Abstract
Opsariichthys bidens (O. bidens) is a fish species native to China and sensitive to temperature changes. In this study, the effects of acute temperature stress on brain gene expression in O. bidens were investigated by sampling brain tissues from specimens exposed to three different temperatures (15 °C, 25 °C, and 35 °C) for varying durations of 2 h, 4 h, 6 h, and 8 h. The study focused on analyzing the expression patterns of key genes implicated in neural function and stress response, including brain-derived neurotrophic factor (BDNF), c-FOS, heat shock proteins (HSP70, HSP90), endoplasmic reticulum stress markers (IRE1, GRP78), oxidative stress enzymes (CAT, SOD), and apoptotic regulators (caspase3, Bax). The findings revealed that upon exposure to acute heat stress, the expression levels of the aforementioned genes in the brain of O. bidens were up-regulated within 2 h, peaking at the 4-h mark. Conversely, following acute cold stress, the expression of c-FOS, BDNF, HSP70, HSP90, SOD, and CAT genes increased significantly after 4 h, while caspase3 expression was notably elevated at the 6-h mark, with no significant impact observed on Bax, IRE1, or GRP78 gene expression levels. The study suggested that the brain of O. bidens responds to high temperatures through mechanisms involving neural activation, heat shock proteins, endoplasmic reticulum stress, oxidative stress, and apoptosis. Similarly, adaptation to low temperatures by O. bidens’ brain was associated with neural activation, regulation of heat shock proteins, oxidative stress responses, and apoptotic processes. Overall, this research aimed to elucidate the impact of temperature stress on brain physiology and the adaptive mechanisms of O. bidens at the genetic level, providing a foundational understanding of its temperature adaptation strategies.
Key Contribution:
Opsariichthys bidens is sensitive to temperature variations in the water column; and the wide-scale promotion of O. bidens culture needs to overcome difficulties in various aspects such as temperature variability. Clarifying the effects of temperature on the brain physiology and response mechanisms of O. bidens at the gene expression level not only enriches and expands the study of this species but also provides corresponding theoretical guidance for the promotion of O. bidens culture and the realization of sustainable aquaculture.
1. Introduction
Opsariichthys bidens (Günther, 1873), belonging to the families Cyprinidae and Opsariichthys, is a small fish that lives in mountain streams and has significant economic value. Water temperature is the main ecophysiological variable of aquatic thermophilic animals, affecting animal behavior, physiology [1], cardiac function [2], swimming performance [3], oxidative stress [4], digestive physiology [5], growth, metabolism, and reproductive performance [6]. As one of the most important environmental factors for fish growth and survival, changes in water temperature can cause a series of hazards [7]. Studies have shown that water temperatures in rivers and lakes in Asia and Europe have increased by 0.2–2 °C due to global climate change [8], resulting in reduced or even extinct fish populations [9] and significant economic losses [10]. O. bidens has a weak anti-stress ability in the external environment and has a specific sensitivity to water temperature. The ideal temperature for the survival of O. bidens ranges from 20 °C to 30 °C. Under the current global warming trend environment, extreme weather becomes more frequent, which may cause adverse effects on O. bidens. Despite these known risks, there is a dearth of research on the impacts of short-term acute temperature fluctuations on the brain physiology of O. bidens.
In response to environmental changes, the fish brain, a key regulator of fish life functions, adjusts the expression levels of relevant genes. Among these genes, brain-derived neurotrophic factor (BDNF) has been extensively investigated in brain tissue due to its crucial physiological roles in synaptic plasticity, neurogenesis, and neuronal maturation [11,12]. BDNF exerts its functions through the activation of tropomyosin-related kinase B (TrkB) and p75 neurotrophin receptor (p75NTR) pathways [13]. The TrkB pathway promotes plasticity and neuronal survival, while the p75NTR pathway triggers apoptosis [14,15]. Additionally, Ma [16] observed that acute temperature stress led to the inhibition of c-Fos expression in the telencephalon and reduced neuronal activity in Batrachuperus pinchoni. c-Fos expression serves as an indicator of neuronal activity and is influenced by various stimuli and signals [17,18]. Studies have demonstrated the involvement of heat shock proteins (HSP70 and HSP90) in mitigating the impacts of high- or low-temperature stress on fish [19,20]. These heat shock proteins play a crucial role in alleviating temperature stress effects on fish physiology [21].
In this study, we investigated the responses of BDNF, c-Fos, heat shock genes (HSP70, HSP90), endoplasmic reticulum stress genes (GRP78, IRE1), oxidative stress genes (CAT, SOD), and apoptosis genes (caspase3, Bax) in the brain of O. bidens under acute temperature stress and explored the mechanism of the effect of temperature stress on gene expression in the brain of O. bidens.
2. Materials and Methods
2.1. Fish
The O. bidens used in this study were obtained from an agricultural science and technology company in Zhejiang Province, China, with an average weight of 4.95 ± 0.50 g. Before the experiment, the fish were temporarily reared in a 150 L culture barrel under controlled conditions. The water temperature was maintained at 25 ± 0.5 °C, with dissolved oxygen levels of ≥6 mg/L, a pH range of 6.5 to 8.5, an ammonia nitrogen content of 0.30 ± 0.11 mg/L, and a nitrite concentration of 0.036 ± 0.009 mg/L. The fish were fed at a daily rate of 0.5%, and the light–dark cycle was set at 12 h:12 h. Additionally, the water in the culture barrel was exchanged daily at a rate of 50%.
2.2. Experimental Methods
2.2.1. Experimental Treatment
The experimental design included acute test temperatures of 15 °C and 35 °C, with 25 °C serving as the control temperature, and each group consisting of three repeated trials. The water temperature was precisely controlled using a constant temperature heater to ensure a maximum temperature deviation of 0.5 °C. For the experiment, temporarily reared O. bidens specimens were directly transferred to individual experimental culture barrels, each containing 20 L of water that was well aerated. Each barrel housed 12 O. bidens specimens for the duration of the experiment. Fasting was implemented throughout the test period, and all other water quality parameters remained consistent with those observed during the temporary incubation phase.
2.2.2. Sample Extraction
Samples were randomly selected at 2 h, 4 h, 6 h, and 8 h after the test, with three O. bidens in each group. We anesthetized every fish with 50 mg/L Methane-Sulfonate-222 (MS-222). The anaesthetic was buffered with sodium bicarbonate to a normal pH (7.0–7.4) before use. Following anesthesia, brain tissue samples were extracted from the skull opening, flash-frozen with liquid nitrogen, and stored at −80 °C for future analysis. The experimental fish were euthanized in accordance with ethical guidelines and protocols.
2.3. Gene Expression Analysis
For the procedures of extraction, quality detection, and reverse transcription of total brain RNA, refer to the literature [22]. The target gene sequence was searched according to the transcriptome data of O. bidens, and primers for real-time quantitative PCR (qRT-PCR) were designed using Primer 3.0 plus (Table 1). The expressions of BDNF, c-Fos, HSP70, HSP90, GRP78, IRE1, CAT, SOD, caspase3, and Bax genes were analyzed by qRT-PCR with the obtained brain cDNA as the template and β-actin as the internal control gene. qRT-PCR reaction procedure: 95 °C for 10 min, 30 cycles of 95 °C for 15 sec, 60 °C for 60 sec, 72 °C for 10 min. The relative expression level of the gene was calculated by the 2−ΔΔCt method.

Table 1.
Real-time PCR primers.
2.4. Data Processing and Statistical
The data were analyzed by SPSS 26.0 software and the expression differences among the groups were compared by two-way analysis of variance (ANOVA). If differences were observed between the groups, Duncan’s method of multiple comparisons was employed to ascertain the significance of these differences in the data across groups. Mapping of the results was conducted through Origin 9.0 software. The mean ± SD values are used to present the results. In the analysis, “*” indicates a significant difference (p < 0.05), while “**” indicates an extremely significant difference (p < 0.01).
