Environmental Influences on Illex argentinus Trawling Grounds in the Southwest Atlantic High Seas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.1.1. Fishing Log Data
2.1.2. Marine Environmental Data
2.2. Data Processing Methods
2.2.1. Center of Fishing Grounds Processing
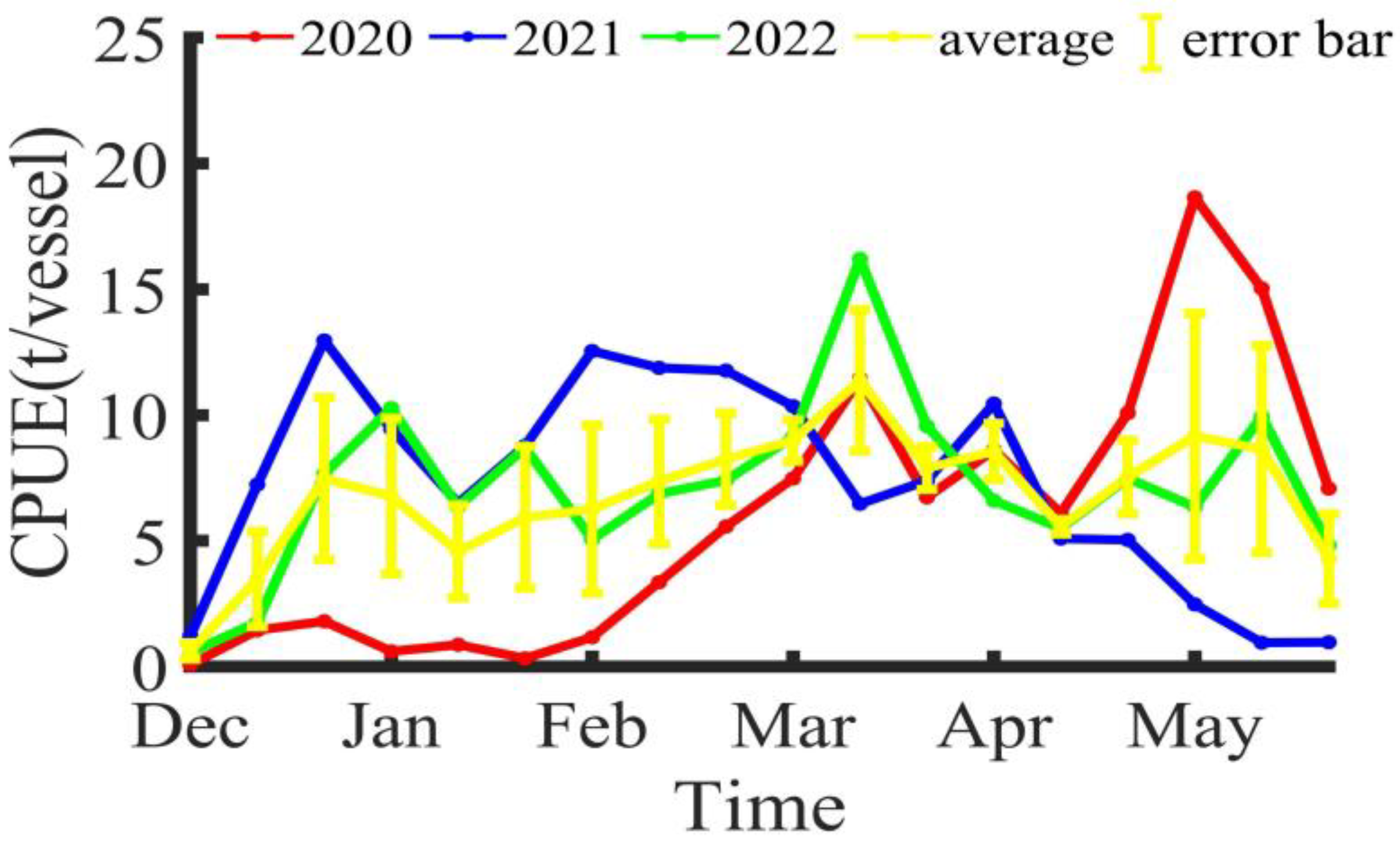
2.2.2. CPUE Processing
2.2.3. Maxent Model
3. Results
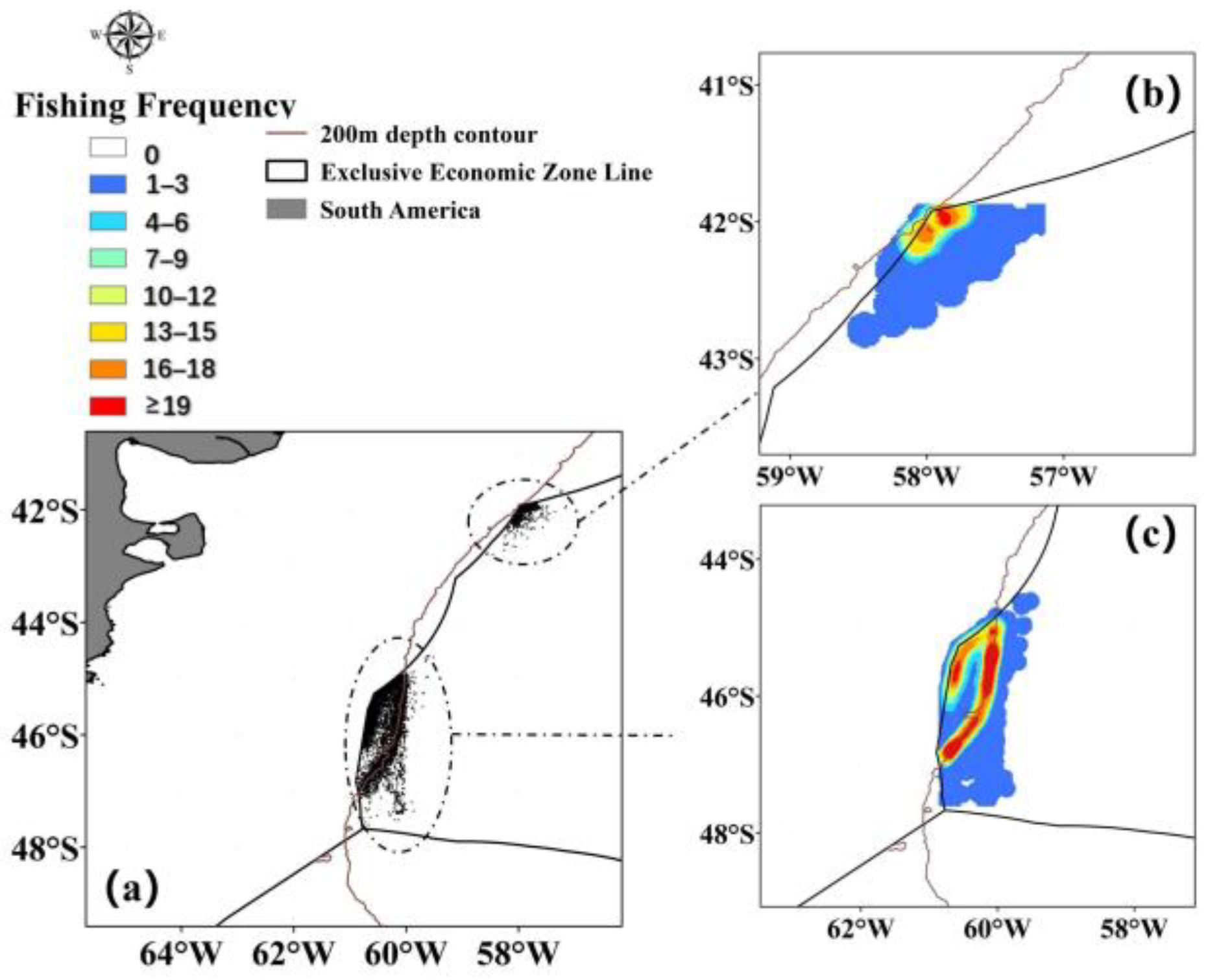
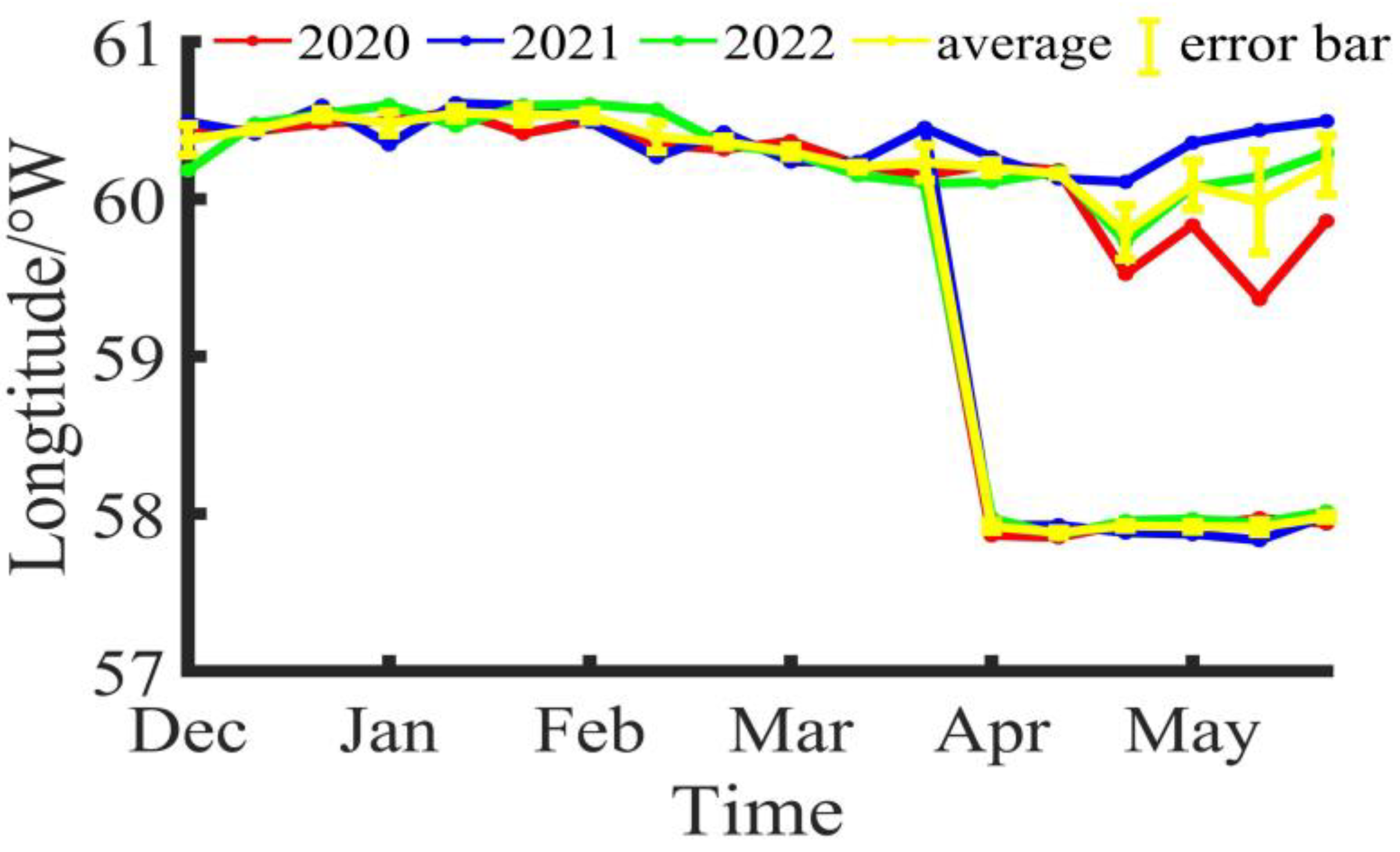
3.1. Changes in Fishing Ground Centers
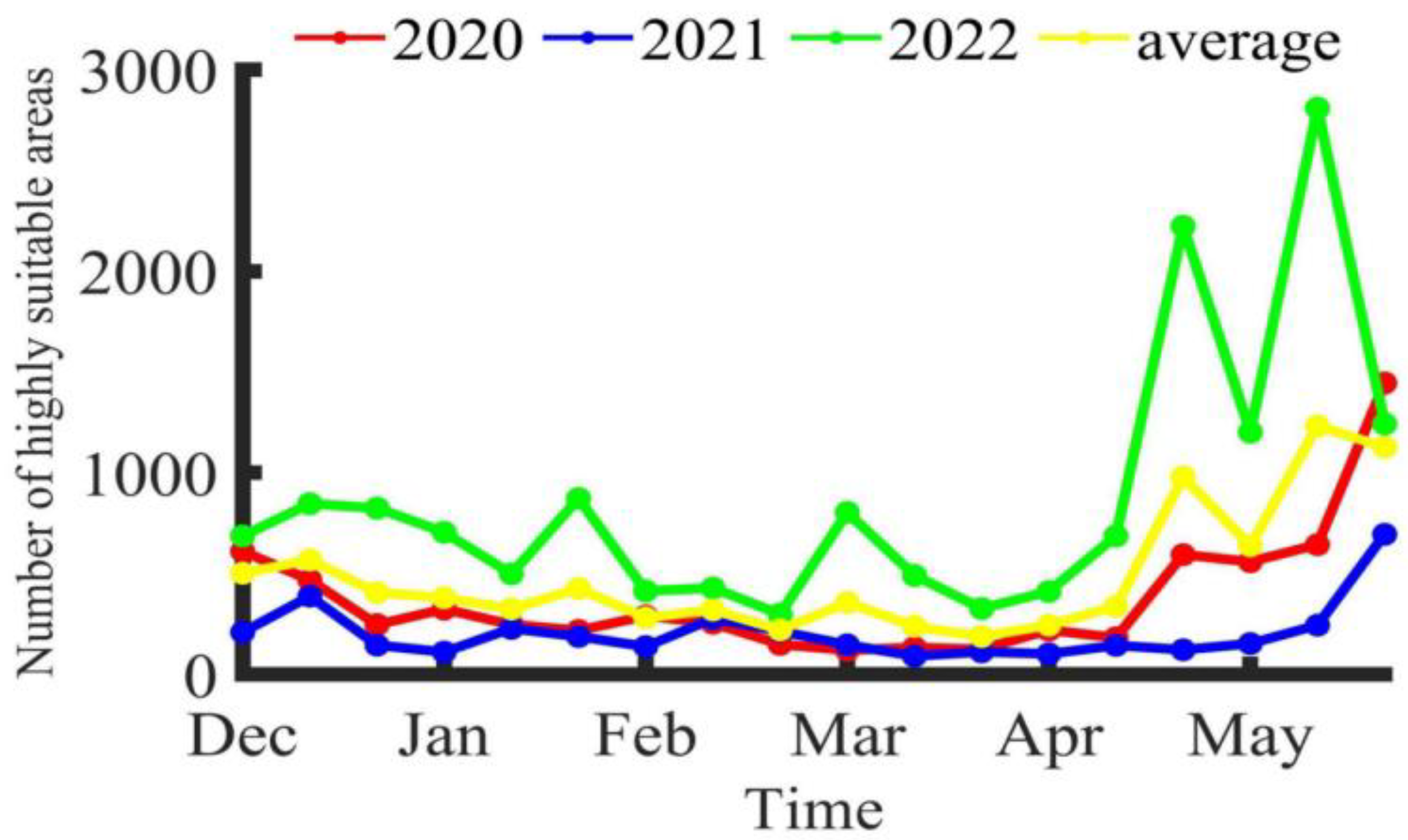
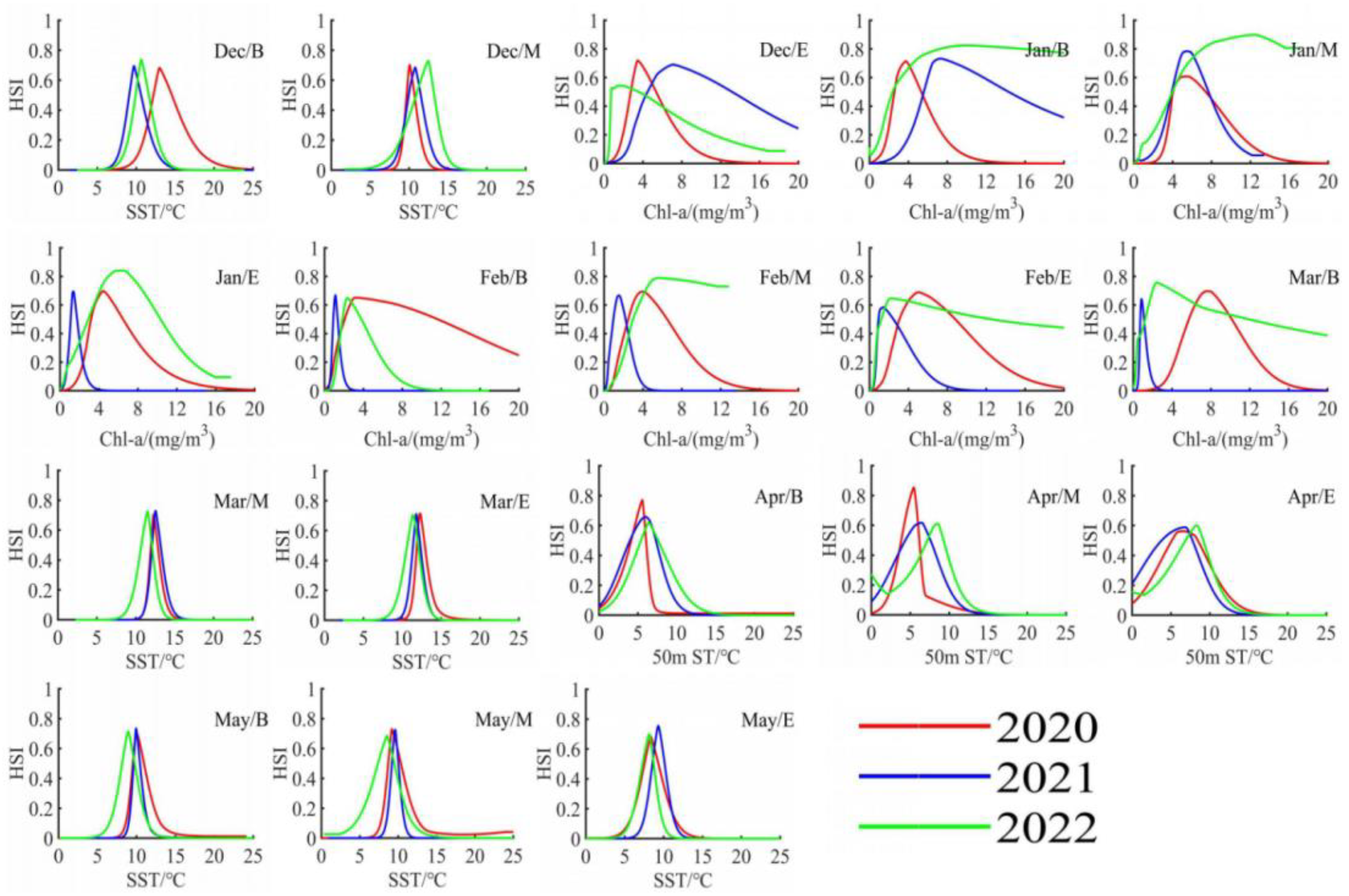
3.2. Characteristics of Potential Habitat Distribution
3.3. Response to Key Environmental Factors
4. Discussion
4.1. Spatiotemporal Distribution of Illex argentinus High Seas Trawl Fishing Grounds
4.2. Environmental Factors Affecting the High Seas Trawl Fishing Grounds for Illex argentinus
4.2.1. The Impact of SST on the Fishing Grounds
4.2.2. Impact of Chlorophyll-a on Fishing Grounds
4.2.3. The Impact of Other Environmental Factors on the Illex argentinus High Sea Trawl Fishing Grounds
4.3. Results and Advantages of the Maxent Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.W.; Yu, W.; Chen, X.J. Research progress on the resources of Illex argentinus in the Southwest Atlantic and its environmental response. J. Fish. China 2020, 27, 1254–1265. [Google Scholar]
- Hou, Q.L.; Chen, X.J.; Wang, J.T. Study on the spatio-temporal distribution of Illex argentinus resources in the Southwest Atlantic. Mar. Sci. 2019, 43, 103–109. [Google Scholar]
- Wu, Y.M.; Yang, S.L.; Shen, J.H.; Zhou, W.F.; Zhang, J. Characteristics of Illex argentinus fishing grounds in the Southwest Atlantic. J. Appl. Ecol. 2009, 20, 1445–1451. [Google Scholar]
- Chen, X.J.; Lu, H.J.; Liu, B.L.; Qian, W.G. Predicting Illex argentinus fishing grounds in the Southwest Atlantic using a habitat index. J. Shanghai Ocean. Univ. 2012, 21, 431–438. [Google Scholar]
