Exploring Cellular Dynamics in the Goldfish Bulbus Arteriosus: A Multifaceted Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Semithin Sections and TEM Preparations
3. Results
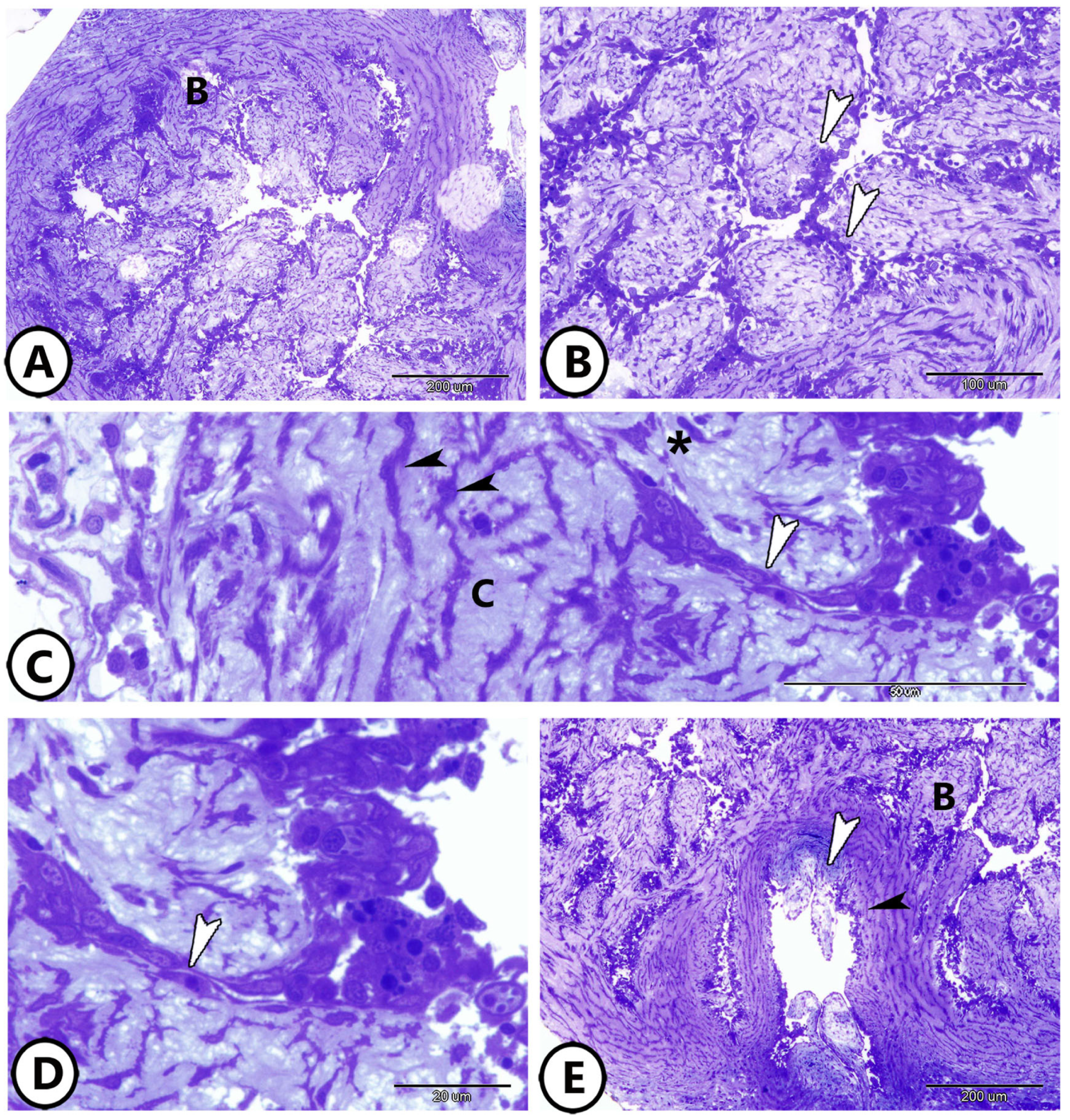
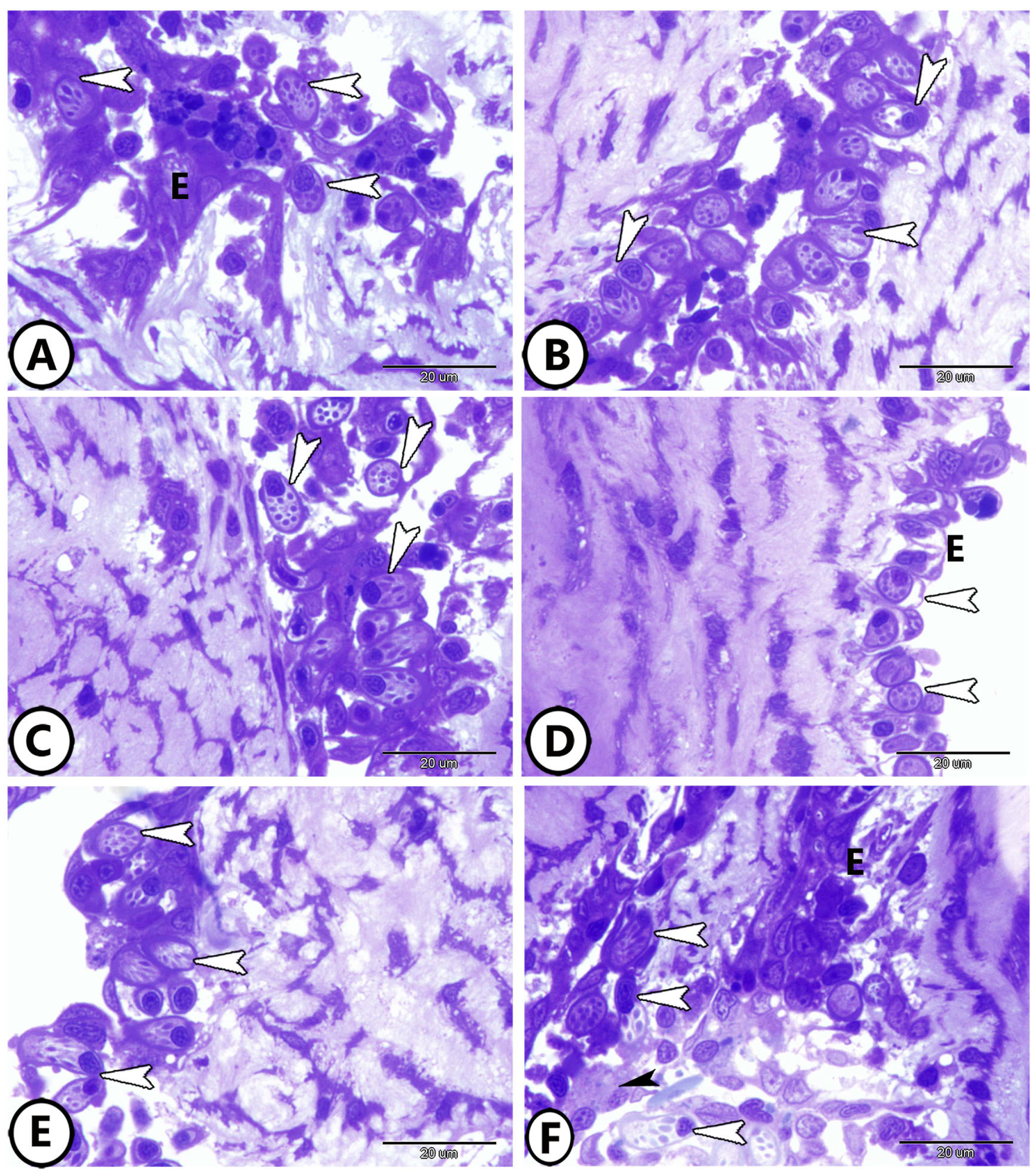
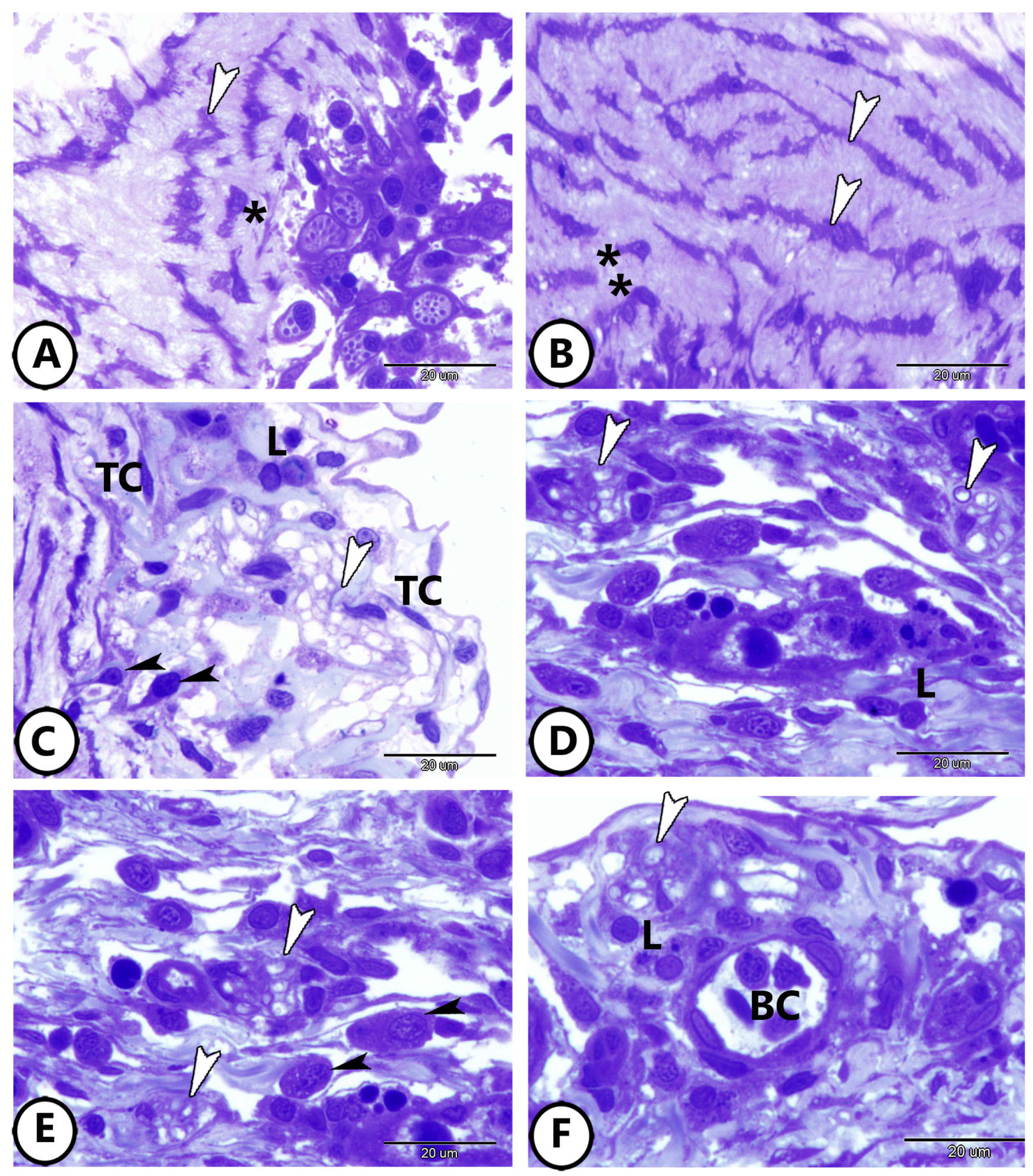
3.1. Light Microscopy
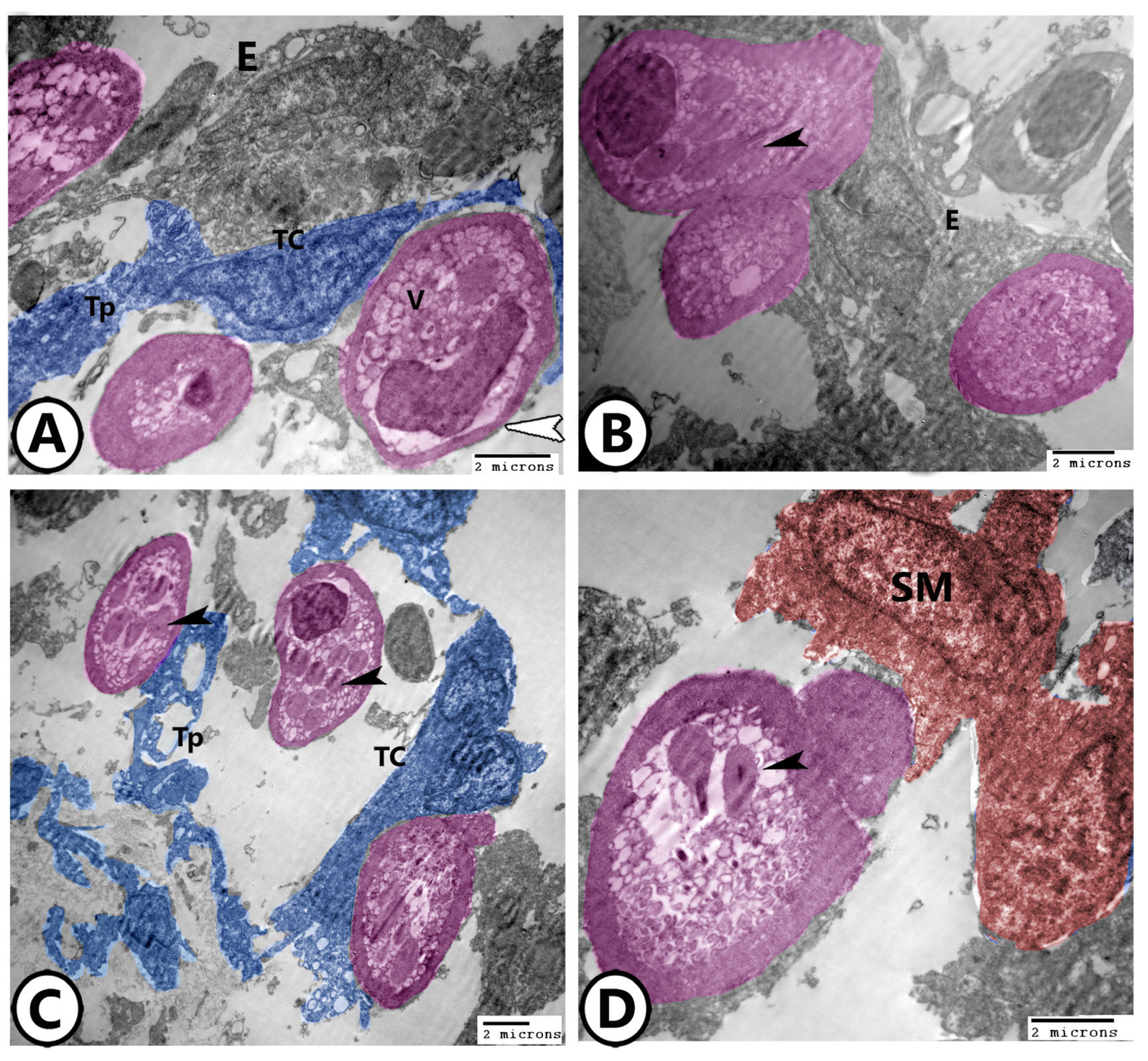
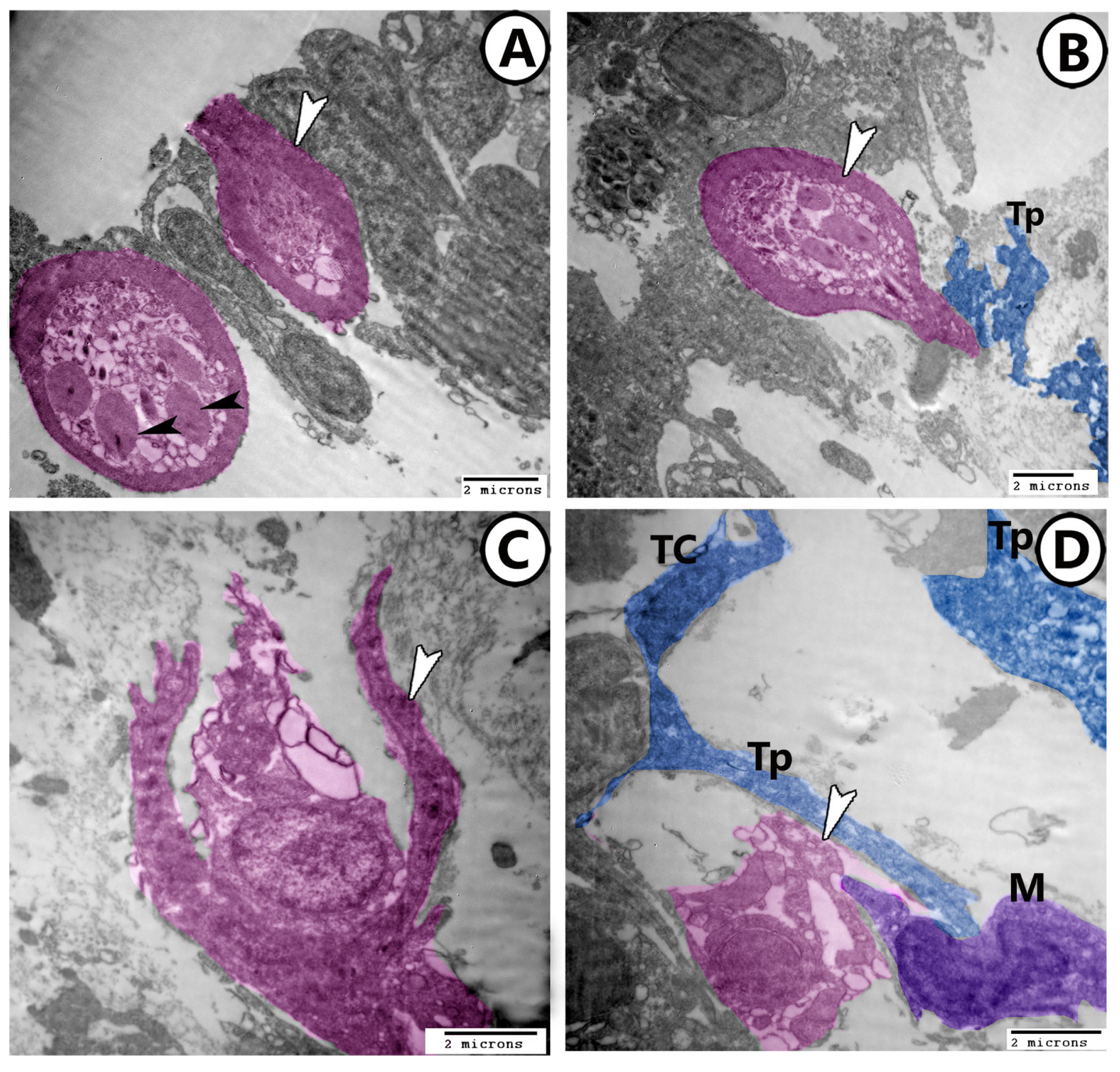
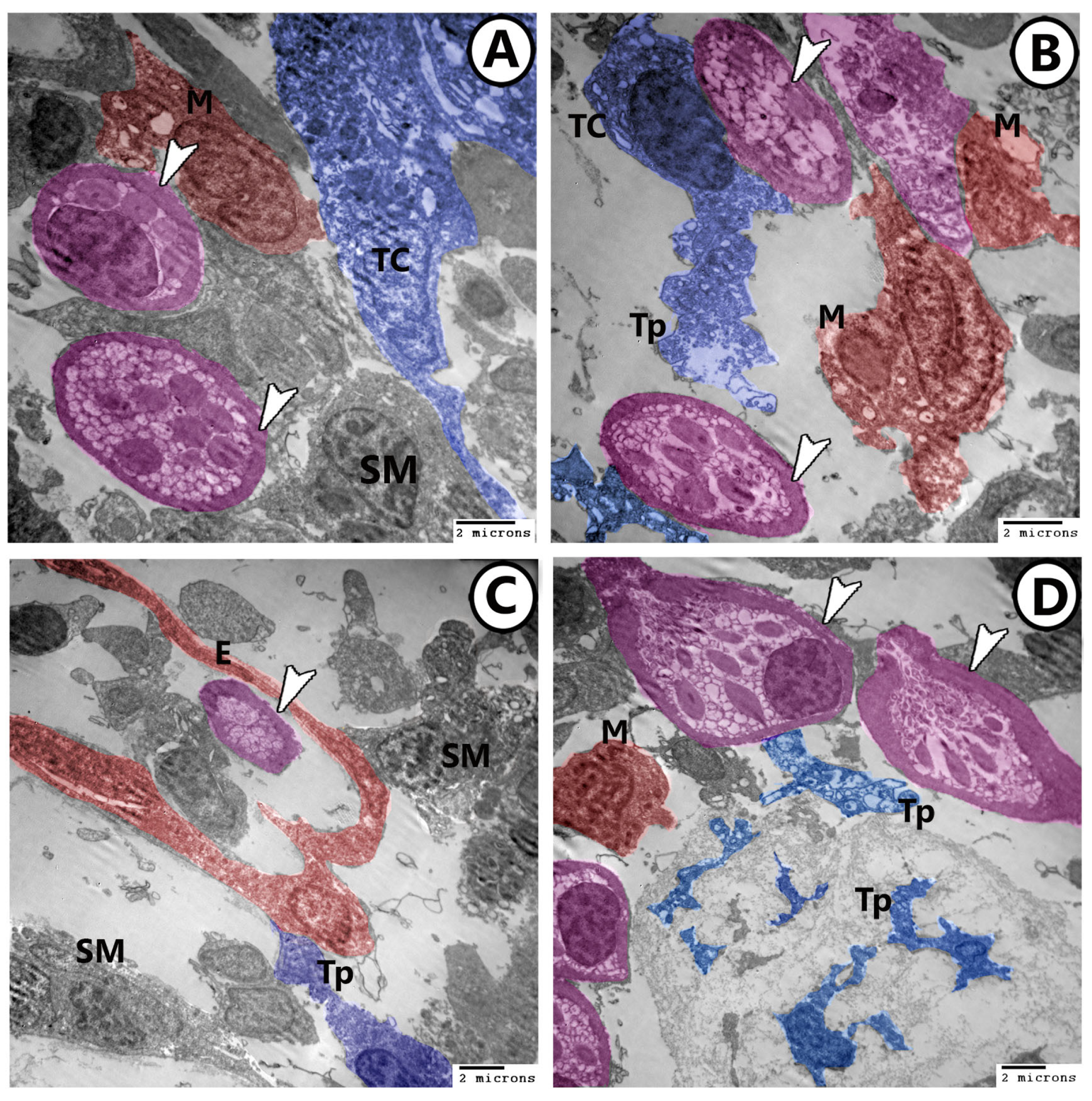
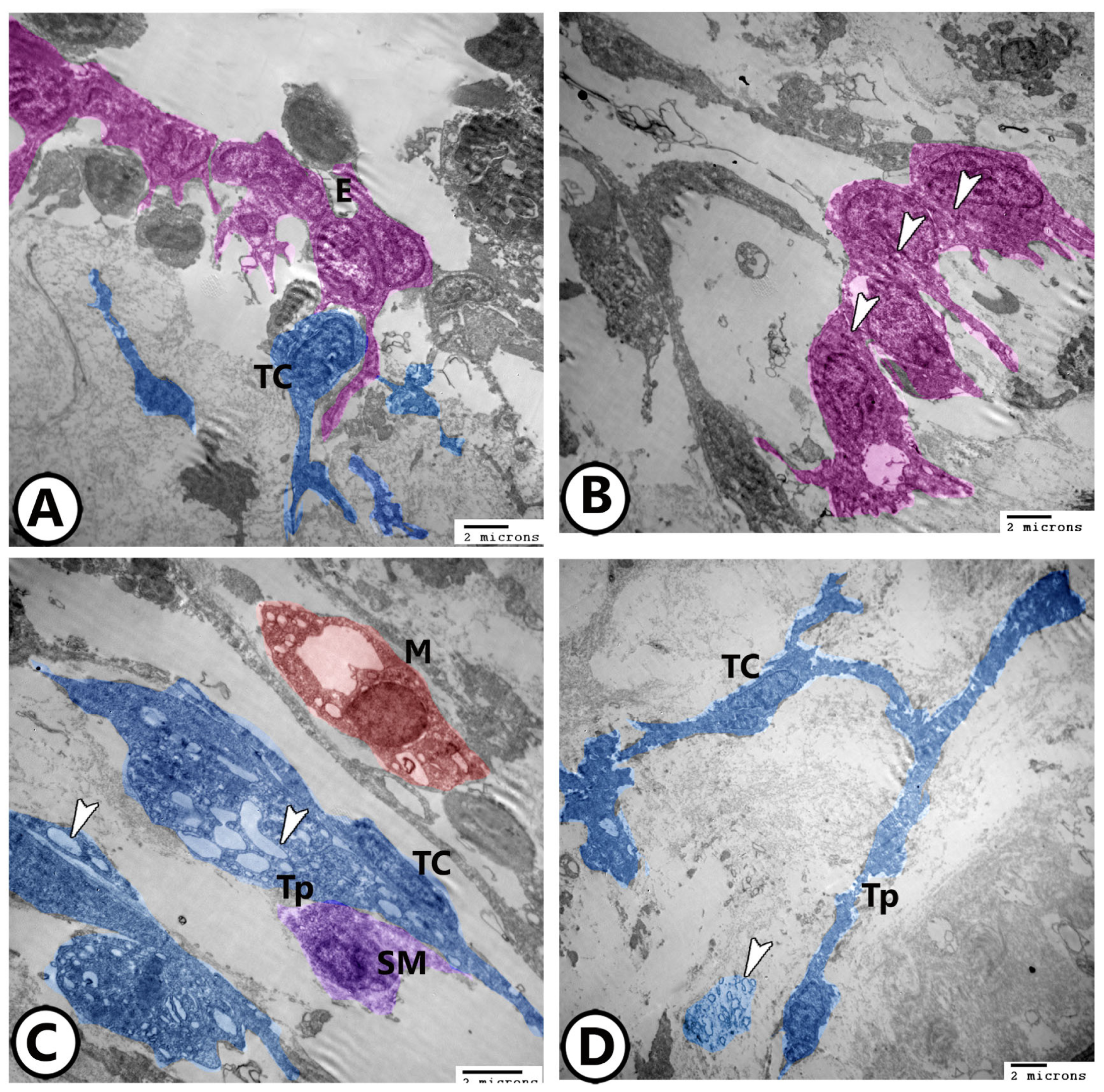
3.2. Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmons, D.B.D.; McCallum, E.S.; Balshine, S.; Chandramouli, B.; Cosgrove, J.; Sherry, J.P. Reduced anxiety is associated with the accumulation of six serotonin reuptake inhibitors in wastewater treatment effluent exposed goldfish Carassius auratus. Sci. Rep. 2017, 7, 17001. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.P.; Jones, D.R. The Heart. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 12, pp. 1–88. [Google Scholar] [CrossRef]
- Icardo, J.M. Conus arteriosus of the teleost heart: Dismissed, but not missed. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Grimes, A.C.; Kirby, M.L. The outflow tract of the heart in fishes: Anatomy, genes and evolution. J. Fish Biol. 2009, 74, 983–1036. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J.M. The Teleost Heart: A Morphological Approach. In Ontogeny and Phylogeny of the Vertebrate Heart; Springer: New York, NY, USA, 2012; pp. 35–53. ISBN 9781461433873. [Google Scholar] [CrossRef]