3. Results
3.1. Expression of BDNF and c-FOS Genes in the Brain of O. bidens
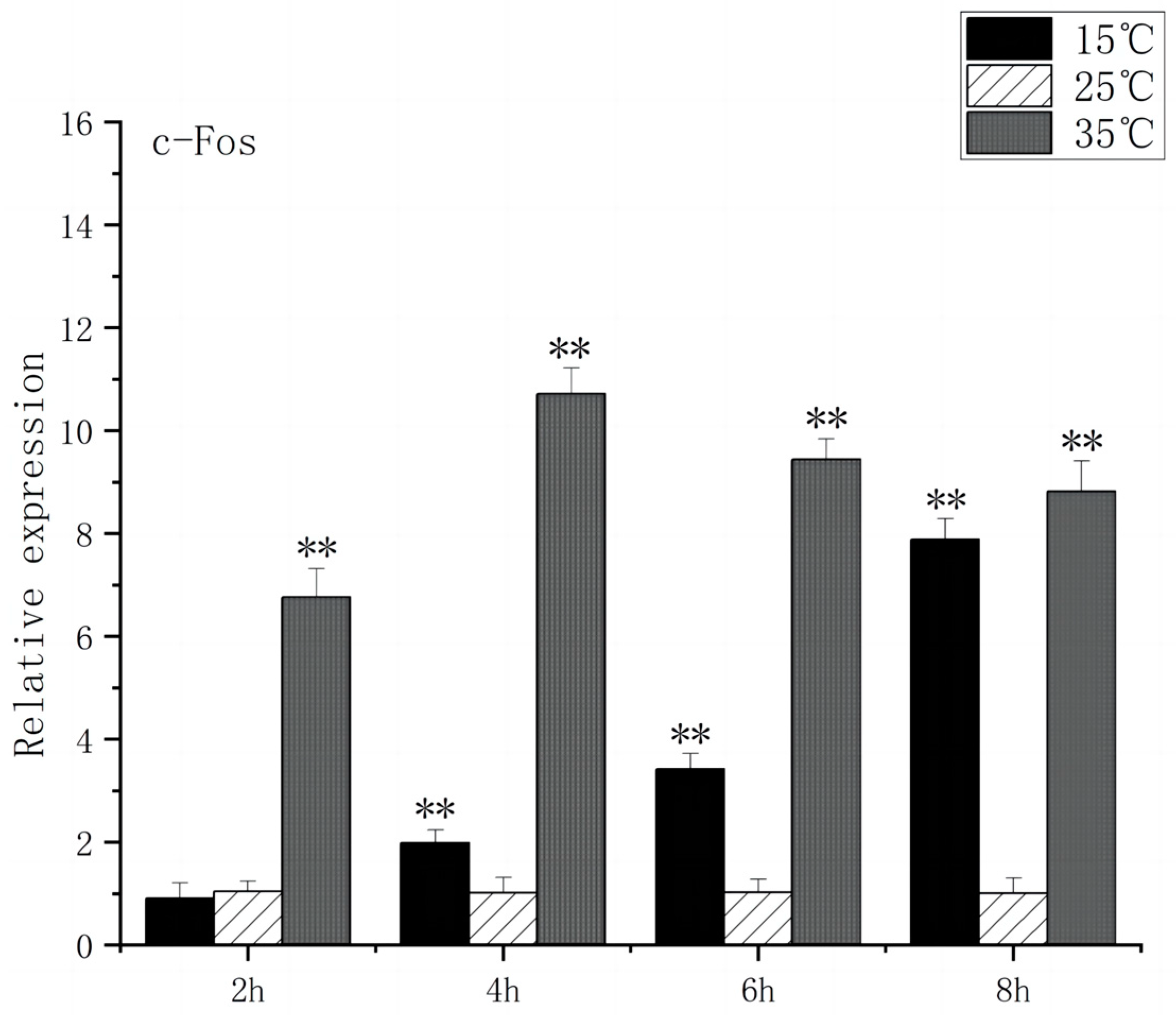
Under acute high-temperature stress, the c-Fos gene expression of O. bidens increased significantly after 2 h (p < 0.01), and reached the peak at 4 h, which was ten times higher than the control expression. In contrast, under acute low-temperature stress, the expression level of c-Fos gene exhibited no significant change at 2 h, increased significantly after 4 h (p < 0.01), and peaked at 8 h (Figure 1). The increase in c-Fos expression in the brain of O. bidens may be related to the apoptosis induced by temperature. Under acute high-temperature stress, the c-Fos up-regulation expression speed was faster and the expression amount was higher, while under acute low-temperature stress, the c-Fos up-regulation expression speed was slower and the expression amount was lower, which indicates that acute high-temperature stress exerts a more detrimental effect on the brain physiology and functionality of O. bidens.
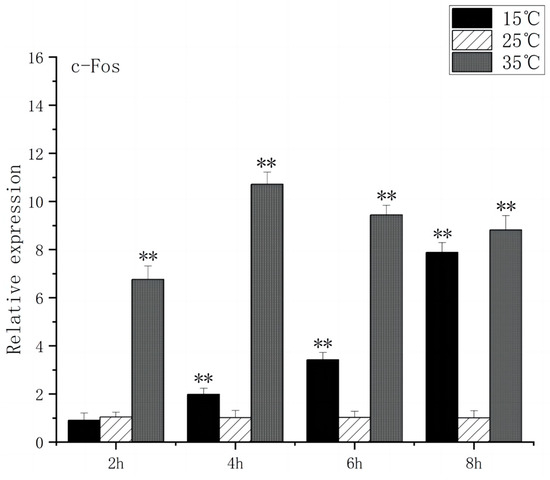
Figure 1.
c-Fos mRNA transcript levels in the brain of O. bidens. “**” indicates an extremely significant difference (p < 0.01).
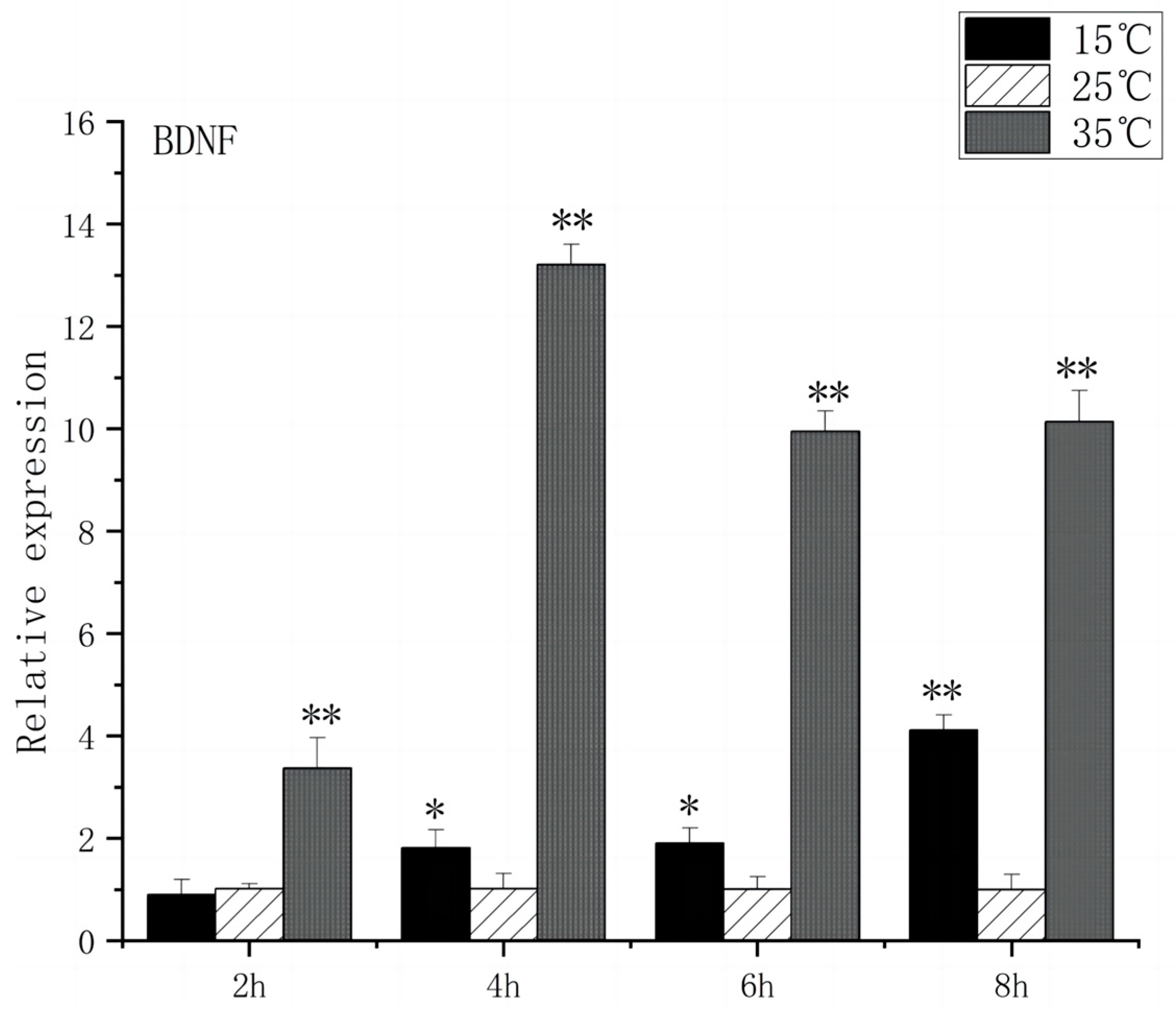
Regarding BDNF expression, under acute high-temperature stress, it significantly increased after 2 h (p < 0.01) and peaked at 4 h, showing a thirteenfold increase compared to the control. The expression of BDNF remained relatively stable during the first 2 h of acute low-temperature stress, before increasing significantly after 4 h (p < 0.01) and reaching its peak at 8 h (Figure 2).
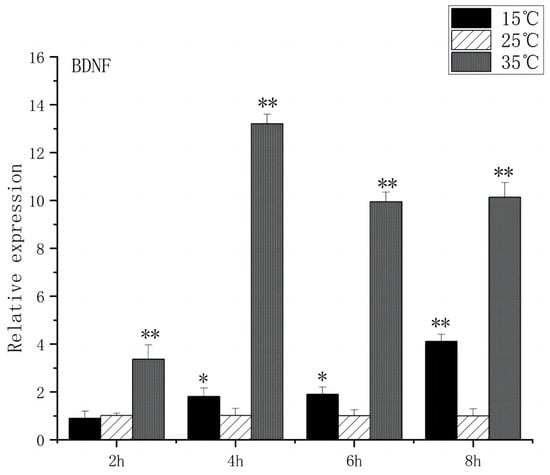
Figure 2.
BDNF mRNA transcript levels in the brain of O. bidens. “*” indicates a significant difference (p < 0.05), “**” indicates an extremely significant difference (p < 0.01).