- Hao, Y.X. Study on the Hydrodynamic Characteristics of Illex argentinus Bottom Trawl. Diploma Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar] [CrossRef]
- Liu, H.W.; Yu, W.; Chen, X.J.; Zhu, W.B. Construction of Illex argentinus habitat model based on seawater temperature at different water layers. J. Dalian Ocean. Univ. 2021, 36, 1035–1043. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.J. Analysis of Illex argentinus habitat distribution in the Southwest Atlantic based on the Maxent model. J. Fish. China 2016, 40, 893–902. [Google Scholar]
- Cui, G.C.; Xuan, W.D.; Wei, Q.Y.; Tao, Y.X.; Su, S.; Yu, Q.C.; Zhu, W.B. Analysis of spatiotemporal changes in Illex argentinus fishing grounds in the Southwest Atlantic based on SST and Chl-a. J. Zhejiang Ocean. Univ. (Nat. Sci. Ed.) 2023, 42, 10–19. [Google Scholar]
- Li, N.; Chen, X.J.; Jin, Y. Assessment and management strategy evaluation of Illex argentinus resources based on a composite population. J. Shanghai Ocean. Univ. 2019, 28, 471–482. [Google Scholar]
- Yue, D.; Wang, L.; Fan, W.; Zhang, X.; Zheng, H.; Tang, F.; Zhang, S. Management of Illex argentinus fisheries resources and its implications for China. China Agric. Sci. Technol. Guide 2014, 16, 124–131. [Google Scholar] [CrossRef]
- Bo, J.N.; Li, R.G.; Lü, D.J.; Chang, Y.F.; Song, W.H. Survey of pair trawling near the coast of Uruguay in the Southwest Atlantic. Ocean. Dev. Manag. 2019, 36, 61–64. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.M.; Sun, M. Composition of trawl catches and biological characteristics of main species on the Patagonian continental shelf in summer and autumn. Prog. Fish. Sci. 2014, 35, 8–18. [Google Scholar]
- Lu, H.J.; Chen, X.J.; Cao, J. Standardization of CPUE in the Chinese mainland Illex argentinus jig fishery based on the GLBM model. Acta Ecol. Sin. 2013, 33, 5375–5384. [Google Scholar]
