In Vitro Culture of Glochidia and Morphological Changes in Juveniles of the Endangered Freshwater Mussel Solenaia oleivora
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials for In Vitro Culture of S. oleivora Glochidia
2.2. Influence of Five Nutritional Resources on the Transformation of Glochidium
2.3. Influence of Cultivation Density on the Transformation of Glochidium
2.4. Cultivation and Growth Determination and Observation of the Juveniles
2.5. Statistical Approach
3. Results
3.1. Influence of Different Sera on the Transformation of Glochidium
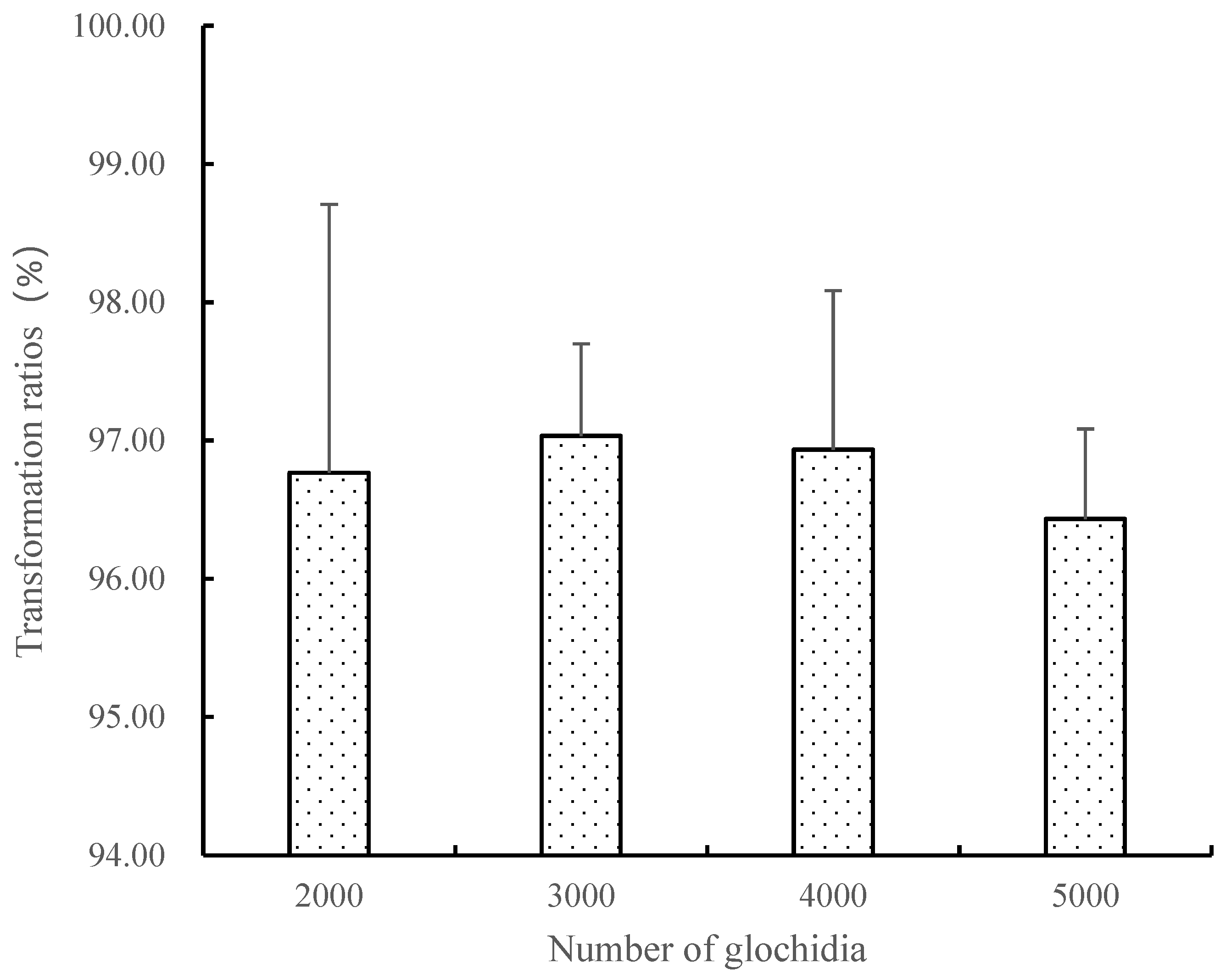
3.2. Influence of Cultivation Density on the Transformation of Glochidium
3.3. Growth Characteristics
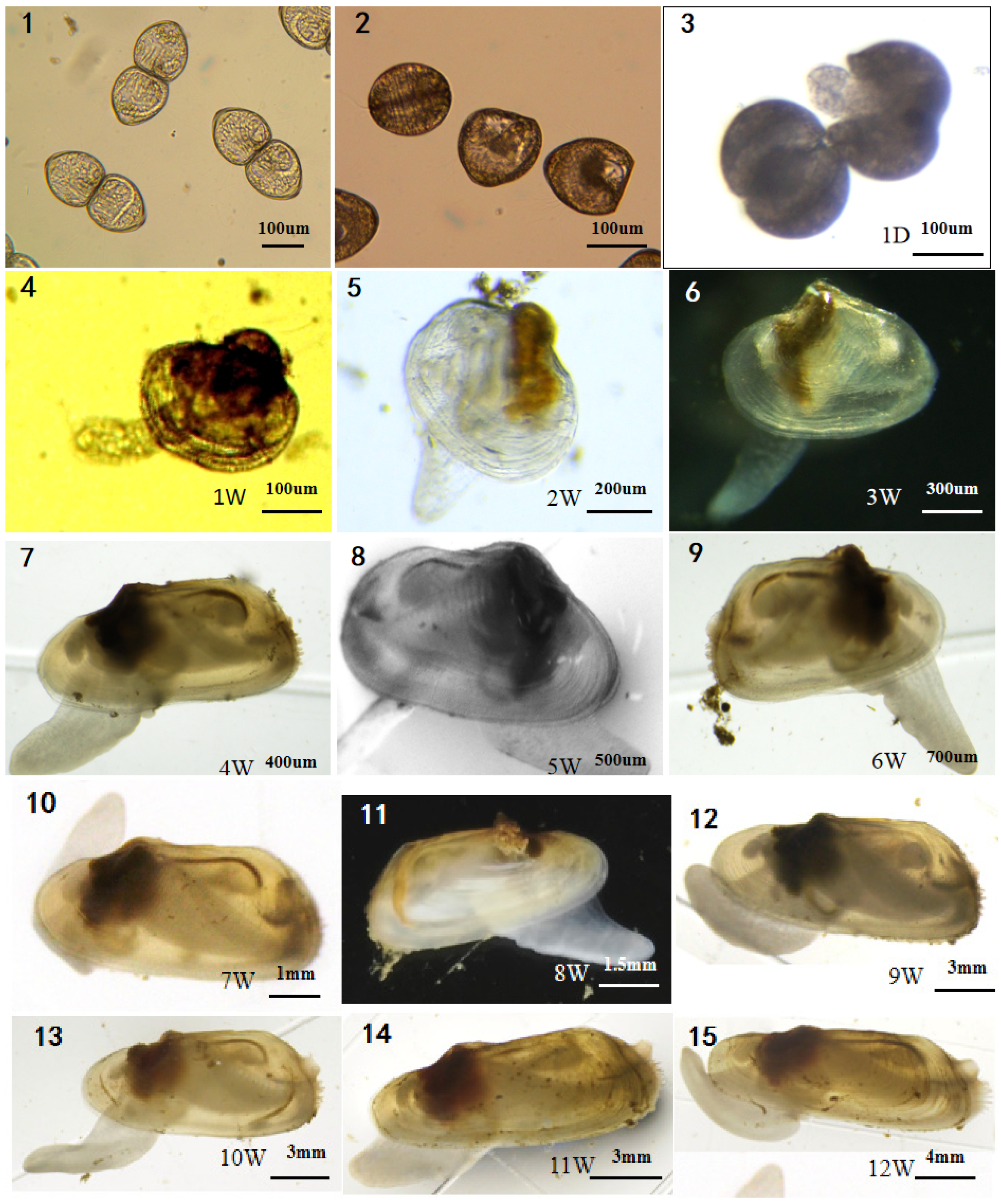
3.4. Morphological Development of the Juvenile
3.4.1. Rapid Anterior Growth Period
3.4.2. Distinct Anterior and Posterior Idiophase
3.4.3. Rapid Posterior Growth Period
4. Discussion
4.1. In Vitro Culture of S. oleivora Glochidium
4.2. Morphological Development of the Juvenile