3.2. Expression of Heat Stress-Related Genes in the Brain of O. bidens
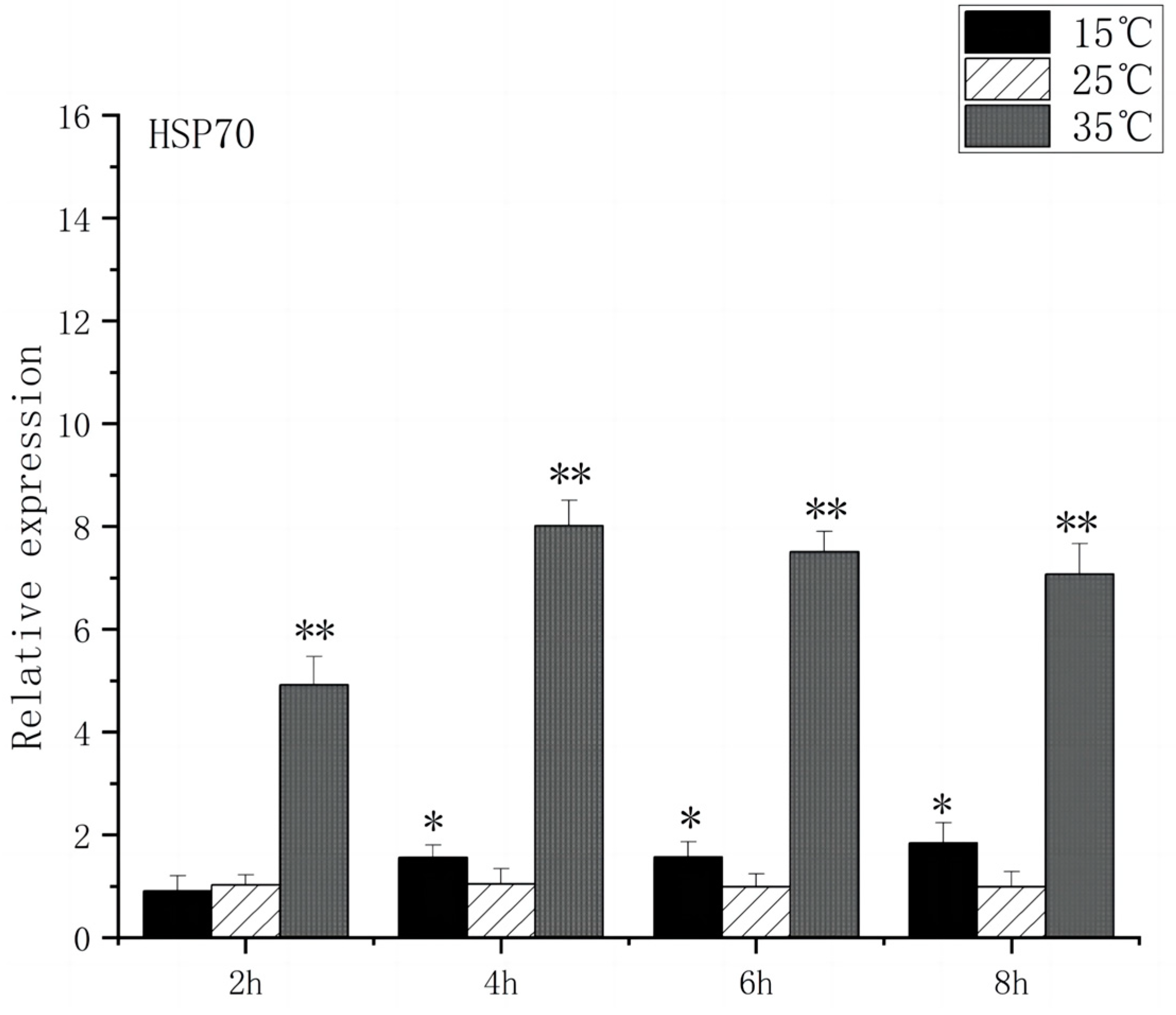
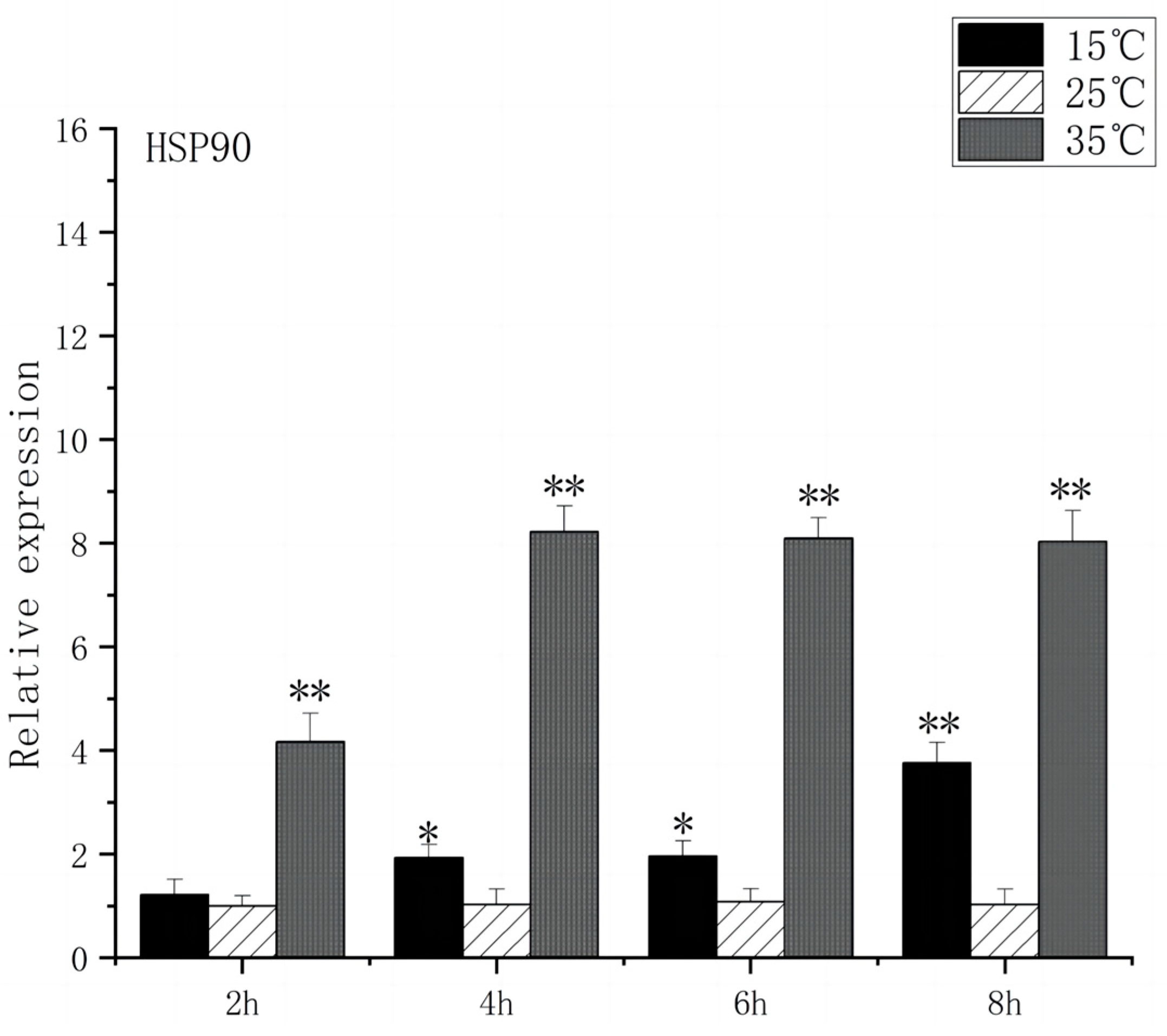
At 2 h after the onset of acute heat stress, the brain tissue of O. bidens exhibited a notable up-regulation in the expression levels of HSP70 and HSP90 compared to the control group (p < 0.01), with peak expression levels observed at 4 h. The peak expression levels were found to be 8 times higher than those in the control group. Conversely, when exposed to acute low-temperature stress, the expression level of HSP70 in O. bidens did not show significant changes after 2 h but increased significantly after 4 h (p < 0.05), reaching its peak at 8 h, which was twice the control level. Meanwhile, HSP90 expression levels significantly increased after 4 h and reached their peak at 8 h, showing a 3.7-fold increase compared to the control group (Figure 3 and Figure 4).
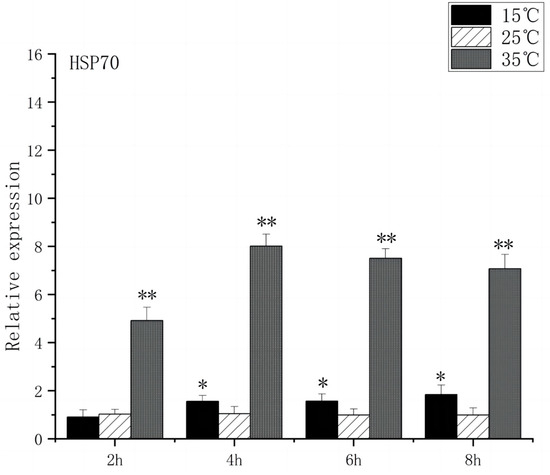
Figure 3.
HSP70 mRNA transcript levels in the brain of O. bidens. “*” indicates a significant difference (p < 0.05), “**” indicates an extremely significant difference (p < 0.01).
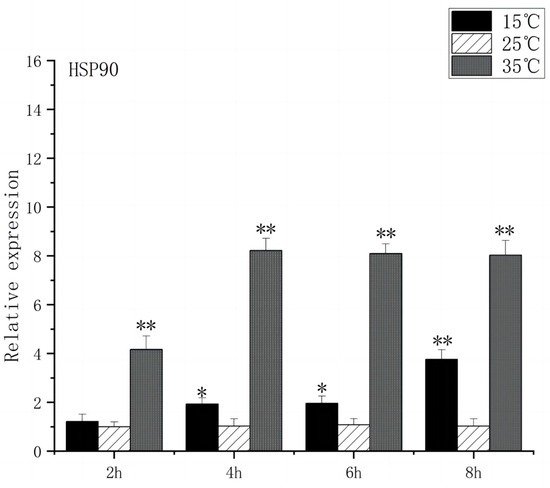
Figure 4.
