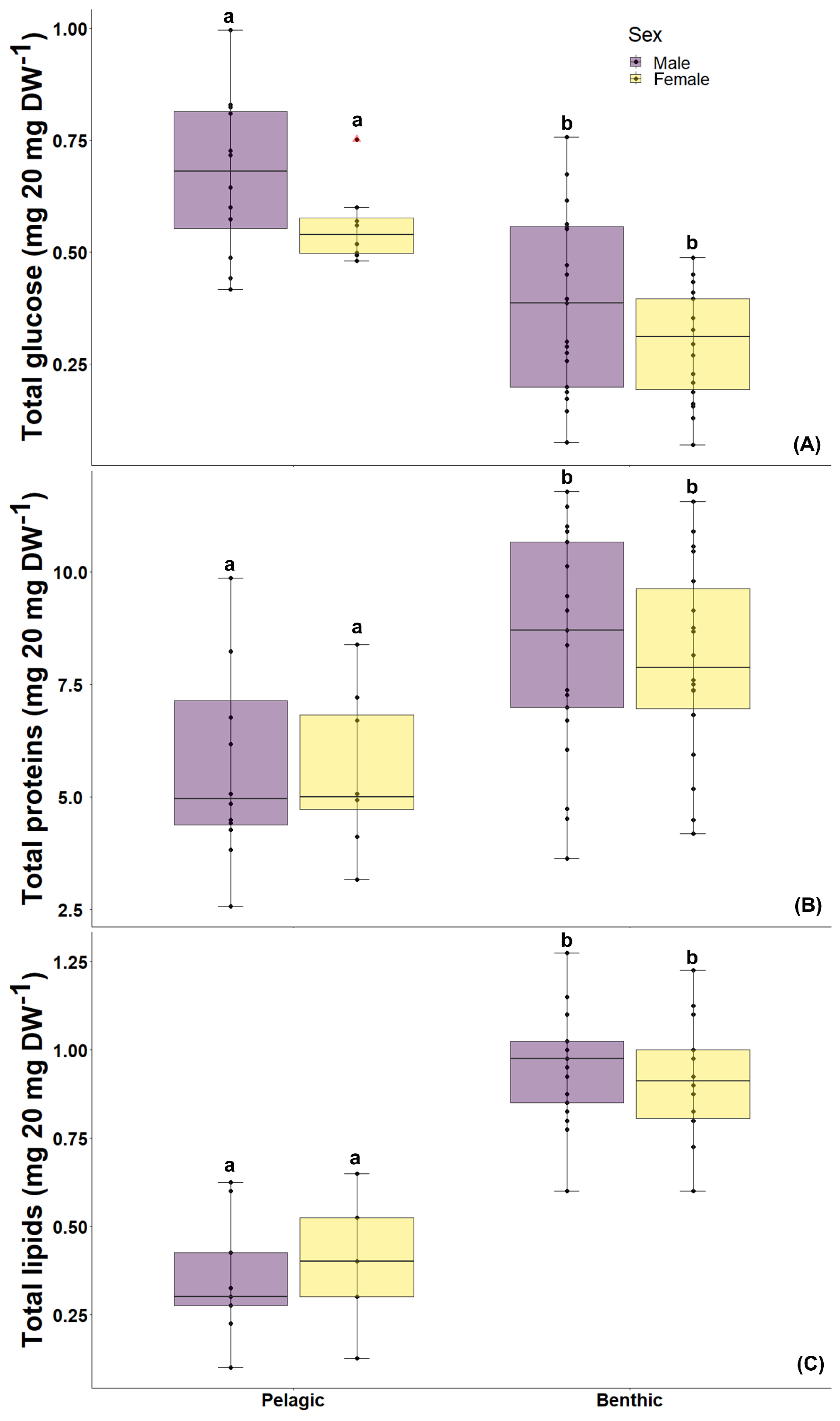
3.2.1. Glucose Content
3.2.2. Protein Content
3.2.3. Lipid Content
3.2.4. Fatty Acid Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geta, K. Importance of Marine Ecosystems. Biol. Med. 2022, 14, 467. [Google Scholar] [CrossRef]
- Tadesse, S. Why are marine ecosystems biologically more diversified than their equivalent terrestrial ecosystems? Int. J. Avian Wildl. Biol. 2018, 3, 304–305. [Google Scholar] [CrossRef]
- Barbier, E.B. Marine Ecosystem Services. Curr. Biol. 2017, 27, R507–R510. [Google Scholar] [CrossRef] [PubMed]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of Marine Organisms to Climate Change across Oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Shalders, T.C.; Champion, C.; Coleman, M.A.; Benkendorff, K. The Nutritional and Sensory Quality of Seafood in a Changing Climate. Mar. Environ. Res. 2022, 176, 105590. [Google Scholar] [CrossRef]
- Wright, L. The Impact of Overfishing on Marine Ecosystems. Poult. Fish. Wildl. Sci. 2023, 11, 221. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2011, 4, 11–37. [Google Scholar] [CrossRef]
- Gerhard, M.; Schlenker, A.; Hillebrand, H.; Striebel, M. Environmental Stoichiometry Mediates Phytoplankton Diversity Effects on Communities’ Resource Use Efficiency and Biomass. J. Ecol. 2022, 110, 430–442. [Google Scholar] [CrossRef]
- Bestion, E.; Soriano-Redondo, A.; Cucherousset, J.; Jacob, S.; White, J.; Zinger, L.; Fourtune, L.; Di Gesu, L.; Teyssier, A.; Cote, J. Altered Trophic Interactions in Warming Climates: Consequences for Predator Diet Breadth and Fitness. Proc. R. Soc. B Biol. Sci. 2019, 286, 20192227. [Google Scholar] [CrossRef]
- Campagne, C.S.; Roy, L.-A.; Langridge, J.; Claudet, J.; Mongruel, R.; Beillouin, D.; Thiébaut, É. Existing Evidence on the Impact of Changes in Marine Ecosystem Structure and Functioning on Ecosystem Service Delivery: A Systematic Map. Environ. Evid. 2023, 12, 13. [Google Scholar] [CrossRef]
- Green, B.S.; Gardner, C.; Hochmuth, J.D.; Linnane, A. Environmental Effects on Fished Lobsters and Crabs. Rev. Fish Biol. Fish. 2014, 24, 613–638. [Google Scholar] [CrossRef]
- Chouvelon, T.; Gilbert, L.; Caurant, F.; Méndez-Fernandez, P.; Bustamante, P.; Brault-Favrou, M.; Spitz, J. Nutritional Grouping of Marine Forage Species Reveals Contrasted Exposure of High Trophic Levels to Essential Micro-Nutrients. Oikos 2022, 2022, e08844. [Google Scholar] [CrossRef]
- Montecino, V.; Lange, C.B. The Humboldt Current System: Ecosystem Components and Processes, Fisheries, and Sediment Studies. Prog. Oceanogr. 2009, 83, 65–79. [Google Scholar] [CrossRef]
- Zuercher, R.; Galloway, A.W.E. Coastal Marine Ecosystem Connectivity: Pelagic Ocean to Kelp Forest Subsidies. Ecosphere 2019, 10, e02602. [Google Scholar] [CrossRef]
- Nwabueze, A.A.; Nwabueze, E.O. Impact of Environmental Variables on Abundance, Growth and Condition factor of Gymnarchus niloticus (Curvier, 1829) from Umueze-Ossissa Lakesystem, Southern Nigeria. Asian J. Agric. Biol. 2021, 2021, 202011567. [Google Scholar] [CrossRef]