- Zhang, W.; Zhang, J. Discussion on the relationship between Illex argentinus fishing grounds in the Southwest Atlantic and major marine environmental factors. J. Shanghai Fish. Univ. 2008, 17, 471–475. [Google Scholar]
- Yang, S.L.; Fan, X.M.; Tang, F.H.; Cheng, T.F.; Fan, W. Spatiotemporal distribution of kingfish fishing grounds in the Arabian Sea and its relationship with marine environment. J. Trop. Oceanogr. 2019, 38, 91–100. [Google Scholar]
- Wang, L.M.; Li, Y.; Zhang, R.; Tian, Y.J.; Zhang, J.; Lin, L.S. Relationship between resource abundance distribution of Japanese mackerel in the Northwest Pacific and surface temperature and vertical temperature structure. J. Ocean. Univ. China (Nat. Sci. Ed.) 2019, 49, 29–38. [Google Scholar] [CrossRef]
- Chen, X.J.; Feng, B.; Xu, L.X. Study and comparison of habitat indices for bigeye tuna in the Indian Ocean. J. Fish. China 2008, 2, 269–278. [Google Scholar]
- Wang, W.S. Study on the Habitat Simulation of Different Populations of Skipjack Tuna in the Central and Western Pacific Based on the MaxEnt Model. Diploma Thesis, Shanghai Ocean University, Shanghai, China, 2023. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.; Mugo, R.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Seasonal potential fishing ground prediction of neon flying squid (Ommastrephes bartramii) in the western and central North Pacific. Fish. Oceanogr. 2015, 24, 190–203. [Google Scholar] [CrossRef]
- Zhang, J.R.; Yang, X.M.; Tian, S.Q. Habitat prediction for longfin tuna in the South Pacific based on the MaxEnt model. J. Fish. China 2020, 27, 1222–1233. [Google Scholar]
- Gong, C.X.; Chen, X.J.; Gao, F. Simulation of potential habitat distribution of squid in the Northwest Pacific based on the MaxEnt model. J. Fish. China 2020, 27, 336–345. [Google Scholar]