HSP90 mRNA transcript levels in the brain of O. bidens. “*” indicates a significant difference (p < 0.05), “**” indicates an extremely significant difference (p < 0.01).
3.3. Expression of Stress Genes in Brain Endoplasmic Reticulum of O. bidens
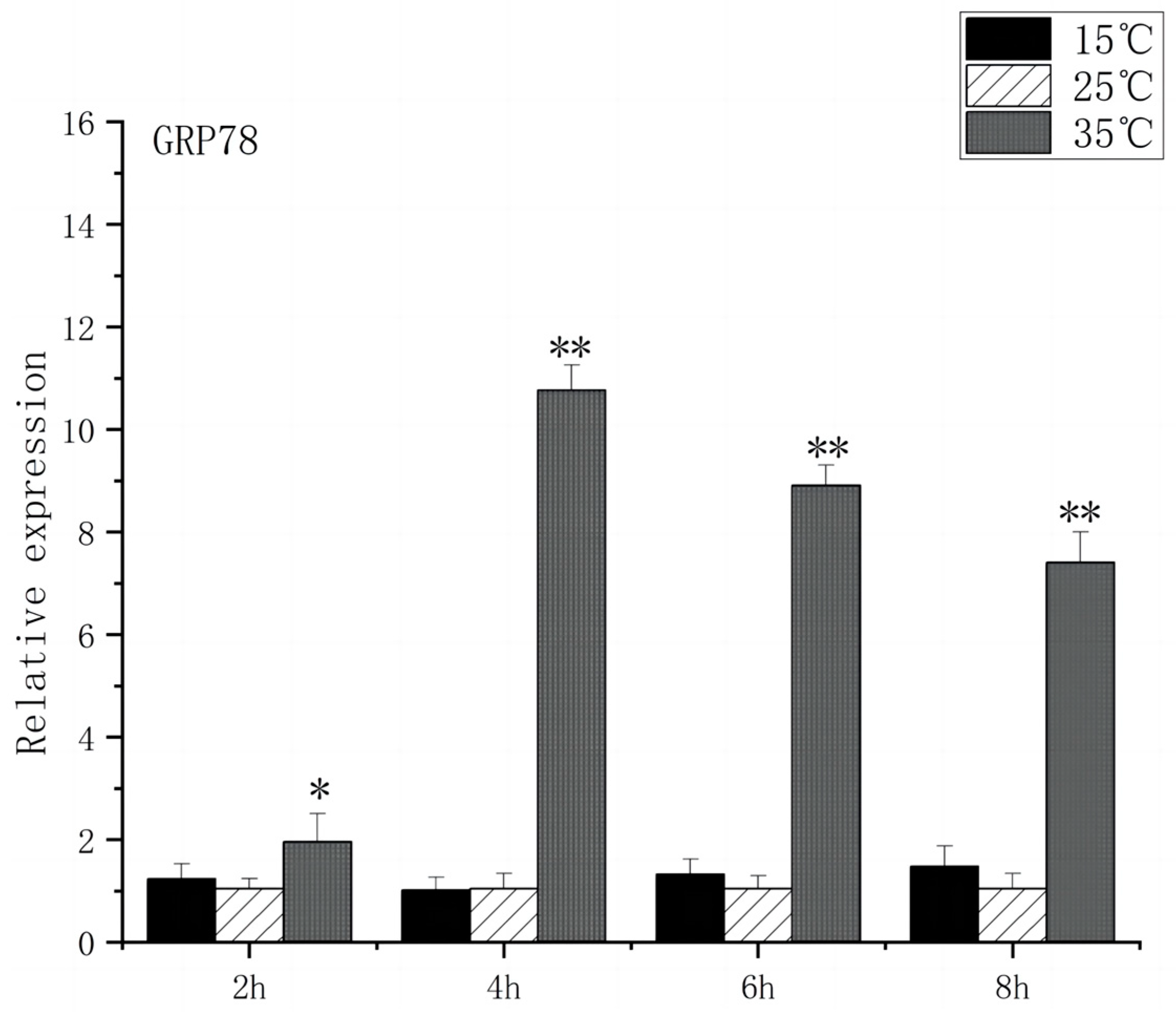
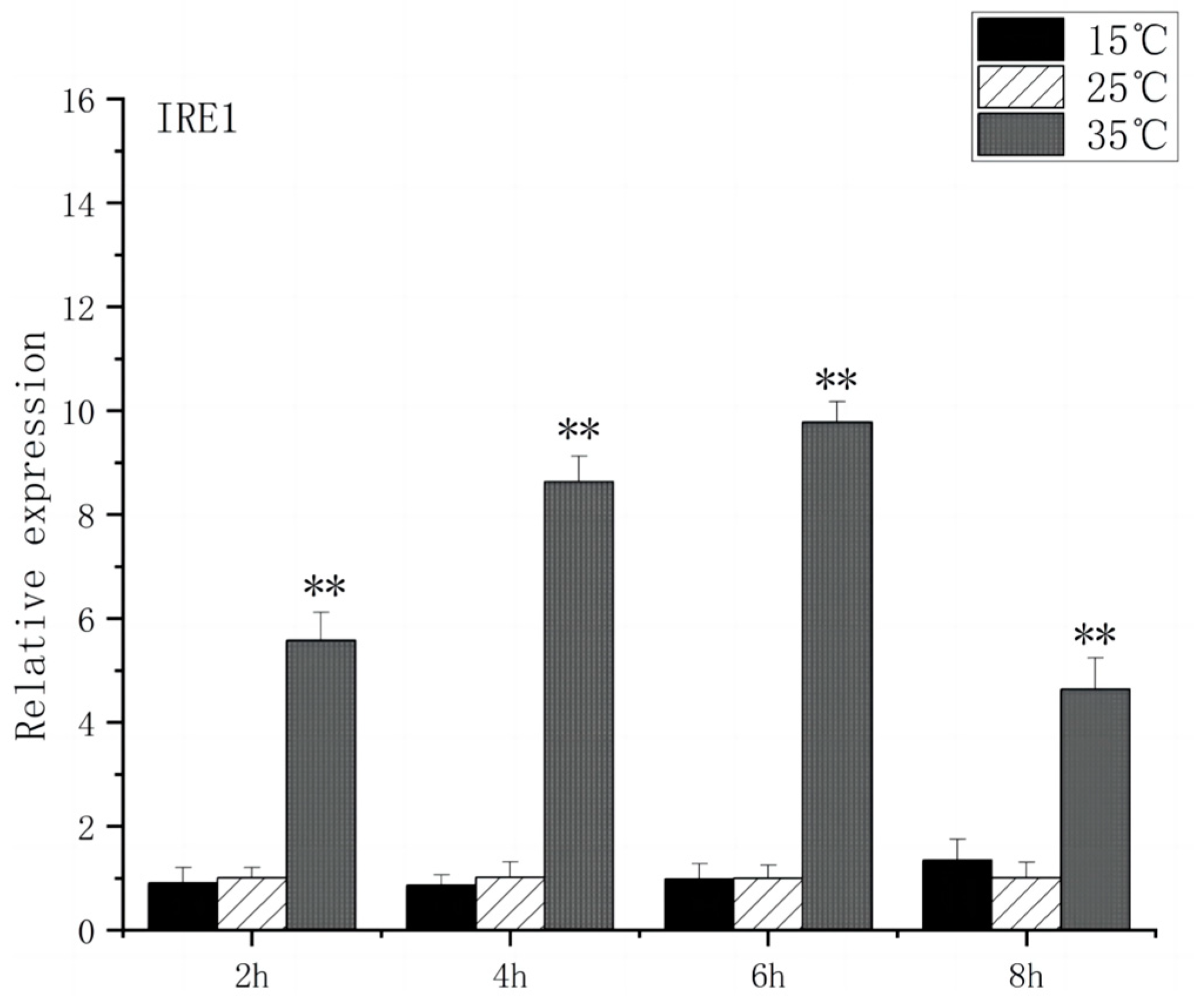
As shown in Figure 5, under acute high-temperature stress, the expression of GRP78 in O. bidens increased significantly after 2 h (p < 0.01) and reached the peak at 6 h, which was 10.7 times that of the control; after 4 h, the expression of GRP78 showed a downward trend. There was no significant difference in the expression of GRP78 between the two groups under acute cold stress. As can be seen from Figure 6, under acute high-temperature stress, the IRE1 expression of Chinese walleye showed a significant increase after 2 h (p < 0.01), and the expression reached a peak at 6 h, which was 9.7 times the control expression, while there was no significant difference between IRE1 expression in acute low-temperature stress and the control group. The expression of GRP78 and IRE1 increased with the increase in water temperature, indicating that high-temperature-induced endoplasmic reticulum stress in the brain of O. bidens, while low-temperature stress had little effect on the expression of GRP78 and IRE1 genes in the brain of O. bidens.
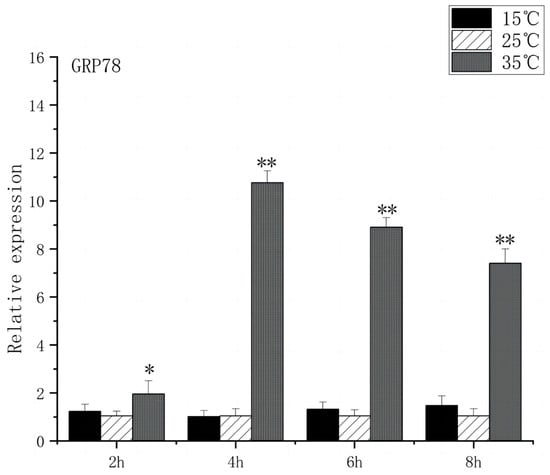
Figure 5.