- Pulgar, J.; Moya, A.; Fernández, M.; Varas, O.; Guzmán-Rivas, F.; Urzúa, Á.; Quijón, P.A.; García-Huidobro, M.R.; Aldana, M.; Duarte, C. Upwelling Enhances Seaweed Nutrient Quality, Altering Feeding Behavior and Growth Rates in an Intertidal Sea Urchin, Loxechinus albus. Sci. Total Environ. 2022, 851, 158307. [Google Scholar] [CrossRef] [PubMed]
- Gravel, M.-A.; Couture, P.; Cooke, S.J. Comparative Energetics and Physiology of Parental Care in Smallmouth Bass Micropterus Dolomieu across a Latitudinal Gradient. J. Fish Biol. 2010, 76, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Machordom, A.; Ahyong, S.T.; Andreakis, N.; Baba, K.; Buckley, D.; García-Jiménez, R.; McCallum, A.W.; Rodríguez-Flores, P.C.; Macpherson, E. Deconstructing the Crustacean Squat Lobster Genus Munida to Reconstruct the Evolutionary History and Systematics of the Family Munididae (Decapoda, Anomura, Galatheoidea). Invertebr. Syst. 2022, 36, 926–970. [Google Scholar] [CrossRef]
- Barros, M.E.; Alarcón, R.; Arancibia, H. Distribución espaciotemporal del potencial reproductivo del stock de hembras de langostino colorado (Pleuroncodes monodon) en la zona centro-sur de Chile. Cienc. Mar. 2023, 49, e3321. [Google Scholar] [CrossRef]
- Barriga-Sánchez, M.; Sanchez-Gonzales, G.; Condori, M.A.V.; Alvites, M.N.S.; Valenzuela, M.E.A.G.D. Extraction of Bioactive Lipids from Pleuroncodes monodon Using Organic Solvents and Supercritical CO2. Grasas Aceites 2023, 74, e492. [Google Scholar] [CrossRef]
- Yapur-Pancorvo, A.L.; Quispe-Machaca, M.; Guzmán-Rivás, F.; Urzúa, Á.; Espinoza, P. The Red Squat Lobster Pleuroncodes monodon in the Humboldt Current System: From Their Ecology to Commercial Attributes as Marine Bioresource. Animals 2023, 13, 2279. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, F.; Bascur, M.; Olavarria, L.; Mora, S.; Riera, R.; Urzúa, Á. Seasonal and Interannual Changes in Reproductive Parameters and Eggs Biochemical Composition of the Fishery Resource Pleuroncodes monodon (Decapoda: Munididae) from the Humboldt Current System. Fish. Res. 2020, 221, 105404. [Google Scholar] [CrossRef]
- Haye, P.A.; Salinas, P.; Acuña, E.; Poulin, E. Heterochronic Phenotypic Plasticity with Lack of Genetic Differentiation in the Southeastern Pacific Squat Lobster Pleuroncodes monodon. Evol. Dev. 2010, 12, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Yannicelli, B.; Castro, L.; Parada, C.; Schneider, W.; Colas, F.; Donoso, D. Distribution of Pleuroncodes monodon Larvae over the Continental Shelf of South-Central Chile: Field and Modeling Evidence for Partial Local Retention and Transport. Prog. Oceanogr. 2012, 92–95, 206–227. [Google Scholar] [CrossRef]
- Sampaio, A.L.A.; Pagotto, J.P.A.; Goulart, E. Relationships between Morphology, Diet and Spatial Distribution: Testing the Effects of Intra and Interspecific Morphological Variations on the Patterns of Resource Use in Two Neotropical Cichlids. Neotrop. Ichthyol. 2013, 11, 351–360. [Google Scholar] [CrossRef]
- Lloret, J.; Shulman, G.; Love, R.M. Condition and Health Indicators of Exploited Marine Fishes; Wiley: Hoboken, NJ, USA, 2014; Available online: https://www.wiley.com/en-br/Condition+and+Health+Indicators+of+Exploited+Marine+Fishes-p-9780470670248 (accessed on 18 October 2024).
- Sardenne, F.; Chassot, E.; Fouché, E.; Ménard, F.; Lucas, V.; Bodin, N. Are Condition Factors Powerful Proxies of Energy Content in Wild Tropical Tunas? Ecol. Indic. 2016, 71, 467–476. [Google Scholar] [CrossRef]