- Fang, X.N.; He, Y.; Yu, W.; Chen, X.J. Spatiotemporal distribution of jumbo flying squid habitat off Peru and their response differences to environmental factors. J. Fish. China 2021, 28, 658–672. [Google Scholar]
- Feng, Z.P.; Yu, W.; Chen, X.J.; Zou, X.R. Study on the habitat of jack mackerel off the coast of Chile based on the MaxEnt model. J. Fish. China 2021, 28, 431–441. [Google Scholar]
- Chen, X.J.; Zhao, X.H. Preliminary study on the relationship between Illex argentinus yield distribution and surface temperature in the Southwest Atlantic. J. Dalian Fish. Univ. 2005, 3, 222–228. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J. Preliminary study on the relationship between Illex argentinus yield distribution and surface temperature in the Southwest Atlantic in 2001. Mar. Fish. 2004, 4, 326–330. [Google Scholar]
- Waluda, C.; Rodhouse, P.; Podestá, G.; Trathan, P.; Pierce, G. Surface oceanography of the inferred hatching grounds of Illex argentinus (Cephalopoda: Ommastrephidae) and influences on recruitment variability. Mar. Biol. 2001, 139, 671–679. [Google Scholar]
- Tang, F.H.; Cui, X.S.; Fan, W.; Zhang, S.M.; Fan, X.M.; Wu, Y.M. Study on the relationship between squid resource abundance and marine environment in international waters. China Agric. Sci. Technol. Guide 2016, 18, 153–162. [Google Scholar] [CrossRef]
- He, Y. Study on the Relationship between Illex argentinus Resource Distribution and Environmental Factors and Fishing Ground Prediction. Diploma Thesis, Shanghai Ocean University, Shanghai, China, 2023. [Google Scholar] [CrossRef]
- Zhang, S.P.; Chen, X.J. Relationship between Illex argentinus fishing grounds and water temperature. In Proceedings of the China Oceanographic Society. “Belt and Road” Strategy and Marine Science and Technology Innovation—Collection of Academic Papers of China Oceanographic Society 2015, Beijing, China, 26 October 2015; Volume 9. [Google Scholar]