GRP78 mRNA transcript levels in the brain of O. bidens. “*” indicates a significant difference (p < 0.05), “**” indicates an extremely significant difference (p < 0.01).
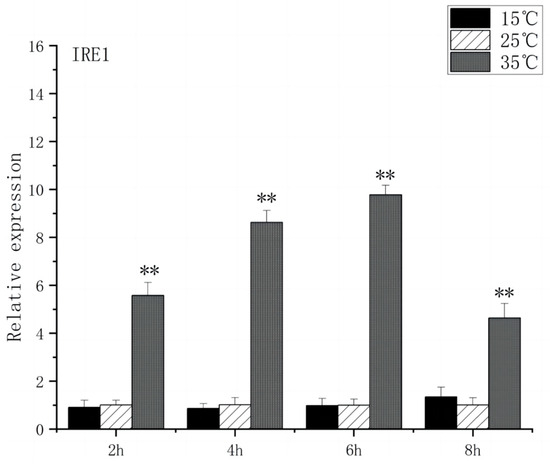
Figure 6.
IRE1 mRNA transcript levels in the brain of O. bidens. “**” indicates an extremely significant difference (p < 0.01).
3.4. Expression of Oxidative Stress Gene in the Brain of O. bidens
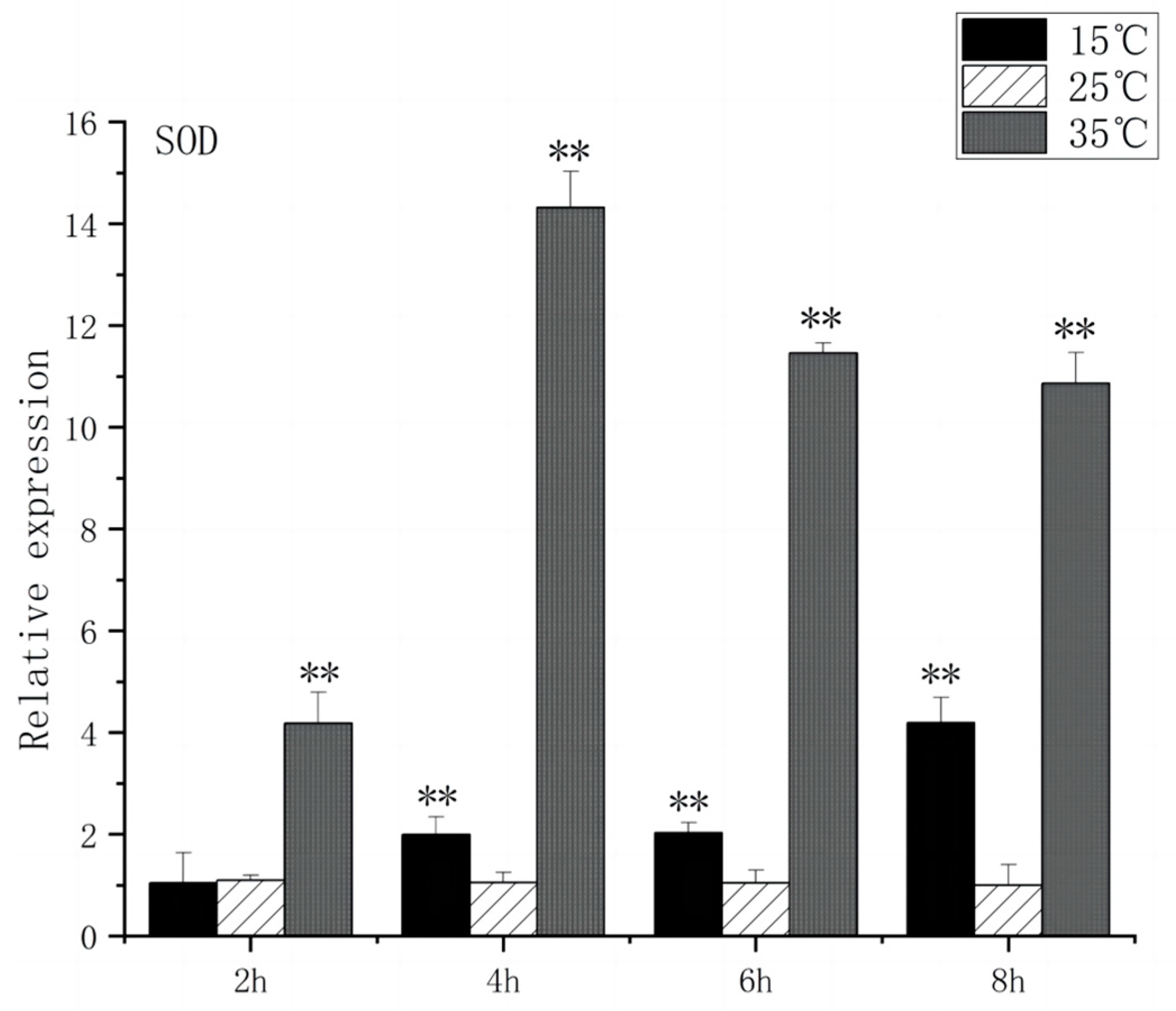
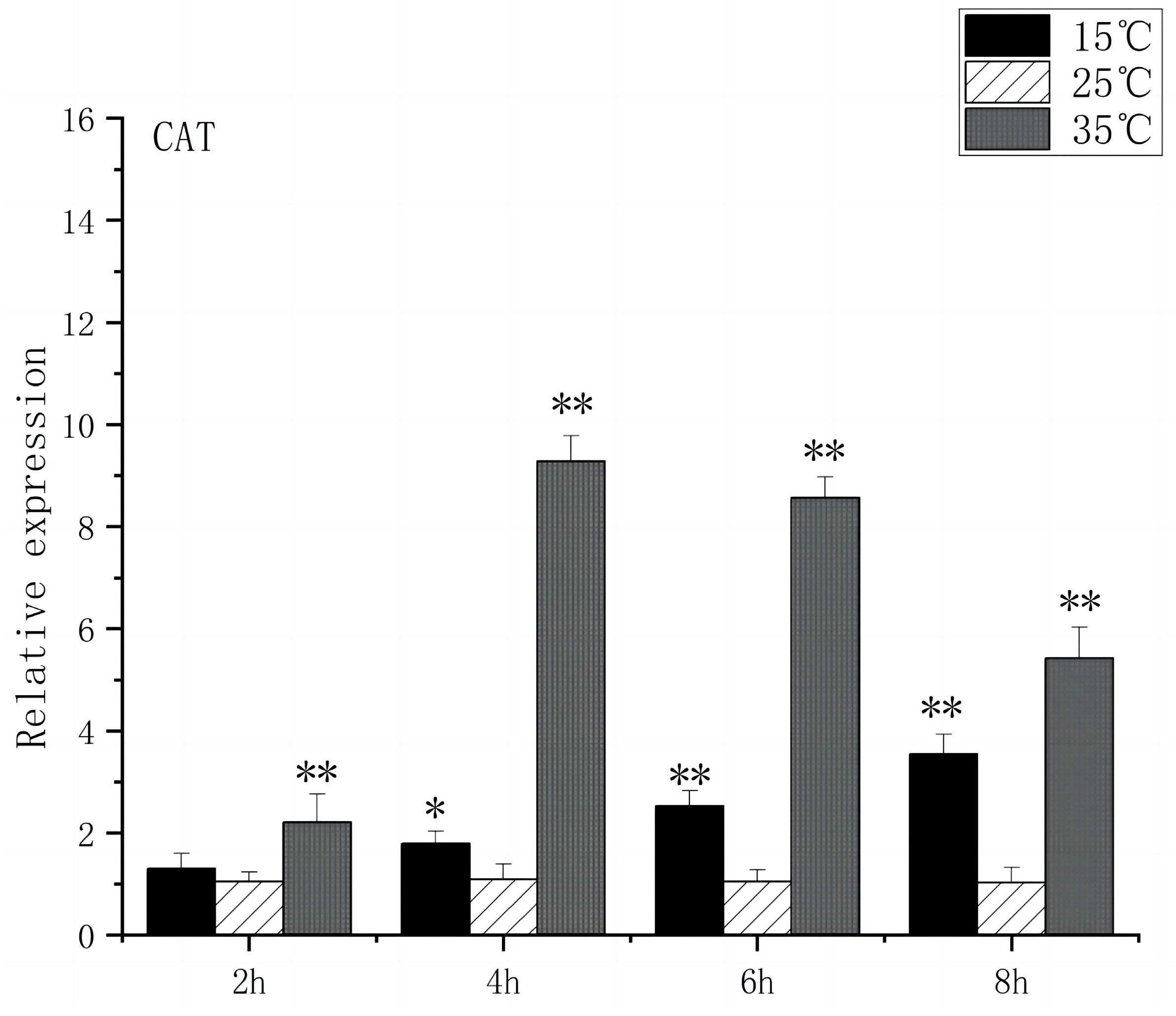
SOD is a substance produced in organisms [23] that helps eliminate superoxide anion-free radicals generated during stress. This process enhances the organism’s resistance to the external environment [24]. CAT plays a crucial role in biological development and stress response by efficiently scavenging hydrogen peroxide from both free radicals and non-radical reactive oxygen species [25]. Under acute high-temperature stress, SOD gene expression increased first and then decreased with the prolongation of high-temperature stress time, reaching its peak at 4 h, which was 14.3 times higher than that of the control (Figure 7). CAT expression increased significantly after 2 h (p < 0.01) and peaked at 4 h, 9.2 times higher than that of control (Figure 8). Under low-temperature stress, SOD gene expression increased significantly after 4 h (p < 0.01) and reached its peak at 8 h, which was 4.1 times higher than that of the control (Figure 7). CAT expression increased significantly after 4 h (p < 0.01) and peaked at 8 h, which was 3.5 times higher than that of control (Figure 8).