- Jones, D.R.; Brill, R.W.; Bushnell, P.G. Ventricular and Arterial Dynamics of Anaesthetised and Swimming Tuna. J. Exp. Biol. 1993, 182, 97–112. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ito, F.; Takeda, H.; Yano, T.; Okabe, M.; Kuraku, S.; Keeley, F.W.; Koshiba-Takeuchi, K. Evolution of the fish heart by sub/neofunctionalization of an elastin gene. Nat. Commun. 2016, 7, 10397. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, P.G.; Jones, D.R.; Farrell, A.P. The Arterial System. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 12, pp. 89–139. ISBN 1546-5098. [Google Scholar]
- Watson, A.D.; Cobb, J.L.S. A comparative study on the innervation and the vascularization of the bulbus arteriosus in teleost fish. Cell Tissue Res. 1979, 196, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Bulbus arteriosus of the antarctic teleosts. I. The white-blooded Chionodraco hamatus. Anat. Rec. 1998, 254, 396–407. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Bulbus Arteriosus of the Antarctic Teleosts. II. The Red-Blooded Trematomus bernacchii. Anat. Rec. 1999, 256, 116–126. [Google Scholar] [CrossRef]
- Posner, L.P.; Scott, G.N.; Law, J.M. Repeated Exposure of Goldfish (Carassius auratus) to Tricaine Methanesulfonate (MS-222). J. Zoo Wildl. Med. 2013, 44, 340–347. [Google Scholar] [CrossRef]