- Shen, X.Q.; Wang, Y.L.; Yuan, Q.; Huang, H.L.; Zhou, A.Z. Distribution characteristics of chlorophyll-a in squid fishing grounds in the North Pacific and its relationship with fishing grounds. Acta Oceanol. Sin. (Chin. Ed.) 2004, 6, 118–123. [Google Scholar]
- Wu, H.Z.; Chen, Z.K.; Lin, D.M. The impact of marine environment on the reproductive characteristics of female Illex argentinus. South China Fish. Sci. 2024, 20, 130–140. [Google Scholar]
- Zhang, S.M.; Dai, Y.; Cheng, T.F.; Wu, Y.M. Thematic map service for Illex argentinus fishing grounds in Southwest Atlantic Ocean. Computer Knowledge and Technology 2017, 13, 192–194. [Google Scholar] [CrossRef]
- Koronkiewicz, A. Growth and life cycle of squid Illex argentinus from Patagonian and Falkland Shelf and Polish fishery of squid for this region, 1978–1985. Gdyn. Sea Fish. Inst. 1986, 27, 25. [Google Scholar]
- Zheng, L.L.; Wu, Y.M.; Fan, W. Distribution of chlorophyll-a in Illex argentinus fishing grounds in the Southwest Atlantic and its relationship with fishing grounds. Bull. Mar. Lake Res. 2011, 1, 63–70. [Google Scholar] [CrossRef]
- Kininmonth, S.; Blenckner, T.; Niiranen, S.; Watson, J.; Orio, A.; Casini, M.; Neuenfeldt, S.; Bartolino, V.; Hansson, M. Is Diversity the Missing Link in Coastal Fisheries Management? Diversity 2022, 14, 90. [Google Scholar] [CrossRef]
- Vaihola, S.; Kininmonth, S. Environmental Factors Determine Tuna Fishing Vessels’ Behavior in Tonga. Fishes 2023, 8, 602. [Google Scholar] [CrossRef]
- Wang, J.T.; Gao, F.; Lei, L.; Guan, W.J.; Chen, X.J. Study on the forecasting model of Illex argentinus stock replenishment based on spawning ground environmental factors. Acta Oceanol. Sin. 2014, 36, 119–124. [Google Scholar]
- Li, N.; Lu, H.J.; Chen, X.J. Comparison of forecasting models for Illex argentinus fishing grounds in the Southwest Atlantic based on different BP neural networks. J. Guangdong Ocean. Univ. 2017, 37, 65–71. [Google Scholar] [CrossRef]