- Lopeztegui-Castillo, A. Assessment of Nutritional Condition in Crustaceans: A Review of Methodologies and Guidelines for Applying Inexpensive and Wide-Ranging Indices to the Spiny Lobster Panulirus argus (Latreille, 1804) (Decapoda: Achelata: Palinuridae). J. Crustac. Biol. 2021, 41, ruab067. [Google Scholar] [CrossRef]
- Froese, R. Cube Law, Condition Factor and Weight–Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Jin, S.; Yan, X.; Zhang, H.; Fan, W. Weight–Length Relationships and Fulton’s Condition Factors of Skipjack Tuna (Katsuwonus pelamis) in the Western and Central Pacific Ocean. PeerJ 2015, 3, e758. [Google Scholar] [CrossRef]
- LeCren, E.D. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Costa, E.F.S.; Teixeira, G.M.; Freire, F.A.M.; Dias, J.F.; Fransozo, A. Effects of Biological and Environmental Factors on the Variability of Paralonchurus brasiliensis (Sciaenidae) Density: An GAMLSS Application. J. Sea Res. 2022, 183, 102203. [Google Scholar] [CrossRef]
- Flores-Valiente, J.; Lett, C.; Colas, F.; Pecquerie, L.; Aguirre-Velarde, A.; Rioual, F.; Tam, J.; Bertrand, A.; Ayón, P.; Sall, S.; et al. Influence of Combined Temperature and Food Availability on Peruvian Anchovy (Engraulis ringens) Early Life Stages in the Northern Humboldt Current System: A Modelling Approach. Prog. Oceanogr. 2023, 215, 103034. [Google Scholar] [CrossRef]
- Zhang, Z.; Yokota, M.; Strüssmann, C.A. Relative Growth Pattern and Relative Condition Factor in the Japanese Mitten Crab Eriocheir japonica (De Haan, 1835) (Brachyura: Varunidae). J. Crustac. Biol. 2017, 37, 571–578. [Google Scholar] [CrossRef]
- Dinh, Q.M.; Nguyen, T.H.D.; Nguyen, T.T.K.; Tran, G.V.; Truong, N.T. Spatiotemporal Variations in Length-Weight Relationship, Growth Pattern and Condition Factor of Periophthalmus variabilis Eggert, 1935 in Vietnamese Mekong Delta. PeerJ 2022, 10, e12798. [Google Scholar] [CrossRef]
- Cifuentes, R.; González, J.; Montoya, G.; Jara, A.; Ortíz, N.; Piedra, P.; Habit, E. Relación Longitud-Peso y Factor de Condición de Los Peces Nativos Del Río San Pedro (Cuenca Del Río Valdivia, Chile). Gayana Concepc. 2012, 76, 86–100. [Google Scholar] [CrossRef]
- Rodríguez-Viera, L.; Perera, E.; Montero-Alejo, V.; Perdomo-Morales, R.; García-Galano, T.; Martínez-Rodríguez, G.; Mancera, J.M. Carbohydrates Digestion and Metabolism in the Spiny Lobster (Panulirus argus): Biochemical Indication for Limited Carbohydrate Utilization. PeerJ 2017, 5, e3975. [Google Scholar] [CrossRef]
- Zuloaga, R.; Varas, O.; Ahrendt, C.; Pulgar, V.M.; Valdés, J.A.; Molina, A.; Duarte, C.; Urzúa, Á.; Guzmán-Rivas, F.; Aldana, M.; et al. Revealing Coastal Upwelling Impact on the Muscle Growth of an Intertidal Fish. Sci. Total Environ. 2023, 858, 159810. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Quispe-Machaca, M.; Olavarría, L.; Zilleruelo, M.; Urzúa, Á. Inter-Sexual Comparison of Body Biomass, Proximate Biochemical Composition, and Fatty Acid Profiles of New Juvenile Squat Lobsters (Pleuroncodes monodon) in the Southeast Pacific Ocean. Mar. Ecol. 2022, 43, e12690. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Quispe, M.; Urzúa, Á. Contrasting Nursery Habitats Promote Variations in the Bioenergetic Condition of Juvenile Female Red Squat Lobsters (Pleuroncodes monodon) of the Southern Pacific Ocean. PeerJ 2022, 10, e13393. [Google Scholar] [CrossRef]