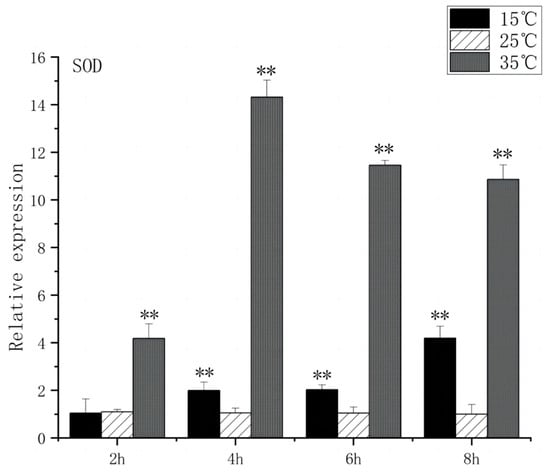
Figure 7.
SOD mRNA transcript levels in the brain of O. bidens. “**” indicates an extremely significant difference (p < 0.01).
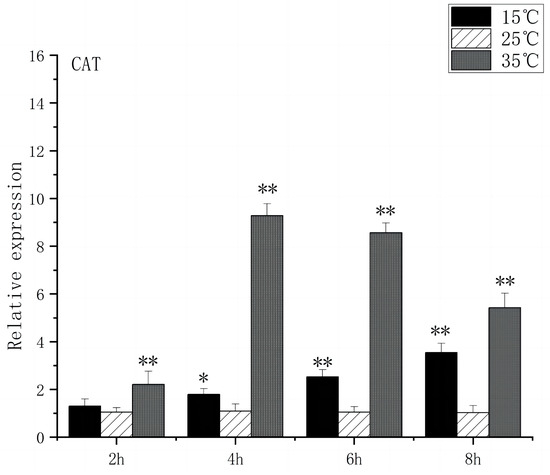
Figure 8.
CAT mRNA transcript levels in the brain of O. bidens. “*” indicates a significant difference (p < 0.05), “**” indicates an extremely significant difference (p < 0.01).
3.5. Expression of Apoptosis Gene in Brain Cells of O. bidens
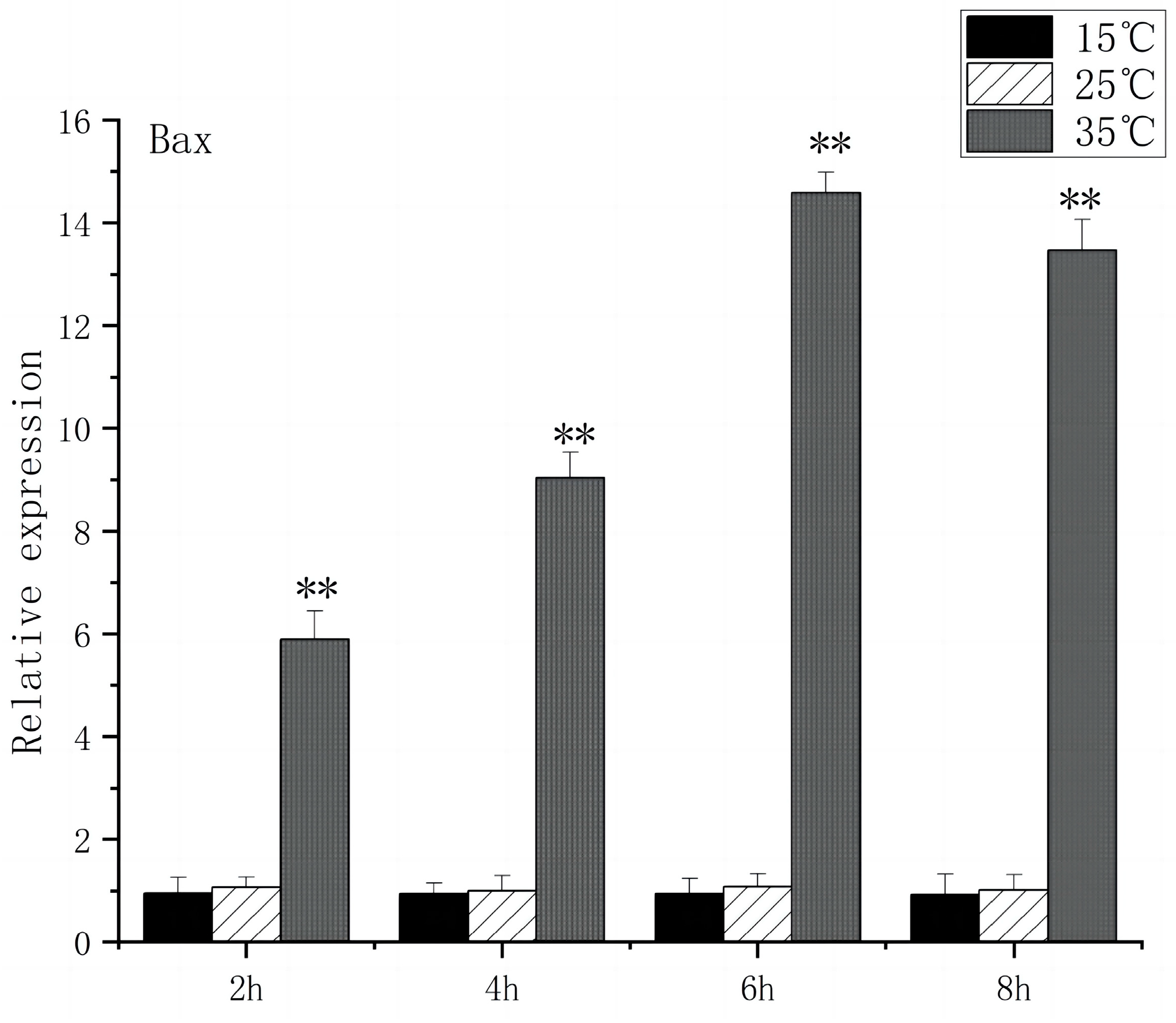
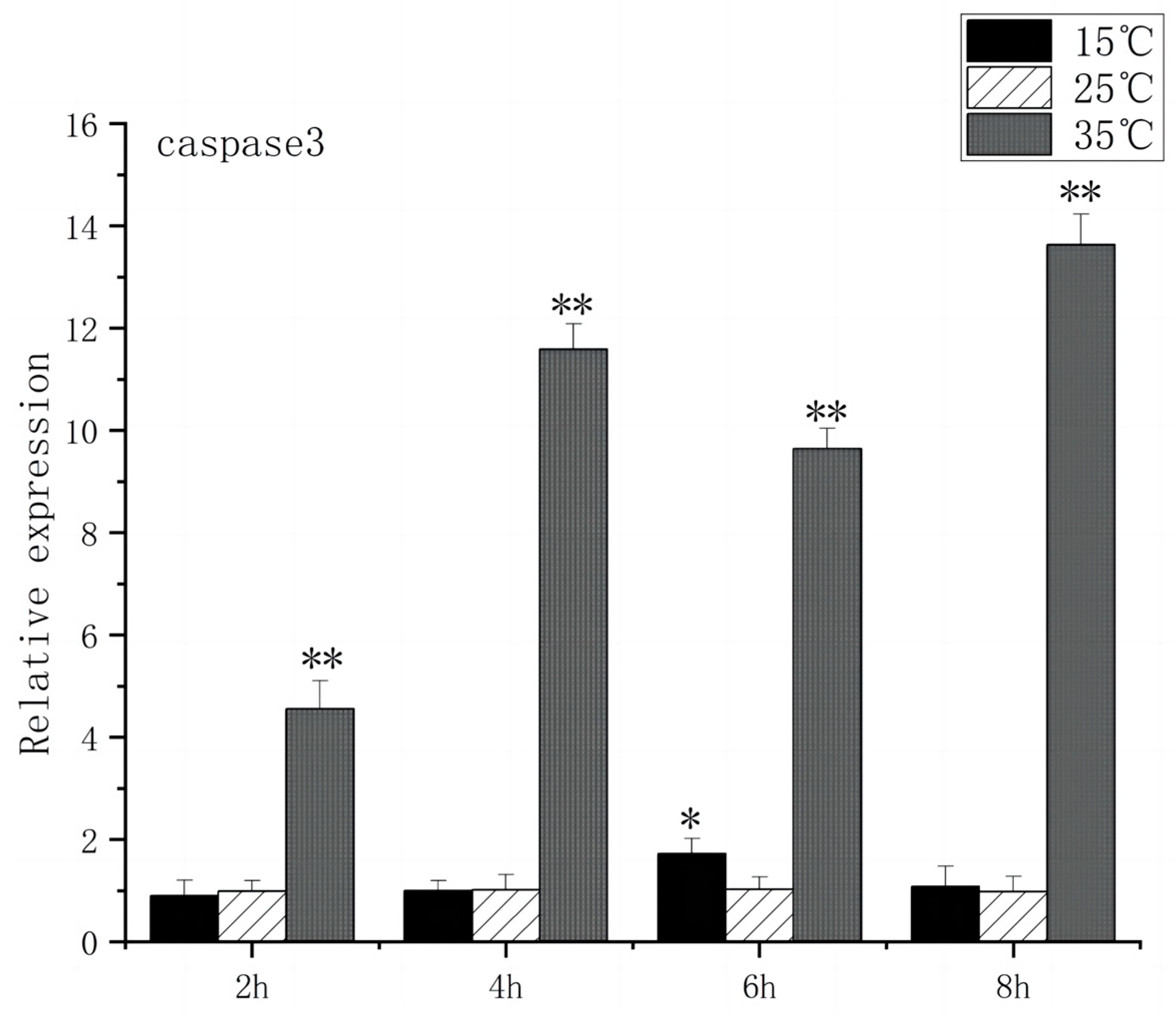
The Caspase family, Bax, and Bcl-2 play key roles in apoptosis signaling. Under high-temperature stress, Bax expression increased significantly after 2 h (p < 0.01), peaked at 6 h, and caspase3 expression increased significantly after 2 h (p < 0.01), peaked at 8 h, and caspase3 expression increased 13.6 times. In contrast, under low-temperature stress, Bax expression had no significant difference compared to the control group, but caspase3 expression increased significantly at 6 h (p < 0.05) and then returned to the control group level (Figure 9 and Figure 10).