- Ding, Q.; Chen, X.J.; Wang, J.T. Comparison of suitable habitat models for Illex argentinus and their application in fishing ground forecasts. Prog. Fish. Sci. 2015, 36, 8–13. [Google Scholar]
- Liu, S.Y.; Zhang, H.; Yang, C.; Fang, Z. Differences in habitat of Far Eastern pilchard and Japanese mackerel in the Northwest Pacific based on the MaxEnt model. J. Shanghai Ocean. Univ. 2023, 32, 806–817. [Google Scholar]
- Yu, W.; Fang, X.N.; Chen, X.J.; Zhang, Z. Monthly distribution of jumbo flying squid habitat in equatorial waters and its association with environmental factors. J. Fish. China 2022, 46, 2315–2329. [Google Scholar]
- Yang, Z.; Chen, W.; Wang, X.; Liu, B.; Dong, J.; Deng, Y. Suitable habitat of the scad fish (Decanters spp.) in Northern South China Sea predicted by MaxEnt model. Reg. Stud. Mar. Sci. 2024, 69, 103315. [Google Scholar] [CrossRef]
- Zlateva, I.; Raykov, V.; Slabakova, V.; Stefanova, E.; Stefanova, K. Habitat suitability models of five keynote Bulgarian Black Sea fish species relative to specific abiotic and biotic factors. Oceanologia 2022, 64, 665–674. [Google Scholar] [CrossRef]
- Lu, H.J.; Chen, X.J.; Liu, B.L.; Gong, C.X. Advances in the study of the biology of Illex argentinus fisheries in the Southwest Atlantic. J. Guangdong Ocean. Univ. 2010, 30, 91–98. [Google Scholar]
- Brunetti, N.E.; Ivanovic, M.L. Distribution and abundance of early life stages of squid (Illex argentinus) in the south-west Atlantic. ICES J. Mar. Sci. 1992, 49, 175–183. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J. Simulation of the spatial distribution of forest fires in Heilongjiang Province based on generalized linear models and the MaxEnt model. Ecol. J. 2013, 32, 1620–1628. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]



| SST | Chl-a | SSH | SSS | 100 m st | 50 m st | 100 m mv | 50 m mv | 100 m zv | 50 m zv | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dec/B | 25.3 | 14.9 | 18.3 | 4.3 | 7.8 | 18.2 | 2.8 | 4.5 | 1.8 | 2.1 |
