Population Genetics and Gene Flow in Cyphotilapia frontosa and Cyphotilapia gibberosa Along the East Coast of Lake Tanganyika
Abstract
:1. Introduction
1.1. The East African Great Lakes
1.2. The Genus Cyphotilapia
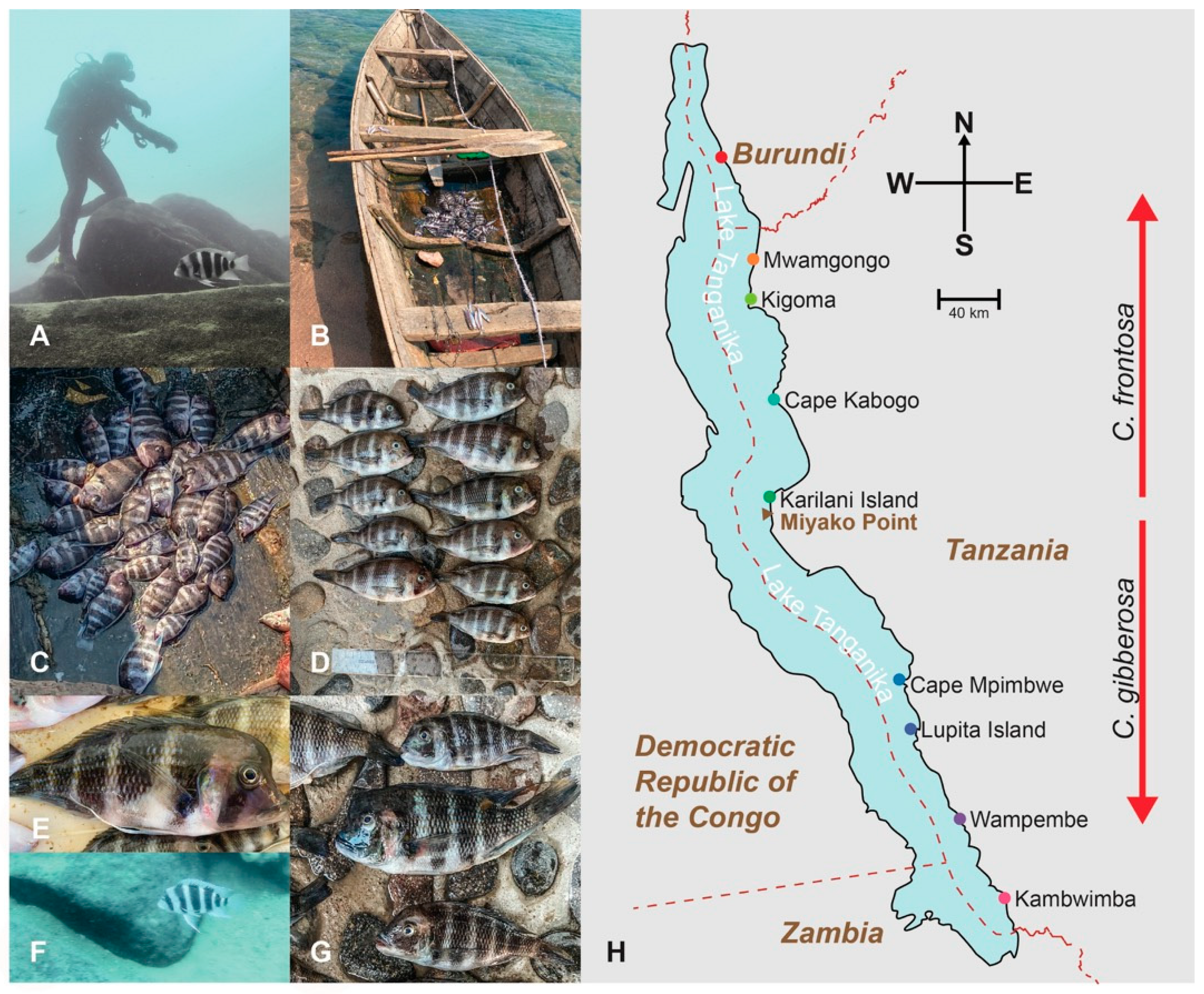
2. Materials and Methods
2.1. DNA Extraction, Preparation, and Sequencing of Genomic Libraries
2.2. Population Genetics Analysis
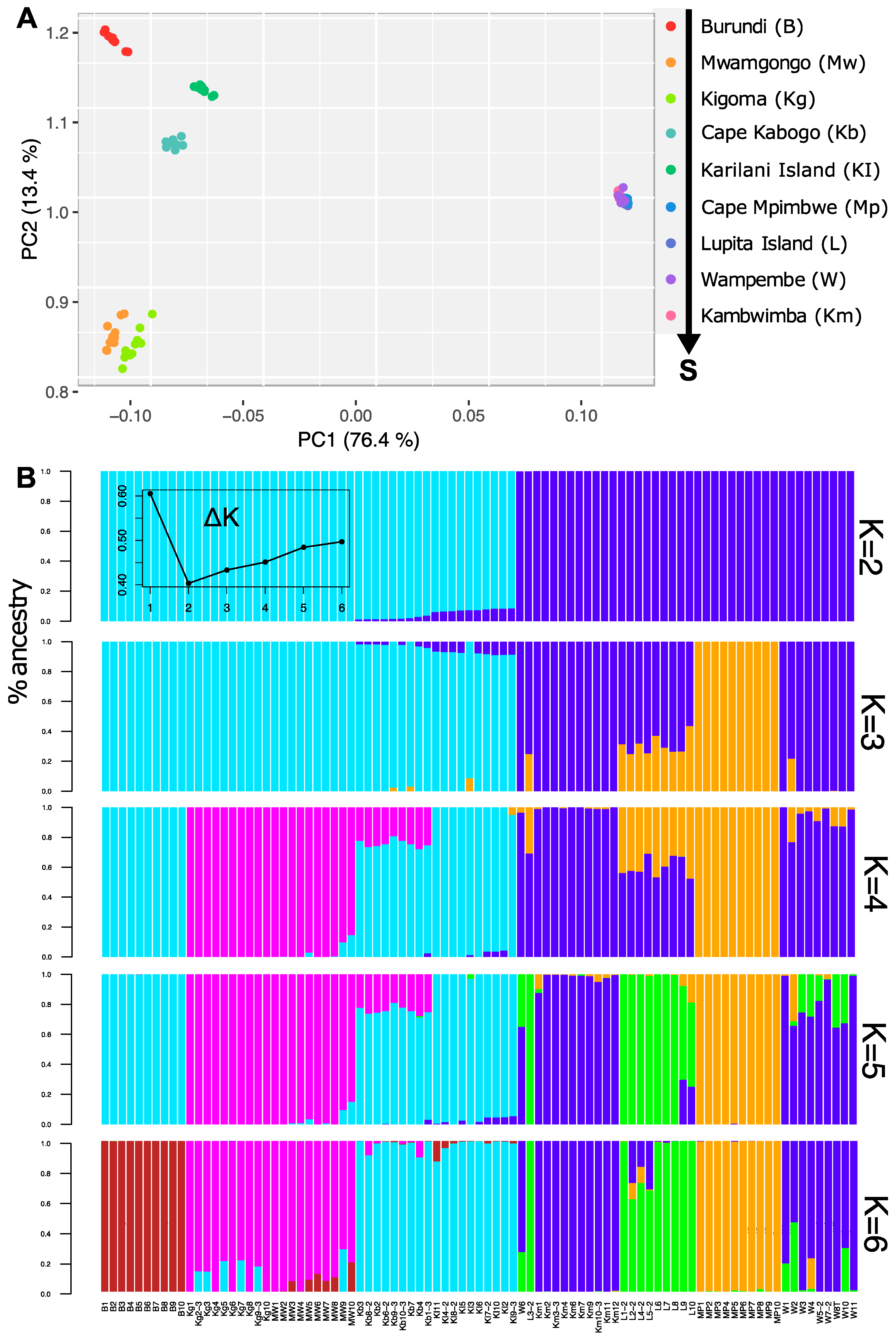
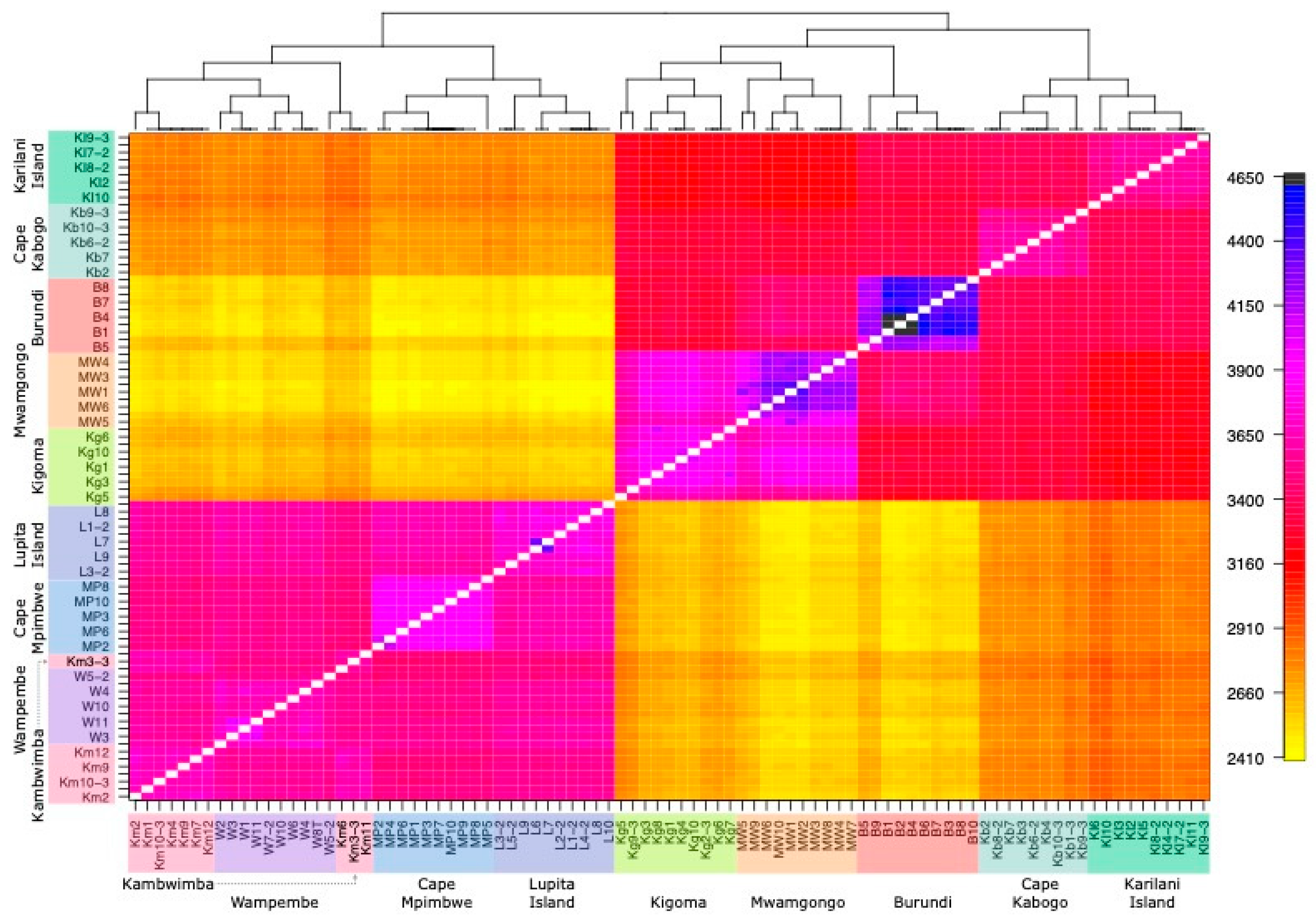
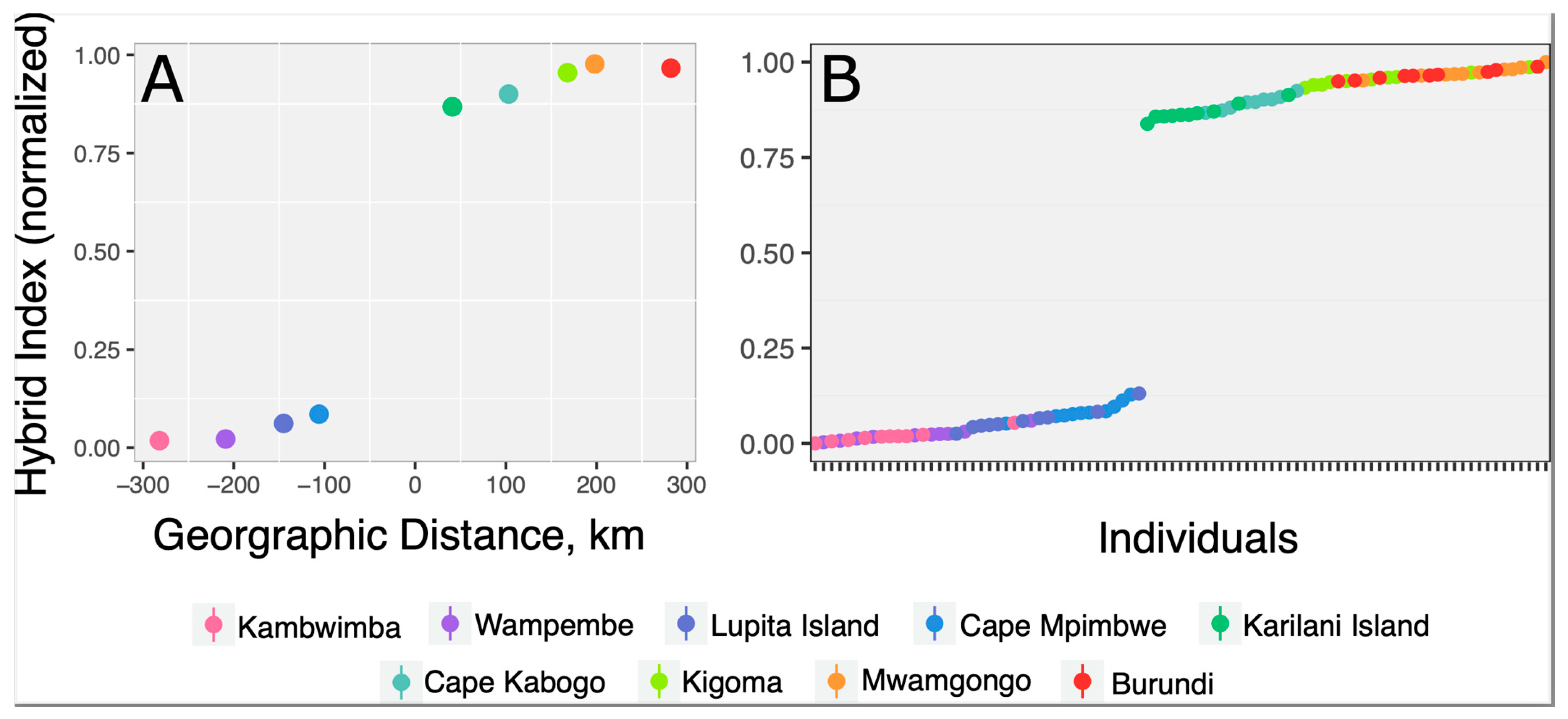
3. Results
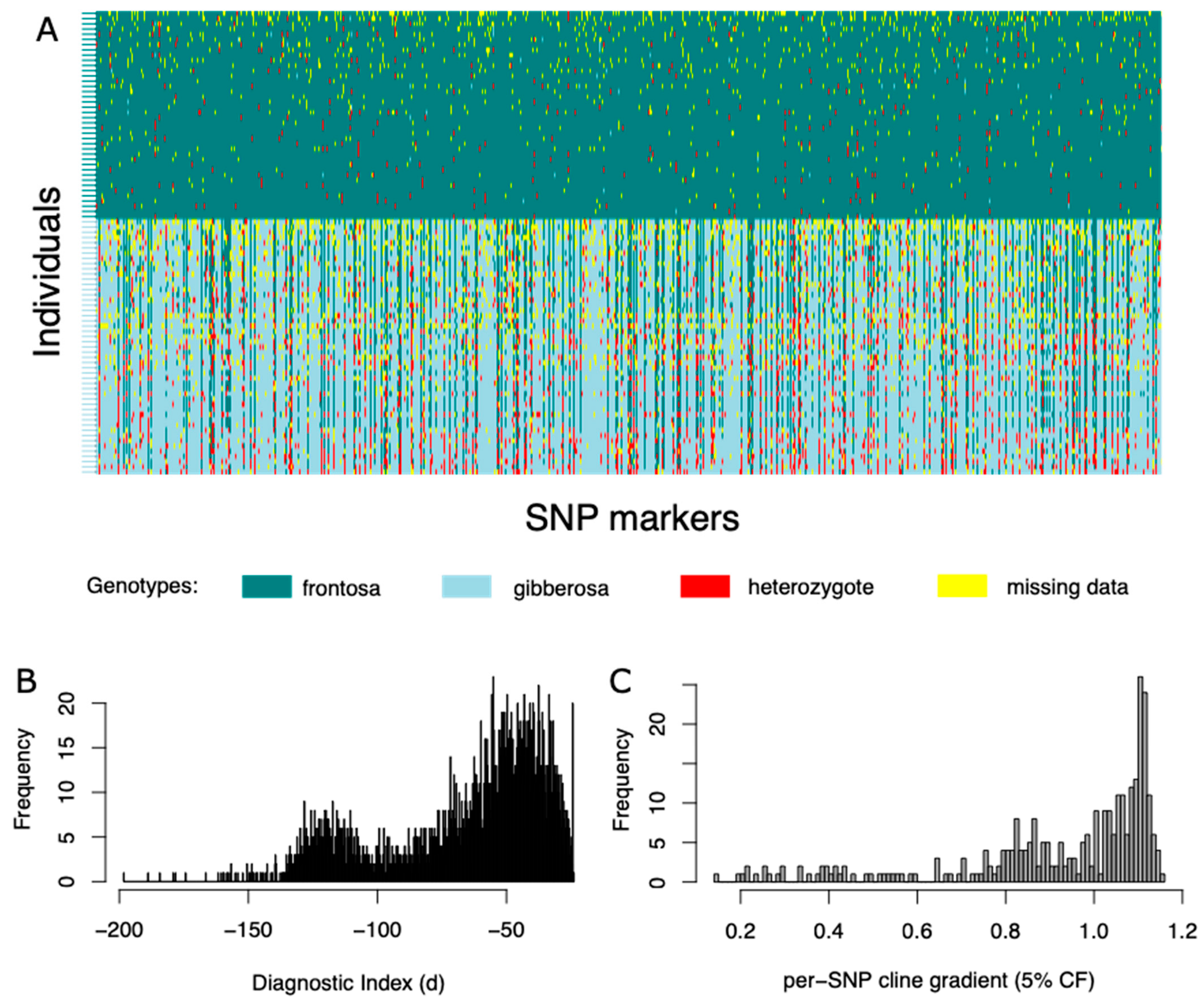
3.1. Genetic Clustering and Visualization Analyses
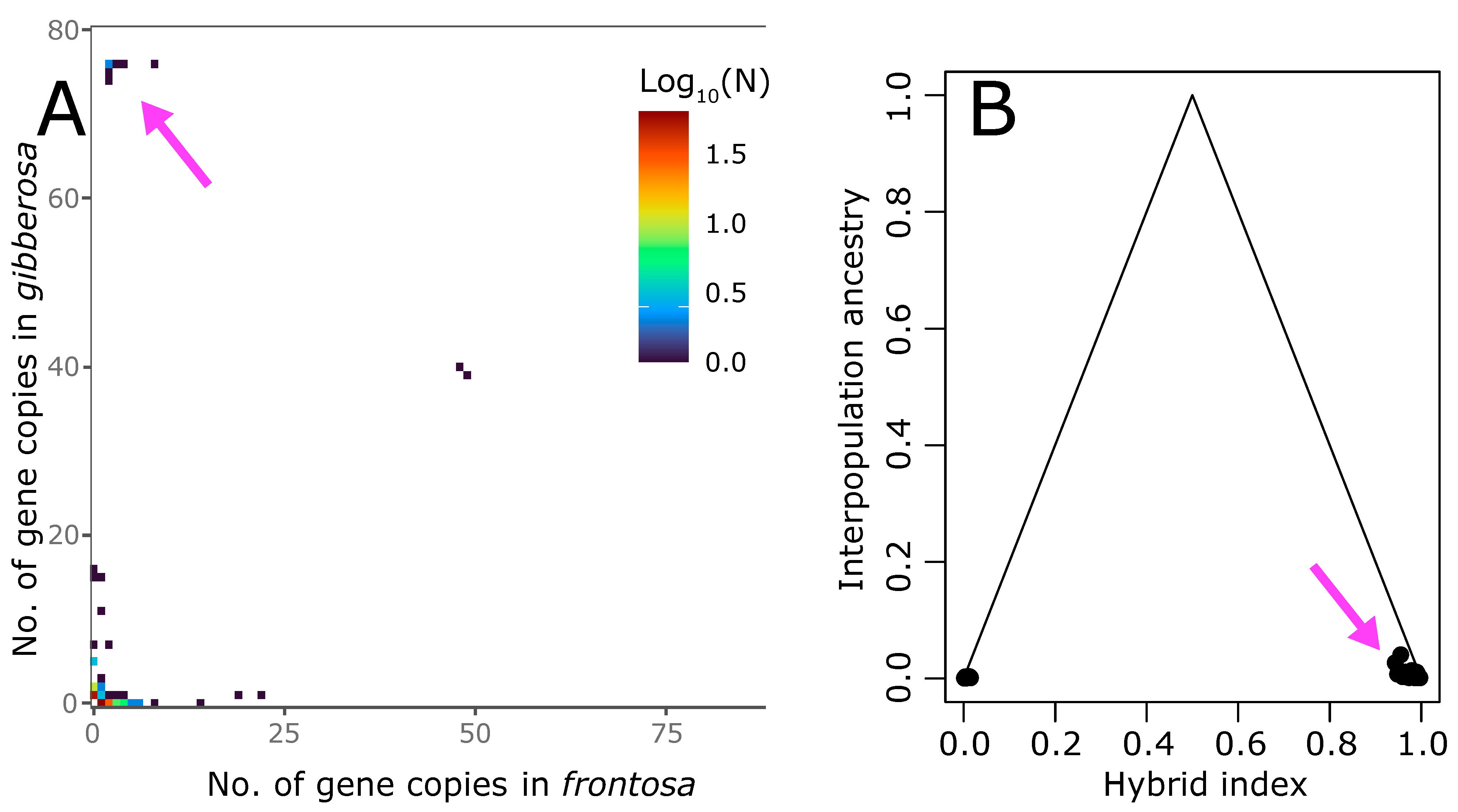
3.2. Clinal Variation and Gene Introgression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salzburger, W.; Mack, T.; Verheyen, E.; Meyer, A. Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol. Biol. 2005, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Kocher, T.D. Ghost of speciation past. Nature 2005, 435, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Egger, B.; Koblmüller, S.; Sturmbauer, C.; Sefc, K.M. Nuclear and mitochondrial data reveal different evolutionary processes in the Lake Tanganyika cichlid genus Tropheus. BMC Evol. Biol. 2007, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Salzburger, W. Understanding explosive diversification through cichlid fish genomics. Nat. Rev. Genet. 2018, 19, 705–717. [Google Scholar] [CrossRef]