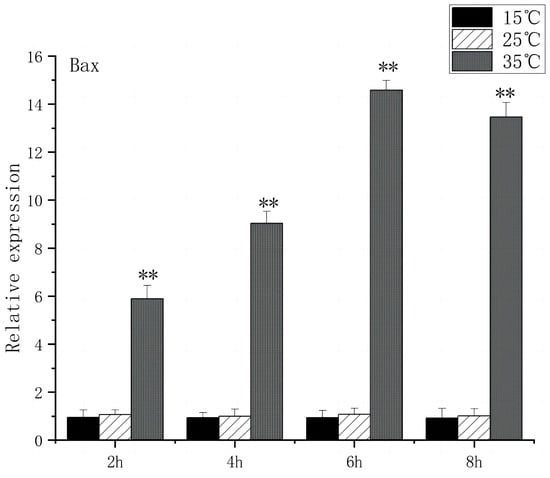
Figure 9.
Bax mRNA transcript levels in the brain of O. bidens. “**” indicates an extremely significant difference (p < 0.01).
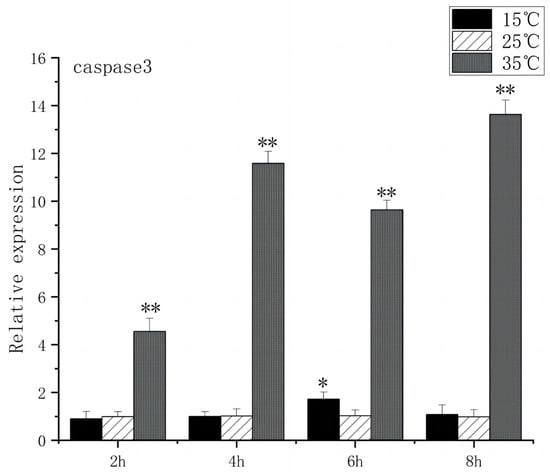
Figure 10.
Caspase3 mRNA transcript levels in the brain of O. bidens. “*” indicates a significant difference (p < 0.05), “**” indicates an extremely significant difference (p < 0.01).
4. Discussion
4.1. Effects of Acute High-Temperature Stress on the Expression of Brain-Related Genes in the O. bidens
Limited research has been conducted on the physiological effects of short-term acute temperature fluctuations on fish brain mechanisms despite the known impact of natural factors, such as natural alternation and frequent extreme weather, on fish survival, physiology, and metabolism. The results showed that high-temperature stress increased the mRNA expression of various genes including BDNF, c-FOS, HSP70, HSP90, ER stress genes (GRP78, IRE1), oxidative stress genes (CAT, SOD), and apoptosis genes (caspase3, Bax). Conversely, low-temperature stress up-regulated the mRNA expression of c-FOS, BDNF, HSP70, HSP90, and oxidative stress genes (SOD, CAT) but had a minimal effect on apoptosis genes (Bax, caspase3) and endoplasmic reticulum stress genes (IRE1, GRP78). Under high-temperature stress, the homeostasis of cells in O. bidens became imbalanced, leading to the accumulation of unfolded or misfolded proteins. This triggered an increase in HSP70 and HSP90 expression, facilitating correct protein folding, the removal of damaged proteins, the regulation of cell metabolism, and an adaptation to environmental changes. It has also been demonstrated in Gillichthys mirabilis that HSP70 and HSP90 expression is significantly elevated in response to high-temperature stress when subjected to heat stress [26]. With the extension of temperature stress time, unfolded or misfolded proteins accumulate excessively in the endoplasmic reticulum cavity, which will cause endoplasmic reticulum stress [27]. At this time, the expression of IER1 and GRP78 genes in the brain of O. bidens is up-regulated, which induces unfolded protein response [28], including degrading unfolded protein to alleviate stress damage, increasing the expression of chaperone protein to assist protein folding correctly, thus improving the survival ability of cells under harmful factors such as high temperature. Research on Coregonus maraena has indicated that the endoplasmic reticulum stress pathway exhibits a crucial stress response [29] under acute heat stress conditions, reducing the concentration of misfolded proteins to counteract heat stress damage. Continuous high-temperature stress will produce a large amount of ROS, such as superoxide anion, hydrogen peroxide, hydroxyl free radicals, etc., in the brain of O. bidens. To prevent or repair oxidative damage caused by ROS, the body up-regulates the gene expression of SOD and CAT. If ER dysfunction persists and oxidative responses continue to be stimulated, cells will initiate an apoptotic program that induces the expression of apoptosis-related genes [30]. The expression of Bax and caspase-3 in the brain of O. bidens was significantly higher than that in the control group, indicating that high-temperature stress finally activated the expression of apoptotic genes and induced apoptosis of brain cells. Moreover, the up-regulation of c-Fos expression is associated with increased BDNF expression and neuronal cell death [31]. During temperature stress, c-Fos potentially regulates BDNF expression, promoting BDNF responses to prevent apoptosis [14]. The interplay between c-Fos and BDNF expression highlights the role of these factors in protecting neurons and modulating apoptosis. This correlation is supported by the up-regulation of c-Fos and BDNF expression observed in the Oncorhynchus mykiss brain following acute heat stress [32].
4.2. Effects of Acute Low-Temperature Stress on the Expression of Brain-Related Genes in the O. bidens
Under low-temperature stress, the balance of homeostasis in brain cells was also induced, denatured proteins accumulated in cells, and the expressions of HSP70 and HSP90 increased, which helped the denatured proteins to fold correctly and prevented the aggregation of proteins whose stability was reduced due to severe mutation. At the same time, under the stimulation of a low-temperature environment, a large amount of ROS will be produced in the brain of O. bidens, the expression of oxidative stress genes will be up-regulated, and the activities of antioxidant enzymes SOD and CAT will be increased to eliminate ROS and protect cells from oxidative damage [33,34]. On the other hand, under low-temperature stress, the expression of c-Fos was significantly increased and induced apoptosis, while the expression level of BNDF was positively correlated with the expression of c-Fos, which was consistent with the study of c-Fos gene expression up-regulation after low-temperature stress in Epinephelus moara embryos [35]. In this study, acute high-temperature stress significantly increased the expression of GRP78 and IRE1 in the brain of Chinese snapper, which were 10.7 and 9.7 times higher than that of the control, respectively. However, there was no significant difference in the expression of GRP78 and IRE1 under acute low-temperature stress compared with that of the control group. The reason may be that compared with high-temperature stress, low-temperature stress caused less damage to the brain of the Chinese snapper, and the homeostasis of cells was not greatly affected. Meanwhile, under the synergistic effect of HSP70 and HSP90, the concentration of denatured protein in the ER cavity decreased, so that ER stress was not induced. When the intensity of external stimulation exceeds the tolerance range of cells, it will lead to DNA structural damage and apoptosis [36]. Li et al. [37] found that the antioxidant defense ability of cobia was low under low-temperature stress, and the expression of the caspase family and Bax was up-regulated, which led to apoptosis. However, the expression levels of apoptosis-related genes (caspase3, Bax) in the brain of the Chinese snapper had no significant change under low-temperature stress but were significantly up-regulated to more than ten times those in the control group under high-temperature stress. This phenomenon may be related to the strong cold tolerance of Chinese snappers. The brain of O. bidens may not be damaged by strong oxidation under low-temperature stress, and the intensity of stimulation is within its tolerance range. At the same time, the oxidative damage is reduced with the help of the antioxidant enzymes SOD and CAT, resulting in the brain not initiating the apoptosis program, so the impact on apoptosis gene expression is small. These results indicated that the adaptability of O. bidens to low-temperature stress was stronger than that to high-temperature stress.
5. Conclusions
In conclusion, our study demonstrated that the brain of O. bidens plays a crucial role in its adaptation to temperature extremes. Specifically, neural activation, heat shock protein regulation, endoplasmic reticulum stress, oxidative stress, and apoptosis were identified as key mechanisms of brain acclimation to high temperatures in O. bidens. However, the mechanisms of adaptation to low temperatures in the brain of O. bidens only involve neural activation, heat shock protein regulation, oxidative stress, and apoptotic processes. By investigating these processes at the gene expression level, this study aims to enhance our comprehension of the interplay between temperature and brain physiology, shedding light on the adaptive mechanisms of O. bidens and contributing to a deeper theoretical understanding of temperature-related responses in this species.