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, T.A.; Moskalenko, I.V.; Strong, A.W.; Orlando, E.; Bouchet, L. Inverse compton origin of the hard X-ray and soft gamma-ray emission from the galactic ridge. Astrophys. J. 2008, 682, 400–407. [Google Scholar] [CrossRef]
- Bogan, A.E. Global diversity of freshwater mussels (mollusca, bivalvia) in freshwater. Hydrobiologia 2008, 595, 139–147. [Google Scholar] [CrossRef]
- Graf, D.L. Patterns of freshwater bivalve global diversity and the state of phylogenetic studies on the unionoida, sphaeriidae and cyrenidae. Am. Malacol. Bull. 2013, 31, 135–153. [Google Scholar] [CrossRef]
- Brusea, R.C.; Brusca, G.J. Phylum Porifera: The Sponge; Invertebrates; Sinauer Press: Sunderland, MA, USA, 1990; pp. 181–210. [Google Scholar]
- Graf, D.L.; Cummings, K.S. A ‘big data’ approach to global freshwater mussel diversity (Bivalvia: Unionoida), with an updated checklist of genera and species. J. Molluscan Stud. 2021, 87, eyab015. [Google Scholar] [CrossRef]
- Hu, Z.Q. Geographical distribution of endemic species of Chinese freshwater bivalves. Chin. J. Zool. 2005, 40, 80–83. [Google Scholar]
- Wen, H.B. Study of Germplasm of Major Economic Freshwater Mollusks of China; Nanjing Agricultural University: Nanjing, China, 2009. [Google Scholar]
- Ma, X.Y.; Sun, G.X.; Wang, L.; Xu, D.P.; Jin, W.; Lv, G.H.; Xu, P.; Ding, T.Q.; Wen, H.B.; Gu, R.B. Seasonal variations of nutrients and mineral elements in Solenaia oleivora from Huaihe River. J. Agron. 2021, 11, 90–94+119. [Google Scholar]
- Liu, Y.Y.; Zhang, W.Z.; Wang, Y.X. Economic Fauna of China: Freshwater Mollusks; Beijing Science Press: Beijing, China, 1979. [Google Scholar]
- Chen, Z.F.; Chen, P. Analysis of Nutritional Components of Solenaia oleivora. In Proceedings of the 65th Annual Meeting of Chinese Animal Society, Beijing, China, 25 April 1999; Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=84413ec88bbeae5a82266a19a787bcc2&site=xueshu_se (accessed on 21 January 2024).