- Meier, J.I.; McGee, M.D.; Marques, D.A.; Mwaiko, S.; Kishe, M.; Wandera, S.; Neumann, D.; Mrosso, H.; Chapman, L.J.; Chapman, C.A. Cycles of fusion and fission enabled rapid parallel adaptive radiations in African cichlids. Science 2023, 381, eade2833. [Google Scholar] [CrossRef]
- Koblmüller, S.; Duftner, N.; Sefc, K.M.; Aibara, M.; Stipacek, M.; Blanc, M.; Egger, B.; Sturmbauer, C. Reticulate phylogeny of gastropod-shell-breeding cichlids from Lake Tanganyika—The result of repeated introgressive hybridization. BMC Evol. Biol. 2007, 7, 7. [Google Scholar] [CrossRef]
- Wagner, C.E. Ecological opportunity, genetic variation, and the origins of African cichlid radiations. In The Behavior, Ecology and Evolution of Cichlid Fishes; Springer: Dordrecht, The Netherlands, 2021; pp. 79–105. [Google Scholar]
- Seehausen, O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004, 19, 198–207. [Google Scholar] [CrossRef]
- Sturmbauer, C.; Koblmüller, S.; Sefc, K.M.; Duftner, N. Phylogeographic history of the genus Tropheus, a lineage of rock-dwelling cichlid fishes endemic to Lake Tanganyika. In Aquatic Biodiversity II; Springer: Dordrecht, The Netherlands, 2005; pp. 335–366. [Google Scholar]