| Dec/M | 39.1 | 24.2 | 18.3 | 5 | 2.4 | 6.4 | 1.2 | 1.4 | 0.5 | 1.5 |
| Dec/E | 31.1 | 47.8 | 7 | 4.6 | 2 | 5.1 | 0.2 | 1.4 | 0.2 | 0.5 |
| Jan/B | 26.7 | 53.2 | 10.8 | 1.2 | 3.8 | 2.2 | 0.4 | 1.2 | 0 | 0.5 |
| Jan/M | 19.7 | 65 | 4.3 | 1.9 | 3.3 | 4.4 | 0.8 | 0.3 | 0.1 | 0.1 |
| Jan/E | 26.7 | 46.1 | 9.3 | 4.5 | 4.3 | 7.9 | 0.3 | 0.3 | 0.2 | 0.4 |
| Feb/B | 33.8 | 36.4 | 16.3 | 1.9 | 2.6 | 7.5 | 0.1 | 0.2 | 0 | 1.2 |
| Feb/M | 27.4 | 44 | 7.3 | 4.7 | 6.5 | 9.2 | 0.4 | 0.2 | 0.2 | 0.2 |
| Feb/E | 29.9 | 37 | 4.2 | 3.4 | 5.7 | 15.1 | 0.8 | 3.5 | 0.2 | 0.2 |
| Mar/B | 30.2 | 42.3 | 3.8 | 1 | 8.2 | 11.5 | 1 | 1.9 | 0 | 0.2 |
| Mar/M | 29.2 | 28.2 | 11 | 0.5 | 3.4 | 19.8 | 0.1 | 7.3 | 0.3 | 0.2 |
| Mar/E | 31.7 | 13.3 | 7.1 | 0.4 | 7.2 | 28.9 | 0.7 | 8.9 | 0.3 | 1.5 |
| Apr/B | 27.4 | 22.8 | 7.3 | 1.5 | 9.2 | 27.6 | 0.9 | 2.3 | 0.5 | 0.5 |
| Apr/M | 23.3 | 12.4 | 18.3 | 1.8 | 8.8 | 24 | 1.3 | 9.6 | 0.1 | 0.4 |
| Apr/E | 17.5 | 23.7 | 14.2 | 4.2 | 9.3 | 25.7 | 1 | 1.6 | 0.6 | 2.2 |
| May/B | 37.4 | 19.5 | 18.7 | 1 | 9.5 | 7.7 | 2 | 2.8 | 0.5 | 1 |
| May/M | 39.2 | 7.9 | 12.1 | 7.8 | 13.6 | 7.3 | 3.8 | 5.3 | 1.3 | 1.6 |
| May/E | 36.1 | 1.5 | 26.9 | 10 | 13.4 | 5.8 | 2.1 | 2.3 | 0.8 | 1 |
| The Primary Key Environmental Factors and Their Ranges | The Secondary Key Environmental Factors and Their Ranges | |
|---|---|---|
| Dec/B | SST (9.6~13.1 °C) | SSH (−14~−9 cm) |
| Dec/M | SST (10.1~12.5 °C) | Chl-a (0.6~3.8 mg/m3) |
| Dec/E | Chl-a (1.2~6.9 mg/m3) | SST (11.7~13.9 °C) |
| Jan/B | Chl-a (3.6~9.1 mg/m3) | SST (12.4~13.7 °C) |
| Jan/M | Chl-a (4.9~12.3 mg/m3) | SST (12.1~14.3 °C) |
| Jan/E | Chl-a (1.3~6.3 mg/m3) | SST (13.0~14.4 °C) |
| Feb/B | Chl-a (1.1~3.5 mg/m3) | SST (13.2~14.5 °C) |
| Feb/M | Chl-a (1.4~5.3 mg/m3) | SST (13.3~14.6 °C) |
| Feb/E | Chl-a (1.5~5.2 mg/m3) | SST (12.0~12.9 °C) |