- Behringer, D.C.; Duermit-Moreau, E. Crustaceans, One Health and the Changing Ocean. J. Invertebr. Pathol. 2021, 186, 107500. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size a biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Kämpf, J.; Chapman, P. The Peruvian-Chilean Coastal Upwelling System. In Upwelling Systems of the World: A Scientific Journey to the Most Productive Marine Ecosystems; Kämpf, J., Chapman, P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 161–201. [Google Scholar] [CrossRef]
- Deville, D.; Sanchez, G.; Barahona, S.P.; Yamashiro, C.; Oré-Chávez, D.; Bazán, R.Q.; Umino, T. Spatio-Temporal Patterns of Genetic Variation of the Silverside Odontesthes Regia in the Highly Productive Humboldt Current System. Fish. Res. 2021, 244, 106127. [Google Scholar] [CrossRef]
- Acha, E.M.; Mianzan, H.W.; Guerrero, R.A.; Favero, M.; Bava, J. Marine Fronts at the Continental Shelves of Austral South America: Physical and Ecological Processes. J. Mar. Syst. 2004, 44, 83–105. [Google Scholar] [CrossRef]
- Ghanawi, J.; Saoud, I.P. Molting, Reproductive Biology, and Hatchery Management of Redclaw Crayfish Cherax quadricarinatus (von Martens 1868). Aquaculture 2012, 358–359, 183–195. [Google Scholar] [CrossRef]
- Waraporn, P.; Kirirat, P.; Pinij, T. Index of Molt Staging in the Black Tiger Shrimp (Penaeus monodon). Songklanakarin J. Sci. Technol. 2004, 26, 765–772. [Google Scholar]
- Araujo, P.; Truzzi, C.; Belghit, I.; Antonucci, M. The Impact of Seawater Warming on Fatty Acid Composition and Nutritional Quality Indices of Trematomus bernacchii from the Antarctic Region. Food Chem. 2021, 365, 130500. [Google Scholar] [CrossRef]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Q.; Zhang, W.; Xia, S.; Tian, H.; Liu, F.; Yang, W.; Yu, Y.; Wu, Y.; Zhu, Y.; et al. Comparison of Morphometric Parameters, Nutritional Composition, and Textural Properties of Seven Crustaceans Species. Fishes 2024, 9, 141. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Cequier-Sánchez, E.; Rodríguez, C.; Ravelo, Á.G.; Zárate, R. Dichloromethane as a Solvent for Lipid Extraction and Assessment of Lipid Classes and Fatty Acids from Samples of Different Natures. J. Agric. Food Chem. 2008, 56, 4297–4303. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, Á.; Anger, K. Seasonal Variations in Larval Biomass and Biochemical Composition of Brown Shrimp, Crangon crangon (Decapoda, Caridea), at Hatching. Helgol. Mar. Res. 2013, 67, 267–277. [Google Scholar] [CrossRef]
- Urzúa, Á.; Anger, K. Larval Biomass and Chemical Composition at Hatching in Two Geographically Isolated Clades of the Shrimp Macrobrachium amazonicum: Intra- or Interspecific Variation? Invertebr. Reprod. Dev. 2011, 55, 236–246. [Google Scholar] [CrossRef]