- Sefc, K.M.; Mattersdorfer, K.; Ziegelbecker, A.; Neuhüttler, N.; Steiner, O.; Goessler, W.; Koblmüller, S. Shifting barriers and phenotypic diversification by hybridisation. Ecol. Lett. 2017, 20, 651–662. [Google Scholar] [CrossRef]
- Sefc, K.M.; Mattersdorfer, K.; Hermann, C.M.; Koblmüller, S. Past lake shore dynamics explain present pattern of unidirectional introgression across a habitat barrier. Hydrobiologia 2017, 791, 69–82. [Google Scholar] [CrossRef]
- Wagner, C.E.; McCUNE, A.R.; Lovette, I.J. Recent speciation between sympatric Tanganyikan cichlid colour morphs. Mol. Ecol. 2012, 21, 3283–3292. [Google Scholar] [CrossRef]
- Konings, A. Tanganyika Cichlids in Their Natural Habitat, 4th ed.; Cichlid Press: El Paso, TX, USA, 2019; p. 662. [Google Scholar]
- Takahashi, T.; Ngatunga, B.; Snoeks, J. Taxonomic status of the six-band morph of Cyphotilapia frontosa (Perciformes: Cichlidae) from Lake Tanganyika, Africa. Ichthyol. Res. 2007, 54, 55–60. [Google Scholar] [CrossRef]
- Konings, A. None other than Cyphotilapia frontosa. Cichlid News, 2019; pp. 11–16. [Google Scholar]
- Poll, M. Poissons Cichlidae. Exploration Hydrobiologique du Lac Tanganika (1946–1947)—Résultats Scientifiques. Institut Royal des Sciences Naturelles de Belgique: Bruxelles, Belgium, 1956; Volume 3:1. [Google Scholar]
- Takahashi, T.; Nakaya, K. New species of Cyphotilapia (Perciformes: Cichlidae) from Lake Tanganyika, Africa. Copeia 2003, 2003, 824–832. [Google Scholar] [CrossRef]