- Karnovsky, M.; Karnovsky, M.; Karnovsky, M.; Karnovsky, M.L.; Karnovsky, M. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron-Microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Reynolds, E.S. The Use of Lead Citrate at High pH as an Electron-Opaque Stain in Electron Microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.P. The Wind-Kessel effect of the bulbus arteriosus in trout. J. Exp. Zoöl. 1979, 209, 169–173. [Google Scholar] [CrossRef]
- Braun, M.H.; Brill, R.W.; Gosline, J.M.; Jones, D.R. Form and function of the bulbus arteriosus in yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus) and blue marlin (Makaira nigricans): Static properties. J. Exp. Biol. 2003, 206, 3311–3326. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Norman, D.; Santer, R.M.; Scarborough, D. Histological, histochemical and ultrastructural studies on the bulbus arteriosus of the sticklebacks, Gasterosteus aculeatus and Pungitius pungitius (Pisces: Teleostei). J. Zoöl. 1983, 200, 325–346. [Google Scholar] [CrossRef]
- Licht, J.; Harris, W.S. The structure, composition and elastic properties of the teleost bulbus arteriosus in the carp, Cyprinus carpio. Comp. Biochem. Physiol. Part A Physiol. 1973, 46, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Serafini-Fracassini, A.; Field, J.; Spina, M.; Garbisa, S.; Stuart, R. The morphological organization and ultrastructure of elastin in the arterial wall of trout (Salmo gairdneri) and salmon (Salmo salar). J. Ultrastruct. Res. 1978, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Norman, D.; Scarborough, D.; Santer, R.M. Carbohydrate-containing endothelial cells lining the bulbus arteriosus of teleosts and the conus arteriosus of elasmobranchs (Pisces). J. Zoöl. 1984, 202, 383–392. [Google Scholar] [CrossRef]
- Walker, M.G.; Santer, R.M.; Benjamin, M.; Norman, D. Heart structure of some deep-sea fish (Teleostei: Macrouridae). J. Zoöl. 1985, 205, 75–89. [Google Scholar] [CrossRef]
- Seternes, T.; Sørensen, K.; Smedsrød, B. Scavenger endothelial cells of vertebrates: A nonperipheral leukocyte system for high-capacity elimination of waste macromolecules. Proc. Natl. Acad. Sci. USA 2002, 99, 7594–7597. [Google Scholar] [CrossRef]
- Cerra, M.C.; Canonaco, M.; Acierno, R.; Tota, B. Different binding activities of A- and B-type natriuretic hormones in the heart of two Antarctic teleosts, the red-blooded Trematomus bernacchii and the hemoglobinless Chionodraco hamatus. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 993–999. [Google Scholar] [CrossRef]
- Imbrogno, S.; Cerra, M.C.; Tota, B. Angiotensin II-induced inotropism requires an endocardial endothelium-nitric oxide mechanism in the in-vitro heart of Anguilla anguilla. J. Exp. Biol. 2003, 206, 2675–2684. [Google Scholar] [CrossRef]