- Quispe-Machaca, M.; Guzmán-Rivas, F.A.; Ibáñez, C.M.; Urzúa, Á. Intra-Individual Variability in Biochemical Constituents and Fatty Acid Composition of Adult Jumbo Squid (Dosidicus gigas) in the Southeastern Pacific Ocean. J. Sea Res. 2021, 174, 102082. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Taipale, S.J.; Aalto, S.L.; Galloway, A.W.E.; Kuoppamäki, K.; Nzobeuh, P.; Peltomaa, E. Eutrophication and Browning Influence Daphnia Nutritional Ecology. Inland Waters 2019, 9, 374–394. [Google Scholar] [CrossRef]
- Hauser, Ú. Condiciones Oceanográficas (Temperatura, Salinidad, Oxígeno Disuelto, Clorofila y Focos de Surgencia) en la Zona Centro-sur de Chile (35.5°S–40°S) Durante la Primavera Austral de 2014, Pontificia Universidad Católica de Valparaíso. 2015. Available online: http://opac.pucv.cl/pucv_txt/txt-2500/UCC2862_01.pdf (accessed on 29 December 2021).
- Graco, M.I.; Ledesma, J.; Flores, G.; Girón, M. Nutrientes, Oxígeno y Procesos Biogeoquímicos En El Sistema de Surgencias de La Corriente de Humboldt Frente a Perú. Rev. Peru. Biol. 2007, 14, 117–128. [Google Scholar] [CrossRef]
- Sobarzo, M.; Bravo, L.; Donoso, D.; Garcés-Vargas, J.; Schneider, W. Coastal Upwelling and Seasonal Cycles That Influence the Water Column over the Continental Shelf off Central Chile. Prog. Oceanogr. 2007, 75, 363–382. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Quispe-Machaca, M.; Queirolo, D.; Ahumada, M.; Urzúa, Á. Latitudinal Changes in the Lipid Content and Fatty Acid Profiles of Juvenile Female Red Squat Lobsters (Pleuroncodes monodon) in Breeding Areas of the Humboldt Current System. PLoS ONE 2021, 16, e0253314. [Google Scholar] [CrossRef]
- Espinoza, P.; Lorrain, A.; Ménard, F.; Cherel, Y.; Tremblay-Boyer, L.; Argüelles, J.; Tafur, R.; Bertrand, S.; Tremblay, Y.; Ayón, P.; et al. Trophic Structure in the Northern Humboldt Current System: New Perspectives from Stable Isotope Analysis. Mar. Biol. 2017, 164, 86. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Steury, T.D.; Sears, M.W. Temperature, Growth Rate, and Body Size in Ectotherms: Fitting Pieces of a Life-History Puzzle. Integr. Comp. Biol. 2004, 44, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.D.; Verdon, R. Development and Evaluation of Condition Indices for the Lake Whitefish. North Am. J. Fish. Manag. 2008, 28, 1270–1293. [Google Scholar] [CrossRef]
- Gubiani, É.A.; Ruaro, R.; Ribeiro, V.R.; Fé, Ú.M.G.d.S. Relative Condition Factor: Le Cren’s Legacy for Fisheries Science. Acta Limnol. Bras. 2020, 32, e3. [Google Scholar] [CrossRef]
- Paramo, J.; Rodriguez, A.; Quintana, C. Growth Type and Relative Condition Factor as a Function of the Body Shape of Deep-Water Crustaceans in the Colombian Caribbean Sea. PeerJ 2024, 12, e16583. [Google Scholar] [CrossRef] [PubMed]
- Kiko, R.; Hauss, H.; Dengler, M.; Sommer, S.; Melzner, F. The Squat Lobster Pleuroncodes monodon Tolerates Anoxic “Dead Zone” Conditions off Peru. Mar. Biol. 2015, 162, 1913–1921. [Google Scholar] [CrossRef]
- Wang, X.; Li, E.; Xu, C.; Qin, J.G.; Wang, S.; Chen, X.; Cai, Y.; Chen, K.; Gan, L.; Yu, N.; et al. Growth, Body Composition, Ammonia Tolerance and Hepatopancreas Histology of White Shrimp Itopenaeus vannamei Fed Diets Containing Different Carbohydrate Sources at Low Salinity. Aquac. Res. 2016, 47, 1932–1943. [Google Scholar] [CrossRef]