| Mar/B | Chl-a (0.9~7.8 mg/m3) | SST (12.0~13.1 °C) |
| Mar/M | SST (11.5~12.6 °C) | Chl-a (0.7~3.0 mg/m3) |
| Mar/E | SST (11.4~12.3 °C) | 50 m st (5.7~6.4 °C) |
| Apr/B | 50 m st (5.6~6.5 °C) | SST (10.8~11.8 °C) |
| Apr/M | 50 m st (5.3~8.0 °C) | SST (10.2~11.5 °C) |
| Apr/E | 50 m st (6.4~8.3 °C) | Chl-a (0.3~0.7 mg/m3) |
| May/B | SST (8.8~10.2 °C) | Chl-a (0.3~0.6 mg/m3) |
| May/M | SST (8.2~9.6 °C) | 100 m st (5.3~5.7 °C) |
| May/E | SST (8.2~9.3 °C) | SSH (−16.1~−9.3 cm) |
| Samples | AUC | Standard Deviation | |
|---|---|---|---|
| Dec/B | 77 | 0.990 | 0.005 |
| Dec/M | 321 | 0.991 | 0.003 |
| Dec/E | 700 | 0.985 | 0.005 |
| Jan/B | 986 | 0.986 | 0.003 |
| Jan/M | 978 | 0.988 | 0.002 |
| Jan/E | 1156 | 0.987 | 0.003 |
| Feb/B | 1038 | 0.992 | 0.002 |
| Feb/M | 1047 | 0.990 | 0.001 |
| Feb/E | 1029 | 0.992 | 0.001 |
| Mar/B | 1230 | 0.987 | 0.003 |
| Mar/M | 1319 | 0.991 | 0.002 |
| Mar/E | 1519 | 0.992 | 0.001 |
| Apr/B | 1149 | 0.989 | 0.003 |
| Apr/M | 1161 | 0.987 | 0.003 |
| Apr/E | 991 | 0.976 | 0.005 |
| May/B | 1111 | 0.985 | 0.003 |
| May/M | 1004 | 0.979 | 0.006 |
| May/E | 843 | 0.973 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, D.; Li, Y.; Jiang, K.; Han, H.; Wang, Y.; Yang, S.; Zhang, H.; Sun, Y. Environmental Influences on Illex argentinus Trawling Grounds in the Southwest Atlantic High Seas. Fishes 2024, 9, 209. https://doi.org/10.3390/fishes9060209
Xiang D, Li Y, Jiang K, Han H, Wang Y, Yang S, Zhang H, Sun Y. Environmental Influences on Illex argentinus Trawling Grounds in the Southwest Atlantic High Seas. Fishes. 2024; 9(6):209. https://doi.org/10.3390/fishes9060209
Chicago/Turabian StyleXiang, Delong, Yang Li, Keji Jiang, Haibin Han, Yuhan Wang, Shenglong Yang, Heng Zhang, and Yuyan Sun. 2024. "Environmental Influences on Illex argentinus Trawling Grounds in the Southwest Atlantic High Seas" Fishes 9, no. 6: 209. https://doi.org/10.3390/fishes9060209