- Icardo, J.M. The Fish Endocardium: A Review on the Teleost Heart. In Endothelial Biomedicine; Cambridge University Press: Cambridge, UK, 2007; pp. 79–84. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Light and Electron Microscopy of the Bulbus arteriosus of the European Eel (Anguilla anguilla). Cells Tissues Organs 2000, 167, 184–198. [Google Scholar] [CrossRef]
- Priede, I.G. Functional morphology of the bulbus arteriosus of rainbow trout (Salmo gairdneri Richardson). J. Fish Biol. 1976, 9, 209–216. [Google Scholar] [CrossRef]
- Hascall, V.C.; Hascall, G.K. Proteoglycans. In Cell Biology of Extracellular Matrix; Springer: Boston, MA, USA, 1981; pp. 39–63. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. The structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart: II. The myocardium, the subepicardium, and the conus-aorta transition. Anat. Rec. 2002, 268, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Picchietti, S.; Taverne-Thiele, J.J.; Taverne, N.; Abelli, L.; Mastrolia, L.; Kemenade, B.M.L.V.-V.; Rombout, J.H.W.M. Distribution of macrophages during fish development: An immunohistochemical study in carp (Cyprinus carpio L.). Anat. Embryol. 1998, 198, 31–41. [Google Scholar] [CrossRef]
- Leknes, I.L. Fine structure and cytochemistry of the endothelial cells and rodlet cells in the bulbus arteriosus in species of Cichlidae (Teleostei). J. Fish Biol. 1986, 28, 29–36. [Google Scholar] [CrossRef]