- Li, W.-F.; Li, S.; Liu, J.; Wang, X.-F.; Chen, H.-Y.; Hao, H.; Wang, K.-J. Vital Carbohydrate and Lipid Metabolites in Serum Involved in Energy Metabolism during Pubertal Molt of Mud Crab (Scylla paramamosain). Metabolites 2021, 11, 651. [Google Scholar] [CrossRef]
- García-Guerrero, M.; Villarreal, H.; Racotta, I.S. Effect of Temperature on Lipids, Proteins, and Carbohydrates Levels during Development from Egg Extrusion to Juvenile Stage of Cherax quadricarinatus (Decapoda: Parastacidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 147–154. [Google Scholar] [CrossRef]
- Fagetti, E.; Campodonico, I. Larval Development of the Red Crab Pleuroncodes monodon (Decapoda Anomura: Galatheidae) under Laboratory Conditions. Mar. Biol. 1971, 8, 70–81. [Google Scholar] [CrossRef]
- Yannicelli, B.; Castro, L.R.; Schneider, W.; Sobarzo, M. Crustacean Larvae Distribution in the Coastal Upwelling Zone off Central Chile. Mar. Ecol. Prog. Ser. 2006, 319, 175–189. [Google Scholar] [CrossRef]
- Buckup, L.; Dutra, B.K.; Ribarcki, F.P.; Fernandes, F.A.; Noro, C.K.; Oliveira, G.T.; Vinagre, A.S. Seasonal Variations in the Biochemical Composition of the Crayfish Parastacus defossus (Crustacea, Decapoda) in Its Natural Environment. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lemos, D.; Hernández-Cortés, M.P.; Navarrete, A.; Garcia-Carreño, F.L.; Phan, V.N. Ontogenetic Variation in Digestive Proteinase Activity of Larvae and Postlarvae of the Pink Shrimp Farfantepenaeus paulensis (Crustacea: Decapoda: Penaeidae). Mar. Biol. 1999, 135, 653–662. [Google Scholar] [CrossRef]
- Kattner, G.; Graeve, M.; Calcagno, J.A.; Lovrich, G.A.; Thatje, S.; Anger, K. Lipid, Fatty Acid and Protein Utilization during Lecithotrophic Larval Development of Lithodes santolla (Molina) and Paralomis granulosa (Jacquinot). J. Exp. Mar. Biol. Ecol. 2003, 292, 61–74. [Google Scholar] [CrossRef]
- Anger, K. The Biology of Decapod Crustacean Larvae, 1st ed.; Balkema, A.A., Ed.; CRC Press: Lisse, The Netherlands, 2001; Volume 14, p. 405. [Google Scholar]
- Cretton, M.; Malanga, G.; Mazzuca Sobczuk, T.; Mazzuca, M. Marine Lipids as a Source of High-Quality Fatty Acids and Antioxidants. Food Rev. Int. 2022, 39, 4941–4964. [Google Scholar] [CrossRef]
- Mejri, S.C.; Tremblay, R.; Audet, C.; Wills, P.S.; Riche, M. Essential Fatty Acid Requirements in Tropical and Cold-Water Marine Fish Larvae and Juveniles. Front. Mar. Sci. 2021, 8, 680003. [Google Scholar] [CrossRef]
- Bascur, M.; Guzmán, F.; Mora, S.; Espinoza, P.; Urzúa, Á. Temporal Variation in the Fatty Acid Composition of Ovigerous Females and Embryos of the Squat Lobster Pleuroncodes monodon (Decapoda, Munididae). J. Mar. Biol. Assoc. UK 2018, 98, 1977–1990. [Google Scholar] [CrossRef]
- Urzúa, Á.; Paschke, K.; Gebauer, P.; Anger, K. Seasonal and Interannual Variations in Size, Biomass and Chemical Composition of the Eggs of North Sea Shrimp, Crangon crangon (Decapoda: Caridea). Mar. Biol. 2012, 159, 583–599. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-Chain Polyunsaturated Fatty Acid Sources and Evaluation of Their Nutritional and Functional Properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Parzanini, C.; Parrish, C.C.; Hamel, J.-F.; Mercier, A. Functional Diversity and Nutritional Content in a Deep-Sea Faunal Assemblage through Total Lipid, Lipid Class, and Fatty Acid Analyses. PLoS ONE 2018, 13, e0207395. [Google Scholar] [CrossRef]