- Ronco, F.; Matschiner, M.; Böhne, A.; Boila, A.; Büscher, H.H.; El Taher, A.; Indermaur, A.; Malinsky, M.; Ricci, V.; Kahmen, A. Drivers and dynamics of a massive adaptive radiation in cichlid fishes. Nature 2021, 589, 76–81. [Google Scholar] [CrossRef]
- Brelsford, A.; Dufresnes, C.; Perrin, N. High-density sex-specific linkage maps of a European tree frog (Hyla arborea) identify the sex chromosome without information on offspring sex. Heredity 2016, 116, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Rochette, N.C.; Rivera-Colón, A.G.; Catchen, J.M. Stacks 2: Analytical methods for paired-end sequencing improve RADseq-based population genomics. Mol. Ecol. 2019, 28, 4737–4754. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4. [Google Scholar] [CrossRef]
- Malinsky, M.; Trucchi, E.; Lawson, D.J.; Falush, D. RADpainter and fineRADstructure: Population inference from RADseq data. Mol. Biol. Evol. 2018, 35, 1284–1290. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Giakoumis, M.; Pinilla-Buitrago, G.E.; Musher, L.J.; Wares, J.P.; Baird, S.J.; Hickerson, M.J. Evidence of introgression, ecological divergence and adaptation in Asterias sea stars. Mol. Ecol. 2023, 32, 5541–5557. [Google Scholar] [CrossRef] [PubMed]
- Gompert, Z.; DeRaad, D.A.; Buerkle, C.A. A next generation of hierarchical Bayesian analyses of hybrid zones enables direct quantification of variation in introgression in R. bioRxiv, 2024. [Google Scholar]