- Reite, O.B. The rodlet cells of teleostean fish: Their potential role in host defence in relation to the role of mast cells/eosinophilic granule cells. Fish Shellfish Immunol. 2005, 19, 253–267. [Google Scholar] [CrossRef]
- Mendonça, I.; Matos, E.; Rodrigues, G.; Matos, P.; Casal, G.; Azevedo, C. Rodlet Cells from the Gills and Kidneys of two Brazilian Freshwater Fishes: An Ultrastructural Study. Braz. J. Morphol. Sci. 2005, 22, 187–192. [Google Scholar]
- Secombes, C.J.; Wang, T. The Innate and Adaptive Immune System of Fish. In Infectious Disease in Aquaculture: Prevention and Control; Woodhead Publishing: Sawston, UK, 2012; pp. 3–68. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdelhafez, E.A. An Overview of the Structural and Functional Aspects of Immune Cells in Teleosts. Histol. Histopathol. 2021, 36, 16. [Google Scholar]
- Mokhtar, D.M.; Hussein, M.M. Microanalysis of Fish Ovarian Follicular Atresia: A Possible Synergic Action of Somatic and Immune Cells. Microsc. Microanal. 2020, 26, 599–608. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Zhang, F.-L.; Tang, X.-L.; Yang, X.-J. Telocytes Enhances M1 Differentiation and Phagocytosis While Inhibits Mitochondria-Mediated Apoptosis Via Activation of NF-κB in Macrophages. Cell Transplant. 2021, 30. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Curici, A.; Wang, E.; Zhang, H.; Hu, S.; Gherghiceanu, M. Telocytes and putative stem cells in ageing human heart. J. Cell. Mol. Med. 2015, 19, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Shim, W. Myocardial Telocytes: A New Player in Electric Circuitry of the Heart. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2016; Volume 913, pp. 241–251. [Google Scholar] [CrossRef]
- Klein, M.; Csöbönyeiová, M.; Žiaran, S.; Danišovič, L’.; Varga, I. Cardiac Telocytes 16 Years on—What Have We Learned So Far, and How Close Are We to Routine Application of the Knowledge in Cardiovascular Regenerative Medicine? Int. J. Mol. Sci. 2021, 22, 10942. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Tay, H.; Vandecasteele, T.; Broeck, W.V.D. Identification of telocytes in the porcine heart. Anat. Histol. Embryol. 2017, 46, 519–527. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.-S.; Bani, D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J. Cell. Mol. Med. 2010, 14, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Popescu, L.M. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: Electron microscope images. J. Cell. Mol. Med. 2010, 14, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Manole, C.G.; Gherghiceanu, M.; Ardelean, A.; Nicolescu, M.I.; Hinescu, M.E.; Kostin, S. Telocytes in human epicardium. J. Cell. Mol. Med. 2010, 14, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Sukhacheva, T.; Nizyaeva, N.; Samsonova, M.; Chernyaev, A.L.; Shchegolev, A.; Serov, R.A. Telocytes in the Myocardium of Children with Congenital Heart Disease Tetralogy of Fallot. Bull. Exp. Biol. Med. 2020, 169, 137–146. [Google Scholar] [CrossRef]