- Xu, Q.Q.; Liu, J.; He, L.R. Analysis of meat content and muscle nutritional components of Solenaia oleivora. Freshw. Fish. 2003, 33, 28–29. [Google Scholar]
- Huang, Y.Y.; OuYang, S.; Wu, X.P.; Liu, H.Z. Phylogeny of the unionidae based on partial mitochondrial 16S rRNA sequences. Acta Hydrobiol. Sin. 2003, 27, 258–263. [Google Scholar]
- Huang, X.C. Complete Paternal and Maternal Mitochondrial Genomes of the Freshwater Mussel Genus Solenaia (Unionidae: Gonideinae) in China and Mitochondrial Phylogenomics of Unionoida; Nanchang University: Nanchang, China, 2014. [Google Scholar]
- Xu, Y. Isolation of Microsatellite Markersand Population Genetic Diversity Analysis in Solenaia oleivora; Huazhong Agriculture University: Wuhan, China, 2004. [Google Scholar]
- Yang, X.L.; Li, H.C.; Song, L. Gonadal development and growth of freshwater mussel Solenaia oleivora. Fish. Sci. 2011, 30, 580–582. [Google Scholar]
- Wang, Y.N. Productive Traits of the Freshwater Mussel Solenaia oleivora (Bivalvia: Unionidae) in the Tianmen River; Huazhong Agriculture University: Wuhan, China, 2013. [Google Scholar]
- Xia, Z.Q.; Han, X.Z.; Zhu, W.; Zhang, Y.F.; Ye, C.Y.; Liu, J. Preliminary study on reproductive biology of Solenaia oleivora. Jiangxi Fish. Sci. Technol. 2014, 21, 17–19. [Google Scholar]
- Xiong, L.F.; OuYang, S.; Chen, T.H.; Qi, T.; Wu, X.P. The resource status and spatio-temporal variation of freshwater mussels in Qinglan Lake of Jiangxi Province. Acta Agric. Univ. Jiangxiensis 2010, 32, 1257–1264. [Google Scholar]
- Bai, Z.Y.; Li, J.L.; Pan, B.B. Comparison of the parasitism effects of glochidia of triangle mussel (Hyriopsis cumingii) in five fishes. Freshw. Fish. 2008, 38, 3–5+43. [Google Scholar]
- Lima, P.; Lima, M.L.; Kovitvadhi, U.; Kovitvadhi, S.; Owen, C.; Machado, J. A review on the “in vitro culture of freshwater mussel (Unionoida). Hydrobiologia 2012, 691, 21–33. [Google Scholar] [CrossRef]
- Wen, H.B.; Gu, R.B.; Hua, D.; Qiu, L.H.; Xu, G.C.; Xu, P. Culture of glochidia of Hyriopsis cumingii in artificial media and morphological variation of transformed juvenile. J. Wuhan Univ. 2011, 57, 57–62. [Google Scholar]
- Ma, X.Y. In Vitro Culture Glochidia and the Development of Juveniles of Cristaria plicata; Nanjing Agricultural University: Nanjing, China, 2015. [Google Scholar]
- Wen, H.B. Study on Basic Biological Characteristics and Transformation and Development of Potamilu salatus; Nanjing Agricultural University: Nanjing, China, 2016. [Google Scholar]
- Ma, X.Y.; Wen, H.B.; Zou, J.; Jin, W.; Hua, D.; Gu, R.B.; Xu, P. An improved method for in vitro culture of glochidia in freshwater mussel Cristaria plicata (Mollusca, Bivalvia). Hydrobiologia 2018, 810, 133–144. [Google Scholar] [CrossRef]
