Effects of Acute Temperature Stress on the Expression of Related Genes in the Brain of Opsariichthys bidens
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Experimental Methods
2.2.1. Experimental Treatment
2.2.2. Sample Extraction
2.3. Gene Expression Analysis
2.4. Data Processing and Statistical
3. Results
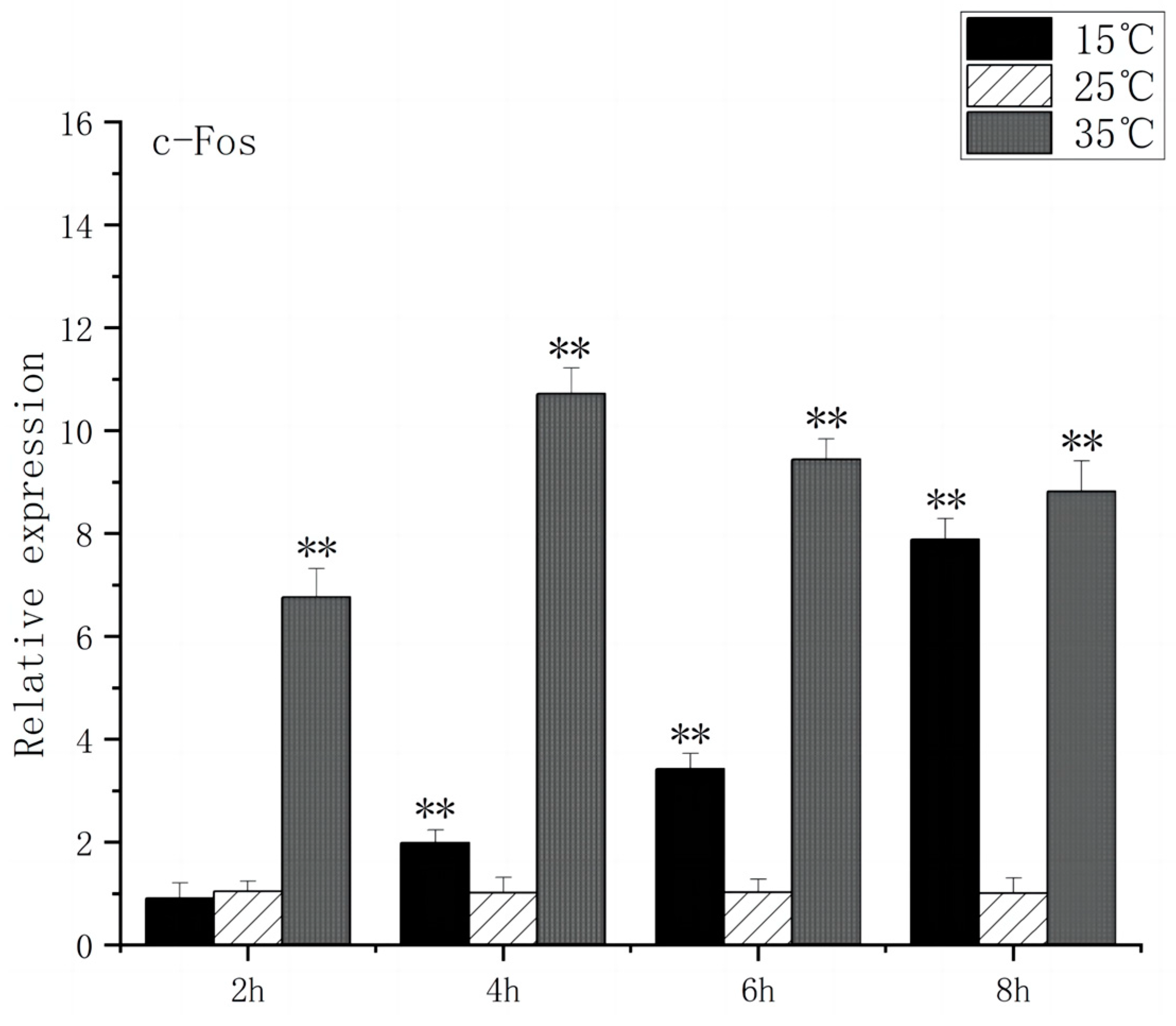
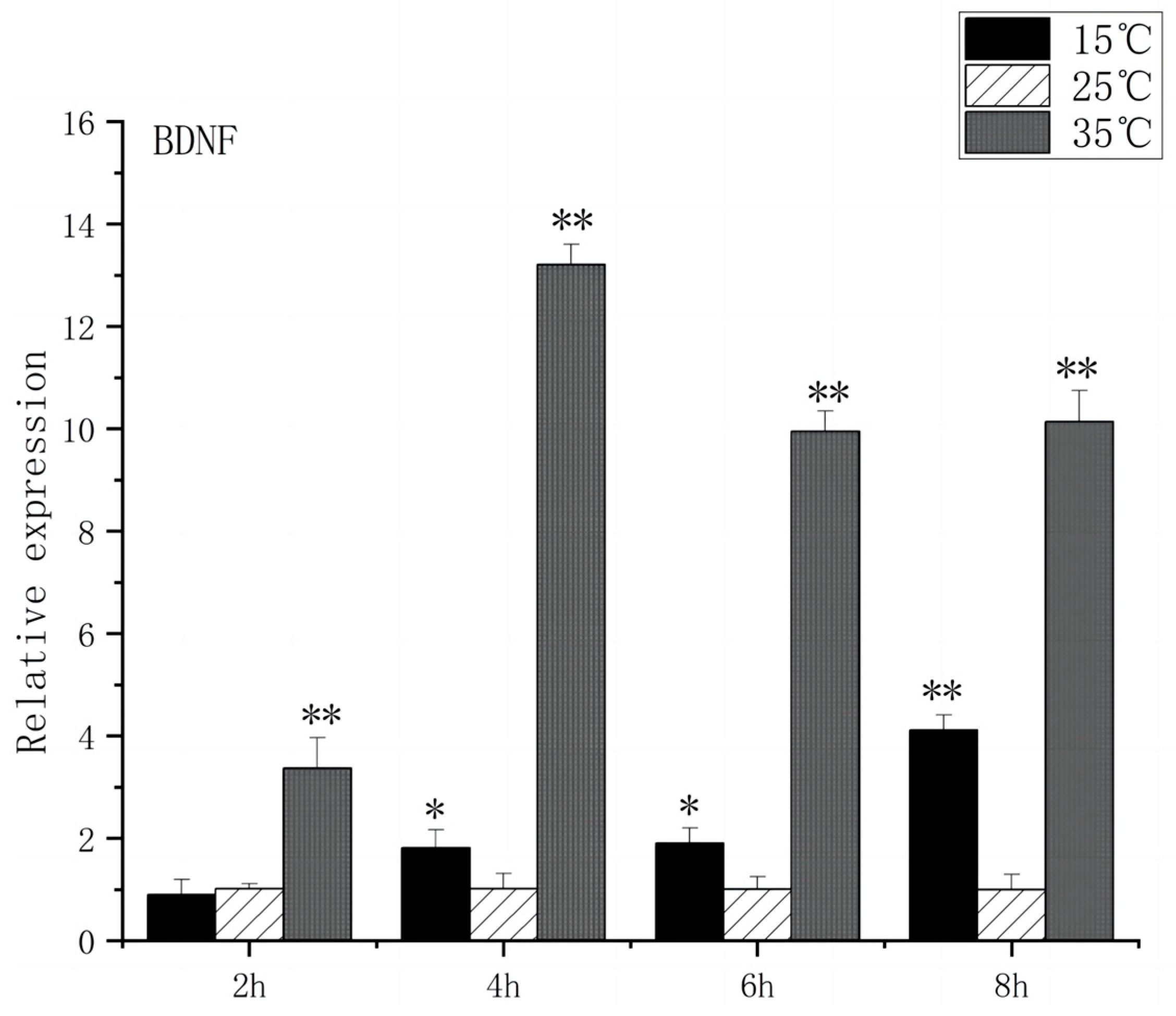