- Hemstrom, W.; Jones, M. snpR: User friendly population genomics for SNP data sets with categorical metadata. Mol. Ecol. Resour. 2023, 23, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.H.; Hewitt, G.M. Analysis of hybrid zones. Annu. Rev. Ecol. Syst. 1985, 16, 113–148. [Google Scholar] [CrossRef]
- Kocher, T.D. Adaptive evolution and explosive speciation: The cichlid fish model. Nat. Rev. Genet. 2004, 5, 288–298. [Google Scholar] [CrossRef]
- Takahashi, T.; Sota, T. A robust phylogeny among major lineages of the East African cichlids. Mol. Phylogenet. Evol. 2016, 100, 234–242. [Google Scholar] [CrossRef]
- Meirmans, P.G. The trouble with isolation by distance. Mol. Ecol. 2012, 21, 2839–2846. [Google Scholar] [CrossRef]
- Szymura, J.M.; Barton, N.H. Genetic analysis of a hybrid zone between the fire-bellied toads, Bombina bombina and B. variegata, near Cracow in southern Poland. Evolution 1986, 40, 1141–1159. [Google Scholar] [CrossRef]
- Yanchukov, A.; Hofman, S.; Szymura, J.M.; Mezhzherin, S.V.; Morozov-Leonov, S.Y.; Barton, N.H.; Nürnberger, B. Hybridization of Bombina bombina and B. variegata (Anura, Discoglossidae) at a sharp ecotone in western Ukraine: Comparisons across transects and over time. Evolution 2006, 60, 583–600. [Google Scholar] [CrossRef]