- Liu, B.; Wang, M.Y.; Xie, J.; Xu, P.; Ge, X.P.; He, Y.J.; Miao, L.H.; Pan, L.K. Effects of acute cold stress onserum biochemical and immune parameters and liver HSP70 gene expression in GIFT strain of Nile tilapia (Oreochromis niloticus). Acta Ecol. Sin. 2011, 37, 4866–4873. [Google Scholar]
- Uthaiwan, K.; Noparatnaraporn, N.; Machado, J. Culture of glochidia of the freshwater pearl mussel Hyriopsis myersiana (Lea, 1856) in artificial media. Aquaculture 2001, 195, 61–69. [Google Scholar] [CrossRef]
- Qian, R.H.; Li, J.L.; Dong, Z.G.; Zheng, H.F.; Li, Y.S.; Yan, W.K. Morphological variations analysis among populations of Hyriopsis cumingii in five large lakes of China. Oceanol. Limnol. Sin. 2003, 34, 436–443. [Google Scholar]
- Uthaiwan, K.; Pakkong, P.; Noparatnaraporn, N.; Vilarinho, L.; Machado, J. Study of a suitable fish plasma for in vitro culture of glochidia Hyriopsis myersiana. Aquaculture 2002, 209, 197–208. [Google Scholar] [CrossRef]
- Davis, G.M.; Fuller, S. Genetic relationships among recent Unionscea (Bivalvia) of North America. Malacologia 1981, 20, 217–253. [Google Scholar]
- Owen, C.T.; Alexander, J.E.; Gregor, M. Control of microbial contamination during in vitro culture of larval unionid mussels. Invertebr. Reprod. Dev. 2010, 54, 187–193. [Google Scholar] [CrossRef]
- Chang, Y.Q. Shellfish Culture; Chinese Agriculture Press: Beijing, China, 2007. [Google Scholar]
- Liu, S.L.; Li, J.L.; Zhang, G.F.; Wang, G.L.; Bai, Z.Y.; Pan, B.B. Morphological development and growth characteristics of juvenile of Hyriopsis cumingii. J. Fish. China 2009, 33, 604–609. [Google Scholar]
- Liu, S.L.; Li, J.L.; Zhang, G.F.; Xu, S.J.; Wang, G.L.; Bai, Z.Y. Morphological development and growth characteristics of juvenile of Sinanodonta woodiana. J. Shanghai Ocean. Univ. 2009, 18, 269–274. [Google Scholar]
- Kovitvadhi, S.; Kovitvadhi, U.; Sawangwong, P.; Thongpan, A.; Machado, J. Optimization of diet and culture environment for larvae and juvenile freshwater pearl mussels, Hyriopsis (Limnoscapha) myersiana Lea, 1856. Invertebr. Reprod. Dev. 2006, 49, 61–70. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wei, Q.S. On reproductive biology of lamprotula scripta (Heude). J. Huazhong Agric. Univ. 1994, 13, 170–174. [Google Scholar]


| Group | L-15 (mL) | Antibiotic Mixture (mL) | Bighead Carp Plasma (mL) | Grass Carp Plasma (mL) | Common Carp Plasma (mL) | Bovine Serum (mL) | Rabbit Serum (mL) | Deionized Water (mL) |
|---|---|---|---|---|---|---|---|---|
| Control group | 2 | 0.5 | 1 | |||||
| A1 | 2 | 0.5 | 1 | |||||
| A2 | 2 | 0.5 | 1 | |||||
| A3 | 2 | 0.5 | 1 | |||||
| A4 | 2 | 0.5 | 1 | |||||
| A5 | 2 | 0.5 | 1 |
| Group | Uncontaminated Transformation Rate | Contamination Rate |
|---|---|---|
| No nutritional resources | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Bighead carp plasma | 97.47 ± 0.93 b | 0.00 ± 0.00 a |
| Grass carp plasma | 98.03 ± 0.71 b | 6.67 ± 11.54 a |