3.1. Expression of BDNF and c-FOS Genes in the Brain of O. bidens
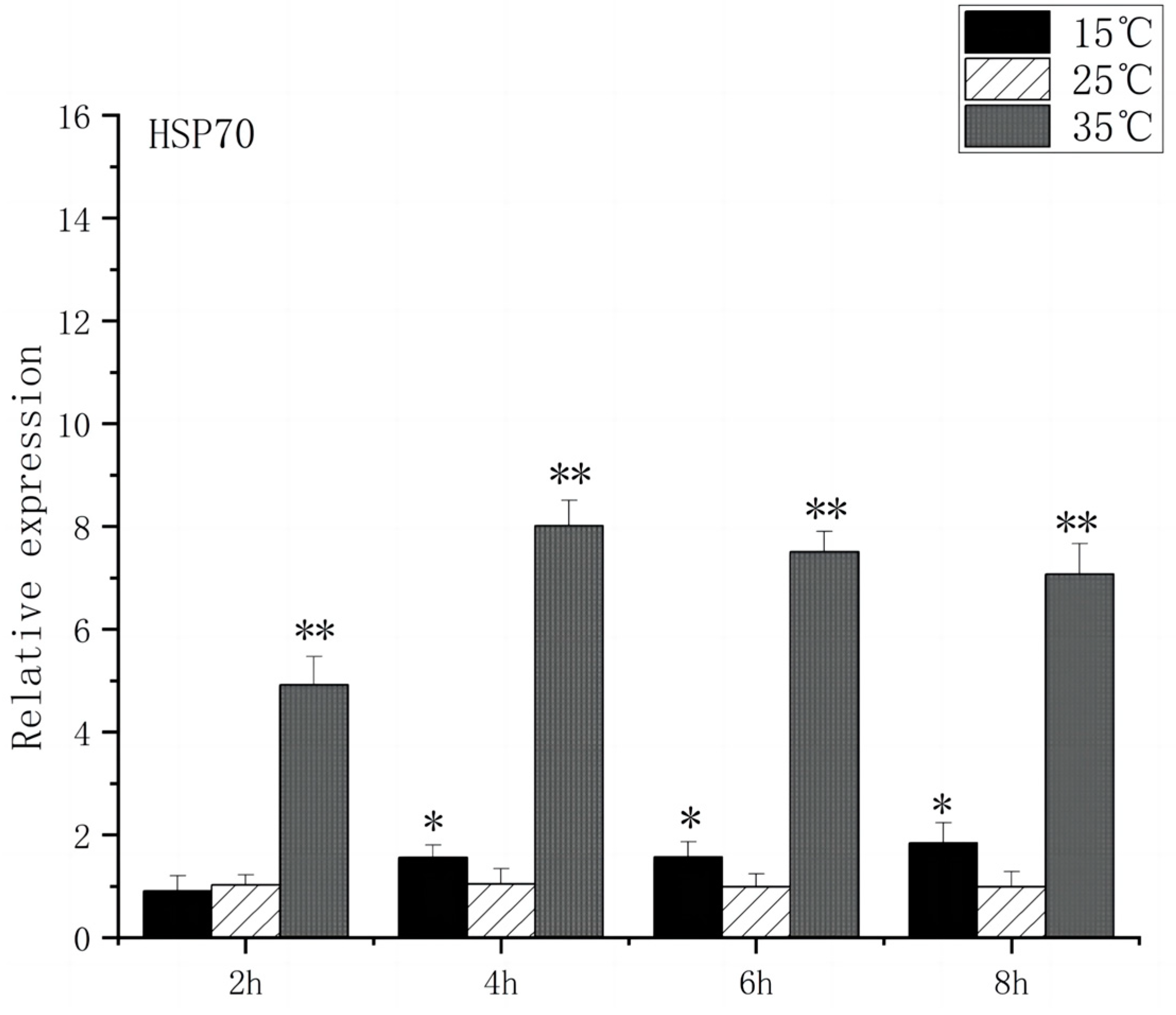