- Gasse, F.; Lédée, V.; Massault, M.; Fontes, J.-C. Water-level fluctuations of Lake Tanganyika in phase with oceanic changes during the last glaciation and deglaciation. Nature 1989, 342, 57–59. [Google Scholar] [CrossRef]
- Scholz, C.A.; King, J.W.; Ellis, G.S.; Swart, P.K.; Stager, J.C.; Colman, S.M. Paleolimnology of Lake Tanganyika, East Africa, over the past 100 kyr. J. Paleolimnol. 2003, 30, 139–150. [Google Scholar] [CrossRef]
- Cohen, A.S.; Stone, J.R.; Beuning, K.R.; Park, L.E.; Reinthal, P.N.; Dettman, D.; Scholz, C.A.; Johnson, T.C.; King, J.W.; Talbot, M.R. Ecological consequences of early Late Pleistocene megadroughts in tropical Africa. Proc. Natl. Acad. Sci. USA 2007, 104, 16422–16427. [Google Scholar] [CrossRef] [PubMed]
- Duftner, N.; Sefc, K.M.; Koblmueller, S.; Nevado, B.; Verheyen, E.; Phiri, H.; Sturmbauer, C. Distinct population structure in a phenotypically homogeneous rock-dwelling cichlid fish from Lake Tanganyika. Mol. Ecol. 2006, 15, 2381–2395. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, K.; Rüber, L.; Genner, M.J. Lake level fluctuations and divergence of cichlid fish ecomorphs in Lake Tanganyika. Hydrobiologia 2017, 791, 21–34. [Google Scholar] [CrossRef]
- Lewanski, A.L.; Golcher-Benavides, J.; Rick, J.A.; Wagner, C.E. Variable hybridization between two Lake Tanganyikan cichlid species in recent secondary contact. Mol. Ecol. 2022, 31, 5041–5059. [Google Scholar] [CrossRef]