- Pond, D.W.; Tarling, G.A.; Mayor, D.J. Hydrostatic Pressure and Temperature Effects on the Membranes of a Seasonally Migrating Marine Copepod. PLoS ONE 2014, 9, e111043. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Vasconi, M.; Aidos, L.; Di Giancamillo, A.; Bellagamba, F.; Domeneghini, C.; Moretti, V.M. Effect of Temperature on Fatty Acid Composition and Development of Unfed Siberian Sturgeon (A. baerii) Larvae. J. Appl. Ichthyol. 2019, 35, 296–302. [Google Scholar] [CrossRef]
- Saha, N.; Ullah, M.R.; Islam, M.S.; Hossain, M.B. Morphometric Relationships between Length-Weight and Length-Length and Condition Factor of Four Small Indigenous Fishes from the Payra River, Southern Bangladesh. Arch. Agric. Environ. Sci. 2019, 4, 230–234. [Google Scholar] [CrossRef]
- Rodriguez, A.; Mendoza, K.; Paramo, J. Length-Weight Relationships and Relative Condition Factor of 53 Species of Shallow-Water Fish in the Colombian Caribbean Sea. J. Appl. Ichthyol. 2023, 2023, 6632464. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Woods, W.A., Jr. Condition Indices for Conservation: New Uses for Evolving Tools. Integr. Comp. Biol. 2006, 46, 1169–1190. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Akester, M.; Naranjo, L. Productivity and Sustainable Management of the Humboldt Current Large Marine Ecosystem under Climate Change. Environ. Dev. 2016, 17, 126–144. [Google Scholar] [CrossRef]
- Völker, D.; Geersen, J.; Contreras-Reyes, E.; Sellanes, J.; Pantoja, S.; Rabbel, W.; Thorwart, M.; Reichert, C.; Block, M.; Weinrebe, W.R. Morphology and Geology of the Continental Shelf and Upper Slope of Southern Central Chile (33°S–43°S). Int. J. Earth Sci. 2014, 103, 1765–1787. [Google Scholar] [CrossRef]


| Morphotype | Sex | N | b | a | R2 |
|---|---|---|---|---|---|
| Pelagic | Male | 101 | 3.1547 | 0.00008 | 0.64 |
| Female | 39 | 3.2338 | 0.00006 | 0.81 | |
| Benthic | Male | 16 | 1.8479 | 0.0169 | 0.86 |
| Female | 18 | 2.9806 | 0.0003 | 0.91 |
| Biochemical Condition (mg 20 mg Dry Weight−1) | Morphotype | Male | Female | ||
|---|---|---|---|---|---|
| N | X ± DS | N | X ± DS | ||
| Glucose | Pelagic | 12 | 0.67 ± 0.18 a | 8 | 0.56 ± 0.09 a |
| Benthic | 21 | 0.39 ± 0.20 b | 18 | 0.29 ± 0.12 b | |
| Protein | Pelagic | 12 | 6.02 ± 2.67 a | 8 | 5.56 ± 1.73 a |
| Benthic | 21 | 8.42 ± 2.45 b | 18 | 8.03 ± 2.18 b | |
| Lipid | Pelagic | 12 | 0.33 ± 0.04 a | 5 | 0.43 ± 0.16 a |
| Benthic | 21 | 0.72 ± 0.06 b | 18 | 0.87 ± 0.17 b |
| Pisco | Faro Carranza | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FA | Male | Female | Male | Female | |||||
| Mean ± SD | % | Mean ± SD | % | Mean ± SD | % | Mean ± SD | % | ||
| SFA | C14:0 | 0.98 ± 0.01 | 14.16 | 0.97 ± 0.00 | 13.45 | 1.09 ± 0.11 | 7.42 | 1.08 ± 0.11 | 7.95 |
| C15:0 | 0.58 ± 0.04 | 3.92 | 0.53 ± 0.15 | 3.88 | |||||
| C16:0 | 1.63 ± 0.26 | 23.60 | 1.72 ± 0.10 | 23.81 | 3.24 ± 0.43 | 22.06 | 3.22 ± 0.39 | 23.72 | |
| C17:0 | 0.54 ± 0.15 | 3.71 | 0.54 ± 0.14 | 3.99 | |||||
| C18:0 | 1.12 ± 0.08 | 16.12 | 1.10 ± 0.05 | 15.22 | 1.50 ± 0.09 | 10.24 | 1.55 ± 0.07 | 11.44 | |