3.2. Expression of Heat Stress-Related Genes in the Brain of O. bidens
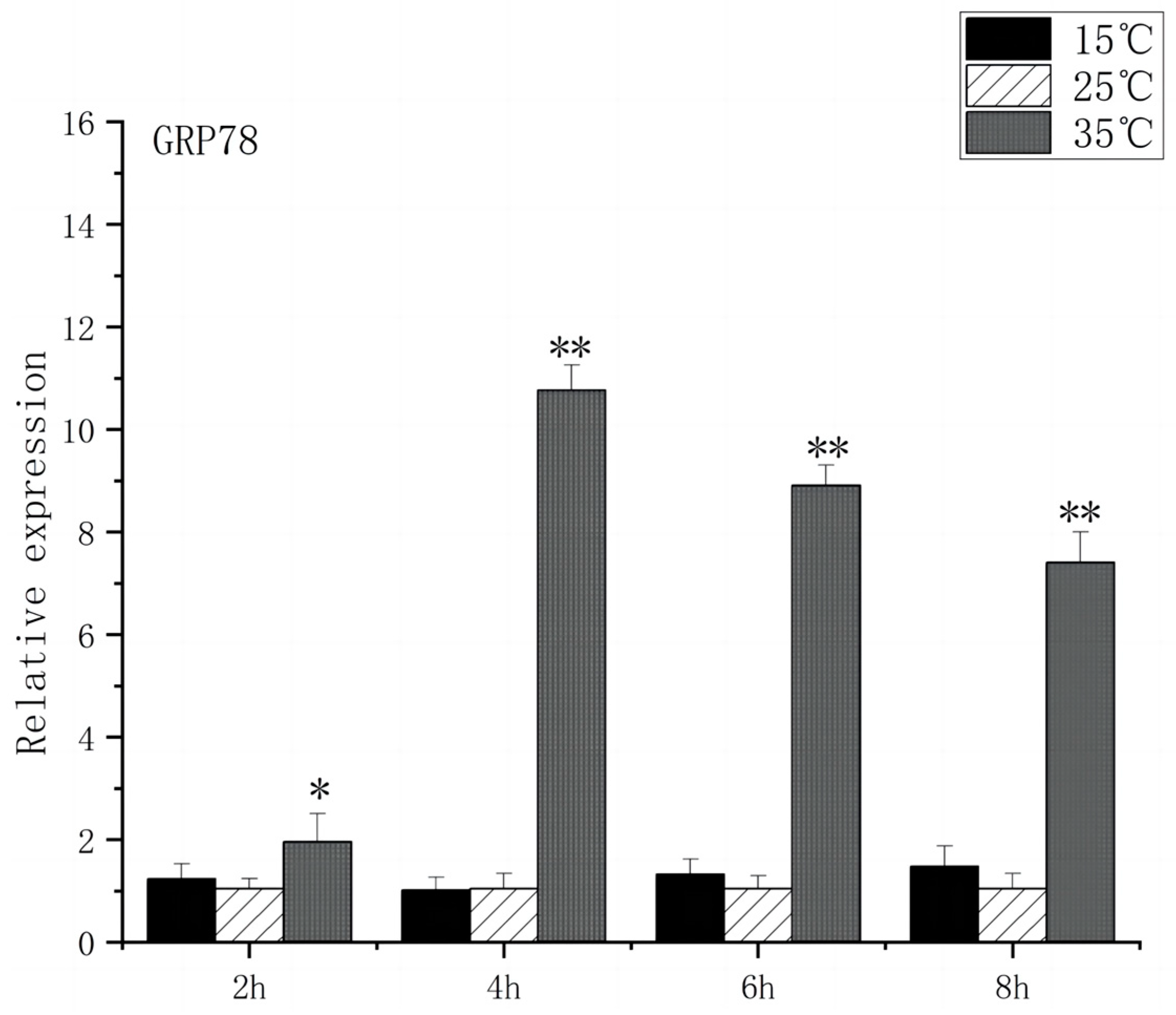
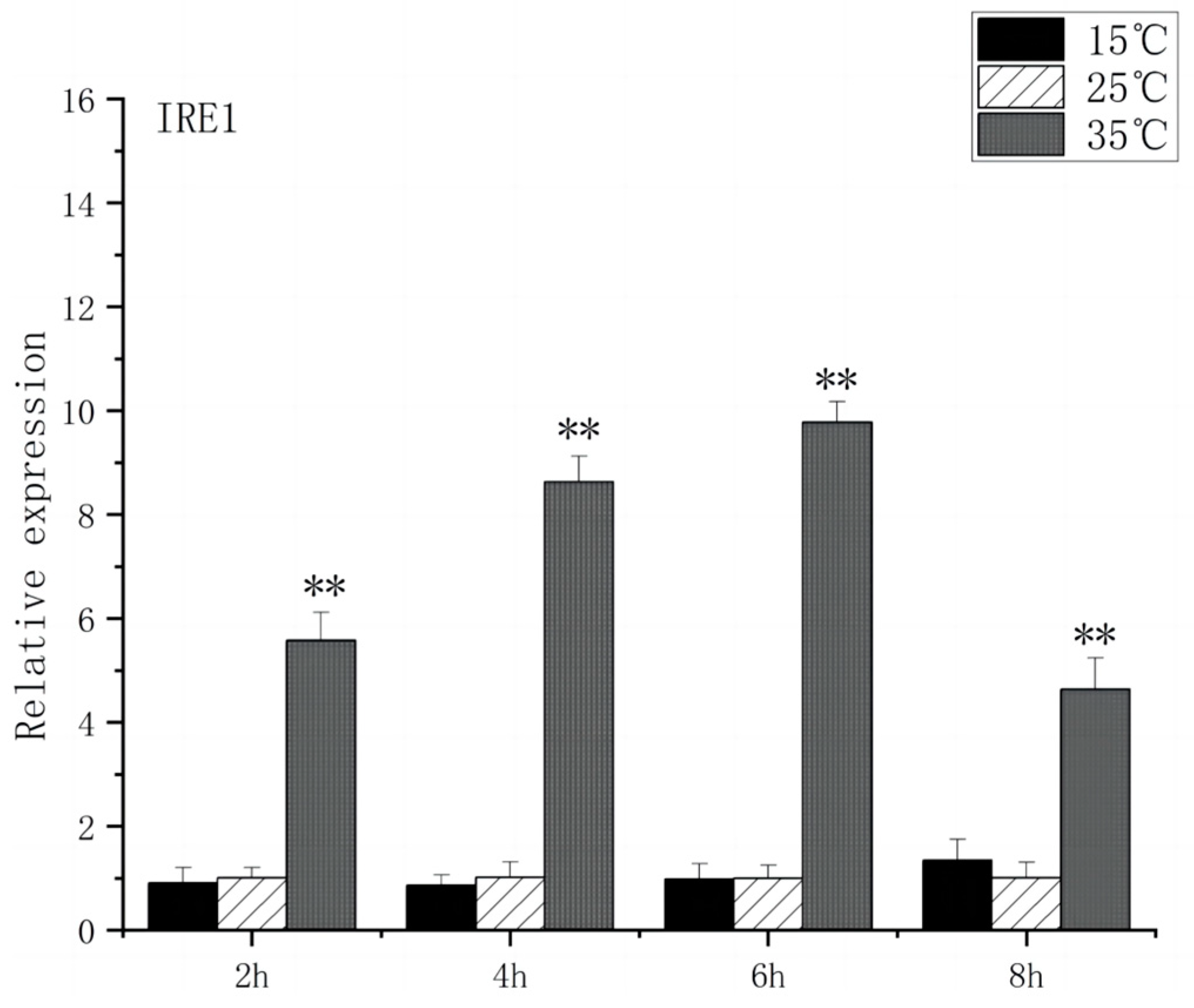