| Common carp plasma | 97.96 ± 0.76 b | 6.67 ± 11.54 a |
| Bovine serum | 60.09 ± 5.96 c | 26.67 ± 11.54 b |
| Rabbit serum | 39.69 ± 3.75 d | 40.00 ± 0.00 b |
| Growth Period | Average Shell Length (mm) | Shell Length Range (mm) | Average Shell Height (mm) | Shell Height Range (mm) | Shell Length/Shell Height | Ratio Range | OB/OA |
|---|---|---|---|---|---|---|---|
| 1 d | 0.090 ± 0.04 | 0.82–0.10 | 0.08 ± 0.05 | 0.08–0.09 | 1.08 ± 0.01 | 0.94–1.20 | 1.07 ± 0.03 |
| 1 W | 0.15 ± 0.02 | 0.13–0.17 | 0.13 ± 0.09 | 0.12–0.15 | 1.10 ± 0.11 | 0.95–1.25 | 0.74 ± 0.11 |
| 2 W | 0.41 ± 0.05 | 0.22–0.47 | 0.34 ± 0.04 | 0.28–0.40 | 1.19 ± 0.05 | 1.14–1.29 | 0.78 ± 0.06 |
| 3 W | 0.76 ± 0.10 | 0.62–0.94 | 0.60 ± 0.05 | 0.55–0.71 | 1.26 ± 0.07 | 1.13–1.36 | 1.04 ± 0.09 |
| 4 W | 1.27 ± 0.32 | 0.79–1.75 | 0.83 ± 0.15 | 0.6–1.06 | 1.51 ± 0.14 | 1.27–1.71 | 1.20 ± 0.12 |
| 5 W | 1.69 ± 0.37 | 1.10–2.75 | 1.01 ± 0.15 | 0.71–1.46 | 1.66 ± 0.15 | 1.40–2.03 | 1.35 ± 0.07 |
| 6 W | 2.12 ± 0.44 | 1.48–3.02 | 1.19 ± 0.18 | 0.90–1.52 | 1.77 ± 0.13 | 1.55–2.03 | 1.49 ± 0.10 |
| 7 W | 3.79 ± 0.83 | 2.25–5.26 | 1.71 ± 0.33 | 1.16–2.20 | 2.22 ± 0.24 | 1.90–2.78 | 1.61 ± 0.05 |
| 8 W | 4.80 ± 0.54 | 3.66–5.89 | 2.06 ± 0.15 | 1.81–2.46 | 2.32 ± 0.16 | 2.01–2.80 | 1.67 ± 0.11 |
| 9 W | 5.86 ± 0.60 | 4.92–7.00 | 2.36 ± 0.20 | 2.08–2.75 | 2.48 ± 0.13 | 2.23–2.74 | 1.70 ± 0.09 |
| 10 W | 6.20 ± 0.78 | 4.83–7.70 | 2.50 ± 0.23 | 2.17–2.97 | 2.48 ± 0.14 | 2.21–2.76 | 1.75 ± 0.08 |
| 11 W | 7.85 ± 0.52 | 7.12–8.69 | 2.76 ± 0.17 | 2.41–3.08 | 2.84 ± 0.14 | 2.59–3.12 | 1.81 ± 0.08 |
| 12 W | 8.97 ± 1.53 | 7.12–8.69 | 3.07 ± 0.41 | 2.04–4.32 | 2.91 ± 0.18 | 2.57–3.32 | 1.84 ± 0.10 |
| 13 W | 12.38 ± 2.33 | 8.39–18.24 | 4.00 ± 0.54 | 3.15–5.07 | 3.08 ± 0.29 | 2.19–3.75 | 1.88 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Jin, W.; Lv, G.; Chen, W.; Xu, D.; Xu, P.; Hua, D.; Wen, H. In Vitro Culture of Glochidia and Morphological Changes in Juveniles of the Endangered Freshwater Mussel Solenaia oleivora. Fishes 2024, 9, 49. https://doi.org/10.3390/fishes9020049
Ma X, Jin W, Lv G, Chen W, Xu D, Xu P, Hua D, Wen H. In Vitro Culture of Glochidia and Morphological Changes in Juveniles of the Endangered Freshwater Mussel Solenaia oleivora. Fishes. 2024; 9(2):49. https://doi.org/10.3390/fishes9020049
Chicago/Turabian StyleMa, Xueyan, Wu Jin, Guohua Lv, Wanwen Chen, Dongpo Xu, Pao Xu, Dan Hua, and Haibo Wen. 2024. "In Vitro Culture of Glochidia and Morphological Changes in Juveniles of the Endangered Freshwater Mussel Solenaia oleivora" Fishes 9, no. 2: 49. https://doi.org/10.3390/fishes9020049
APA StyleMa, X., Jin, W., Lv, G., Chen, W., Xu, D., Xu, P., Hua, D., & Wen, H. (2024). In Vitro Culture of Glochidia and Morphological Changes in Juveniles of the Endangered Freshwater Mussel Solenaia oleivora. Fishes, 9(2), 49. https://doi.org/10.3390/fishes9020049