- Koblmüller, S.; Odhiambo, E.A.; Sinyinza, D.; Sturmbauer, C.; Sefc, K.M. Big fish, little divergence: Phylogeography of Lake Tanganyika’s giant cichlid, Boulengerochromis microlepis. Hydrobiologia 2015, 748, 29–38. [Google Scholar] [CrossRef]
| Population | private.al. | al.rich. | Fis | Tajima’s D | π |
|---|---|---|---|---|---|
| Burundi | 8445 | 1.173 | 0.164 | 0.194 | 0.074 |
| Kambwimba | 4937 | 1.162 | 0.296 | 0.116 | 0.061 |
| Cape Kabogo | 1313 | 1.150 | 0.234 | 0.078 | 0.057 |
| Karilani Island | 3559 | 1.167 | 0.298 | 0.629 | 0.060 |
| Kigoma | 4000 | 1.185 | 0.083 | 0.273 | 0.072 |
| Lupita Island | 6701 | 1.167 | 0.166 | 0.133 | 0.074 |
| Cape Mpimbwe | 5597 | 1.147 | 0.214 | 0.391 | 0.071 |
| Mwamgongo | 8937 | 1.269 | −0.083 | −0.080 | 0.111 |
| Wampembe | 6247 | 1.206 | 0.254 | −0.051 | 0.081 |
| Kabwimba (g) | Cape Kabogo (f) | Karalani Island (f) | Kigoma (f) | Lupita Island (g) | Cape Mpimbwe (g) | Mwamgongo (f) | Wampembe (g) | |
|---|---|---|---|---|---|---|---|---|
| Burundi (f) | 0.443 | 0.148 | 0.154 | 0.196 | 0.473 | 0.483 | 0.150 | 0.390 |
| Kabwimba (g) | 0.349 | 0.349 | 0.381 | 0.039 | 0.070 | 0.294 | 0.015 | |
| Kabogo (f) | 0.048 | 0.090 | 0.424 | 0.438 | 0.079 | 0.327 | ||
| Karalani_Island (f) | 0.086 | 0.403 | 0.417 | 0.069 | 0.304 | |||