| Total SFA | 3.37 ± 0.33 | 53.88 | 3.79 ± 0.34 | 52.48 | 6.96 ± 1.04 | 47.35 | 6.91 ± 1.02 | 50.98 | |
| MUFAs | C16:1 | 0.63 ± 0.04 | 8.69 | 0.64 ± 0.04 | 4.33 | 0.69 ± 0.06 | 5.11 | ||
| C18:1n9 | 1.56 ± 0.09 | 22.59 | 1.52 ± 0.09 | 21.02 | 2.40 ± 0.29 | 16.36 | 2.35 ± 0.38 | 17.33 | |
| Total MUFAs | 1.56 ± 0.09 | 22.59 | 2.14 ± 0.44 | 29.71 | 3.04 ± 0.88 | 20.68 | 3.04 ± 0.78 | 22.44 | |
| PUFAs n6 | C18:3n6 | 0.59 ± 0.02 | 3.98 | 0.57 ± 0.02 | 4.24 | ||||
| C20:2n6 | 0.68 ± 0.00 | 4.61 | |||||||
| C20:3n6 | 0.73 ± 0.02 | 4.97 | 0.79 ± 0.04 | 5.83 | |||||
| Total PUFAs n6 | 1.99 ± 0.07 | 13.56 | 1.36 ± 0.11 | 10.06 | |||||
| PUFAs n3 | C20:5n3 | 0.77 ± 0.09 | 11.05 | 0.68 ± 0.40 | 9.43 | 1.43 ± 0.52 | 9.75 | 1.19 ± 1.13 | 8.79 |
| C22:6n3 | 0.86 ± 0.13 | 12.48 | 0.60 ± 0.34 | 8.38 | 1.27 ± 0.45 | 8.66 | 1.05 ± 0.94 | 7.73 | |
| Total PUFAs n3 | 1.63 ± 0.12 | 23.53 | 1.28 ± 0.35 | 17.81 | 2.70 ± 0.49 | 18.41 | 2.24 ± 1.03 | 16.52 | |
| Total PUFAs | 0.163 ± 0.38 | 23.53 | 1.28 ± 0.35 | 17.81 | 4.70 ± 0.51 | 31.97 | 3.61 ± 0.95 | 26.58 | |
| Total FA | 6.93 ± 0.38 | 100.00 | 7.22 ± 0.49 | 100.00 | 14.69 ± 0.92 | 100.00 | 13.57 ± 1.01 | 100.00 | |
| Groups | R Statistic | Significance Level % |
|---|---|---|
| Pelagic-Male, Benthic-Male | 0.654 | 0.001 |
| Pelagic-Male, Pelagic-Female | 0.193 | 0.069 |
| Pelagic-Male, Benthic-Female | 0.631 | 0.001 |
| Benthic-Male, Pelagic-Female | 0.443 | 0.003 |
| Benthic-Male, Benthic-Female | 0.014 | 0.276 |
| Pelagic-Female, Benthic-Female | 0.431 | 0.002 |
| Morphotype | Fatty Acid | Av. Value | Av. Sq. Dist | Sq. Dist/SD | Contrib % |
|---|---|---|---|---|---|
| Pelagic | C14:0 | 0.185 | 4.55E-02 | 0.42 | 8.77 |
| C20:5n3-EPA | 0.31 | 8.41E-02 | 0.58 | 16.21 | |
| C22:6n3-DHA | 0.314 | 9.87E-02 | 0.57 | 19.02 | |
| C18:1n9 | 0.548 | 0.205 | 0.54 | 39.55 | |
| Benthic | C16:1 | 0.199 | 6.36E-02 | 0.57 | 9.44 |
| C22:6n3-DHA | 0.631 | 0.176 | 0.5 | 26.17 | |
| C20:5n3-EPA | 0.683 | 0.211 | 0.5 | 31.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe-Machaca, M.; Zilleruelo, M.; Espinoza, P.; Torres, G.; Urzúa, Á. Living Along Distribution Margins: Differences in the Body and Biochemistry of Red Squat Lobster Morphotypes (Grimothea monodon) from the Humboldt Current System. Fishes 2024, 9, 445. https://doi.org/10.3390/fishes9110445
Quispe-Machaca M, Zilleruelo M, Espinoza P, Torres G, Urzúa Á. Living Along Distribution Margins: Differences in the Body and Biochemistry of Red Squat Lobster Morphotypes (Grimothea monodon) from the Humboldt Current System. Fishes. 2024; 9(11):445. https://doi.org/10.3390/fishes9110445
Chicago/Turabian StyleQuispe-Machaca, Marco, Maximiliano Zilleruelo, Pepe Espinoza, Gabriela Torres, and Ángel Urzúa. 2024. "Living Along Distribution Margins: Differences in the Body and Biochemistry of Red Squat Lobster Morphotypes (Grimothea monodon) from the Humboldt Current System" Fishes 9, no. 11: 445. https://doi.org/10.3390/fishes9110445
APA StyleQuispe-Machaca, M., Zilleruelo, M., Espinoza, P., Torres, G., & Urzúa, Á. (2024). Living Along Distribution Margins: Differences in the Body and Biochemistry of Red Squat Lobster Morphotypes (Grimothea monodon) from the Humboldt Current System. Fishes, 9(11), 445. https://doi.org/10.3390/fishes9110445