- Lv, L.; Liao, Z.; Luo, J.; Chen, H.; Guo, H.; Yang, J.; Huang, R.; Pu, Q.; Zhao, H.; Yuan, Z.; et al. Cardiac telocytes exist in the adult Xenopus tropicalis heart. J. Cell. Mol. Med. 2020, 24, 2531–2541. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, D.M.; Abd-Elhafez, E.A.; Albano, M.; Zaccone, G.; Hussein, M.T. Exploring Cellular Dynamics in the Goldfish Bulbus Arteriosus: A Multifaceted Perspective. Fishes 2024, 9, 203. https://doi.org/10.3390/fishes9060203
Mokhtar DM, Abd-Elhafez EA, Albano M, Zaccone G, Hussein MT. Exploring Cellular Dynamics in the Goldfish Bulbus Arteriosus: A Multifaceted Perspective. Fishes. 2024; 9(6):203. https://doi.org/10.3390/fishes9060203
Chicago/Turabian StyleMokhtar, Doaa M., Enas A. Abd-Elhafez, Marco Albano, Giacomo Zaccone, and Manal T. Hussein. 2024. "Exploring Cellular Dynamics in the Goldfish Bulbus Arteriosus: A Multifaceted Perspective" Fishes 9, no. 6: 203. https://doi.org/10.3390/fishes9060203
APA StyleMokhtar, D. M., Abd-Elhafez, E. A., Albano, M., Zaccone, G., & Hussein, M. T. (2024). Exploring Cellular Dynamics in the Goldfish Bulbus Arteriosus: A Multifaceted Perspective. Fishes, 9(6), 203. https://doi.org/10.3390/fishes9060203