Author Contributions
Conceptualization, Q.L.; software, Q.L., L.X., Y.Z. and A.Z.; validation, S.Z.; formal analysis, Q.L.; investigation, Q.L., L.X. and Y.Z.; resources, L.X., Y.Z. and A.Z.; data curation, Q.L. and A.Z.; writing—original draft preparation, Q.L.; writing—review and editing, S.Z.; visualization, L.X., Y.Z. and A.Z.; supervision, S.Z.; project administration, S.Z.; funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the ‘Sannong Jiufang’ Project of Zhejiang Province of China (No. 2023SNJF07) and the Key Science and Technology Projects in Longyou County, Zhejiang Province, China (No. JHXM2022008).
Institutional Review Board Statement
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of Zhejiang Normal University (approval no. IAC-2023-22) and conducted in accordance with institutional ethical guidelines for experimental animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data in this study are available on public platforms.
Acknowledgments
The authors thank the technology company for supplying the experiment samples. Meanwhile, fundings from the ‘Sannong Jiufang’ Project of Zhejiang Province of China and the Key Science and Technology Projects in Longyou County, Zhejiang Province, China are gratefully acknowledged. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that they do not have any conflicts of interest in this work. We also declare that we have no commercial or associative interests that could pose a conflict of interest about the submitted work.
References
- Toni, M.; Angiulli, E.; Miccoli, G.; Cioni, C.; Alleva, E.; Frabetti, F.; Pizzetti, F.; Grassi Scalvini, F.; Nonnis, S.; Negri, A.; et al. Environmental temperature variation affects brain protein expression and cognitive abilities in adult zebrafish (Danio rerio): A proteomic and behavioural study. J. Proteom. 2019, 204, 103396. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.M.; Castro, V.; Krasnov, A.; Torgersen, J.; Timmerhaus, G.; Hevrøy, E.M.; Hansen, T.J.; Susort, S.; Breck, O.; Takle, H. Cardiac responses to elevated seawater temperature in Atlantic salmon. BMC Physiol. 2014, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Stitt, B.C.; Burness, G.; Burgomaster, K.A.; Currie, S.; McDermid, J.L.; Wilson, C.C. Intraspecific variation in thermal tolerance and acclimation capacity in brook trout (Salvelinus fontinalis): Physiological implications for climate change. Physiol. Biochem. Zool. 2014, 87, 15–29. [Google Scholar] [CrossRef]
- Carney Almroth, B.; Asker, N.; Wassmur, B.; Rosengren, M.; Jutfelt, F.; Gräns, A.; Sundell, K.; Axelsson, M.; Sturve, J. Warmer water temperature results in oxidative damage in an Antarctic fish, the bald notothen. J. Exp. Mar. Biol. Ecol. 2015, 468, 130–137. [Google Scholar] [CrossRef]
- Bowyer, J.; Booth, M.; Qin, J.; D’Antignana, T.; Thomson, M.; Stone, D. Temperature and dissolved oxygen influence growth and digestive enzyme activities of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquac. Res. 2014, 45, 2010–2020. [Google Scholar] [CrossRef]
- Donelson, J.; Munday, P.; McCormick, M.; Pankhurst, N.; Pankhurst, P. Effects of elevated water temperature and food on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–243. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yang, F.-F.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L.; Tan, J.-W.; Chen, X.-Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Shahjahan, M.; Uddin, M.H.; Bain, V.; Haque, M.M. Increased water temperature altered hemato-biochemical parameters and structure of peripheral erythrocytes in striped catfish Pangasianodon hypophthalmus. Fish Physiol. Biochem. 2018, 44, 1309–1318. [Google Scholar] [CrossRef]
- Qi, Z.-H.; Liu, Y.-F.; Luo, S.-W.; Chen, C.-X.; Liu, Y.; Wang, W.-N. Molecular cloning, characterization and expression analysis of tumor suppressor protein p53 from orange-spotted grouper, Epinephelus coioides in response to temperature stress. Fish Shellfish Immunol. 2013, 35, 1466–1476. [Google Scholar] [CrossRef]
- Cacialli, P.; Gueguen, M.M.; Coumailleau, P.; D’Angelo, L.; Kah, O.; Lucini, C.; Pellegrini, E. BDNF Expression in Larval and Adult Zebrafish Brain: Distribution and Cell Identification. PLoS ONE 2016, 11, e0158057. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Wurzelmann, M.; Romeika, J.; Sun, D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen. Res. 2017, 12, 7–12. [Google Scholar] [PubMed]
- Çomakli, S.; Köktürk, M.; Topal, A.; Özkaraca, M.; Ceyhun, S.B. Immunofluorescence/fluorescence assessment of brain-derived neurotrophic factor, c-Fos activation, and apoptosis in the brain of zebrafish (Danio rerio) larvae exposed to glufosinate. NeuroToxicology 2018, 69, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Effects of Acute Temperature Stress on the Expression of c-Fos and 5-HT1A Receptors in the Brain of Batrachuperus pinchoni. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2008. [Google Scholar]
- Topal, A.; Atamanalp, M.; Oruç, E.; Halıcı, M.B.; Şişecioğlu, M.; Erol, H.S.; Gergit, A.; Yılmaz, B. Neurotoxic effects of nickel chloride in the rainbow trout brain: Assessment of c-Fos activity, antioxidant responses, acetylcholinesterase activity, and histopathological changes. Fish Physiol. Biochem. 2015, 41, 625–634. [Google Scholar] [CrossRef]
- Varani, A.P.; Moutinho Machado, L.; Balerio, G.N. Baclofen prevented the changes in c-Fos and brain-derived neutrophic factor expressions during mecamylamine-precipitated nicotine withdrawal in mice. Synapse 2014, 68, 508–517. [Google Scholar] [CrossRef]
- Huang, L. Transcriptional Expression of Immune-Related Genes and Heat Shock Protein Genes of Japanese Flounder (Paralichthys olivaceus). Ph.D. Thesis, Graduate School of Chinese Academy of Sciences (Institute of Oceanology), Beijing, China, 2015. [Google Scholar]
- Wang, Q.; Liu, Y.; Zheng, Z.; Deng, Y.; Jiao, Y.; Du, X. Adaptive response of pearl oyster Pinctada fucata martensii to low water temperature stress. Fish Shellfish Immunol. 2018, 78, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Kennedy, C.J.; Iwama, G.K. The effects of stress on the association between hsp70 and the glucocorticoid receptor in rainbow trout. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 134, 655–663. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Yang, L.; Yang, E.; Xie, R.; Chen, G.; Huang, J. Transcription level of immune related genes of juvenile cobia (Rachycentron canadum) under hypoxia stress. Haiyang Xuebao 2021, 43, 92–101. [Google Scholar]
- Tian, X.R.; Wu, H.; Li, J. Accumulation effect of fresh heavy metals and antioxidant system response of Cd, Pb composite stress in wetland. J. Agro-Environ. Sci. 2015, 34, 844–851. [Google Scholar]
- Chen, K.; Ma, P.; Huang, J.; Huang, D.; Wang, T.; Huang, X. Effects of hypoxic stress on superoxide dismutase SOD content in juvenile pearl grouper. Jiangxi Aquat. Sci. Technol. 2021, 4, 16–18. [Google Scholar]
- Shi, Z.-H.; Zhang, C.-J.; Peng, S.-M.; Wang, J.-G.; Gao, Q.-X. Effect of salinity on serum osmolality, catalase and gill ion-regulatory enzyme activities in silver pomfret. J. Fish. 2013, 37, 1697–1705. [Google Scholar] [CrossRef]
- Buckley, B.A.; Gracey, A.Y.; Somero, G.N. The cellular response to heat stress in the goby Gillichthys mirabilis: A cDNA microarray and protein-level analysis. J. Exp. Biol. 2006, 209, 2660–2677. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Xu, M.; Wang, T. Endoplasmic reticulum stress response and associated molecular chaperones. Prog. Anat. Sci. 2014, 20, 381–384. [Google Scholar]
- Rebl, A.; Verleih, M.; Nipkow, M.; Altmann, S.; Bochert, R.; Goldammer, T. Gradual and Acute Temperature Rise Induces Crossing Endocrine, Metabolic, and Immunological Pathways in Maraena Whitefish (Coregonus maraena). Front. Genet. 2018, 9, 241. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Dong, M.; Wu, Y.; Fan, Y.; Xu, M.; Zhang, J. c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci. Lett. 2006, 400, 177–180. [Google Scholar] [CrossRef]
- Topal, A.; Özdemir, S.; Arslan, H.; Çomaklı, S. How does elevated water temperature affect fish brain? (A neurophysiological and experimental study: Assessment of brain derived neurotrophic factor, cFOS, apoptotic genes, heat shock genes, ER-stress genes and oxidative stress genes). Fish Shellfish Immunol. 2021, 115, 198–204. [Google Scholar] [CrossRef]
- Kim, B.-S.; Jung, S.J.; Choi, Y.J.; Kim, N.N.; Choi, C.Y.; Kim, J.-W. Effects of different light wavelengths from LEDs on oxidative stress and apoptosis in olive flounder (Paralichthys olivaceus) at high water temperatures. Fish Shellfish Immunol. 2016, 55, 460–468. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Tian, Y.-S.; Ma, W.-H.; Zhai, J.-M. Gene expression changes in response to low temperatures in embryos of the kelp grouper, Epinephelus moara. Cryobiology 2020, 97, 159–167. [Google Scholar] [CrossRef]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Chen, Y. Effects of low-temperature stress on serum biochemical, antioxidant enzymes activity and apoptosis-related gene expression in liver of juvenile Cobia (Rachycentron canadum). J. Guangdong Ocean. Univ. 2022, 42, 18–26. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).