| Kigoma (f) | 0.417 | 0.429 | 0.014 | 0.319 | ||||
| Lupita_Island (g) | 0.039 | 0.329 | 0.041 | |||||
| Cape Mpimbwe (g) | 0.334 | 0.067 | ||||||
| Mwamgongo (f) | 0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, G.D.; Standish, T.; Çetintaş, O.; Zinenko, O.; Shechonge, A.H.; Yanchukov, A. Population Genetics and Gene Flow in Cyphotilapia frontosa and Cyphotilapia gibberosa Along the East Coast of Lake Tanganyika. Fishes 2024, 9, 481. https://doi.org/10.3390/fishes9120481
Jackson GD, Standish T, Çetintaş O, Zinenko O, Shechonge AH, Yanchukov A. Population Genetics and Gene Flow in Cyphotilapia frontosa and Cyphotilapia gibberosa Along the East Coast of Lake Tanganyika. Fishes. 2024; 9(12):481. https://doi.org/10.3390/fishes9120481
Chicago/Turabian StyleJackson, George D., Timothy Standish, Ortaç Çetintaş, Oleksandr Zinenko, Asilatu H. Shechonge, and Alexey Yanchukov. 2024. "Population Genetics and Gene Flow in Cyphotilapia frontosa and Cyphotilapia gibberosa Along the East Coast of Lake Tanganyika" Fishes 9, no. 12: 481. https://doi.org/10.3390/fishes9120481
APA StyleJackson, G. D., Standish, T., Çetintaş, O., Zinenko, O., Shechonge, A. H., & Yanchukov, A. (2024). Population Genetics and Gene Flow in Cyphotilapia frontosa and Cyphotilapia gibberosa Along the East Coast of Lake Tanganyika. Fishes, 9(12), 481. https://doi.org/10.3390/fishes9120481