3.3. Expression of Stress Genes in Brain Endoplasmic Reticulum of O. bidens
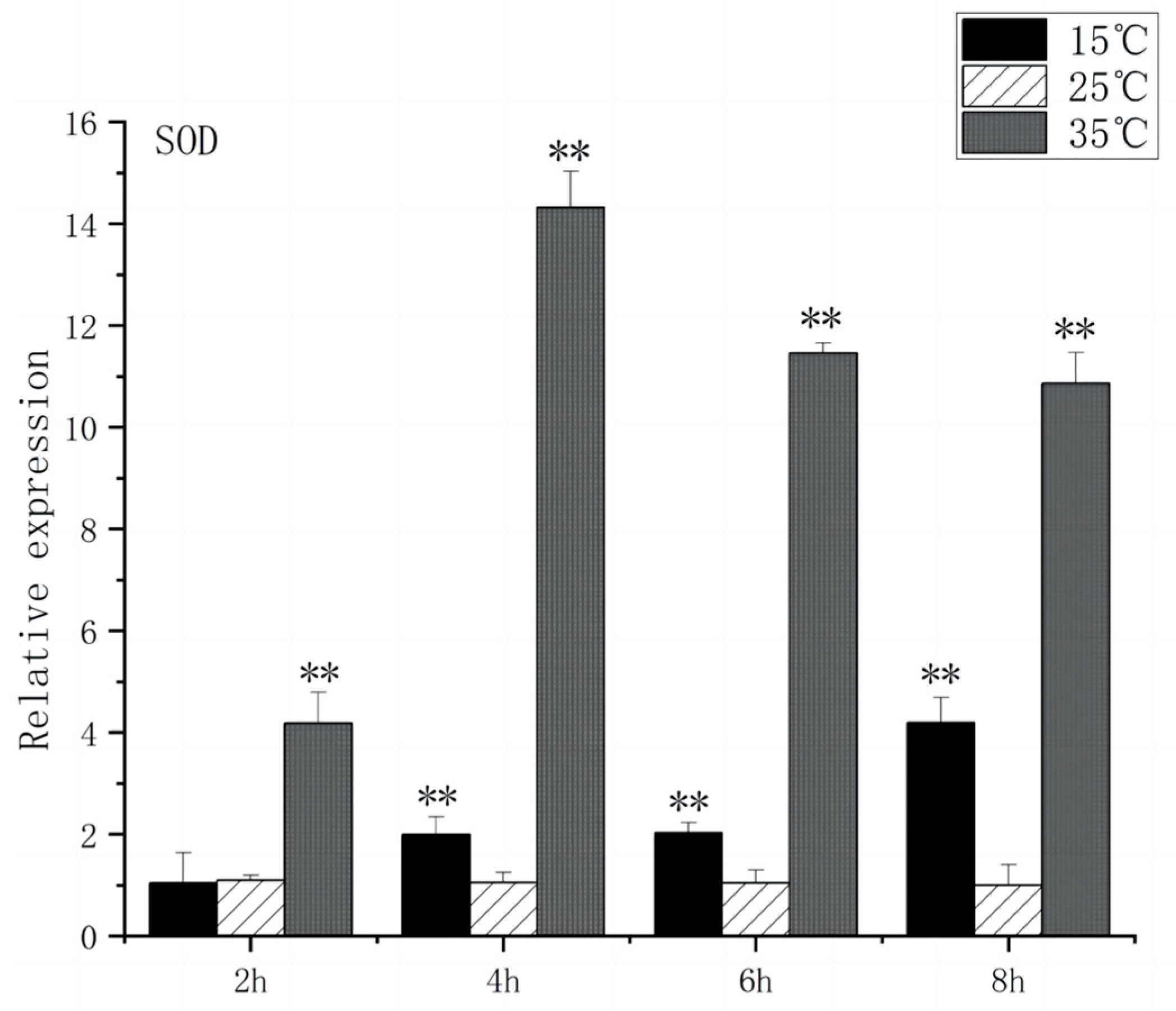
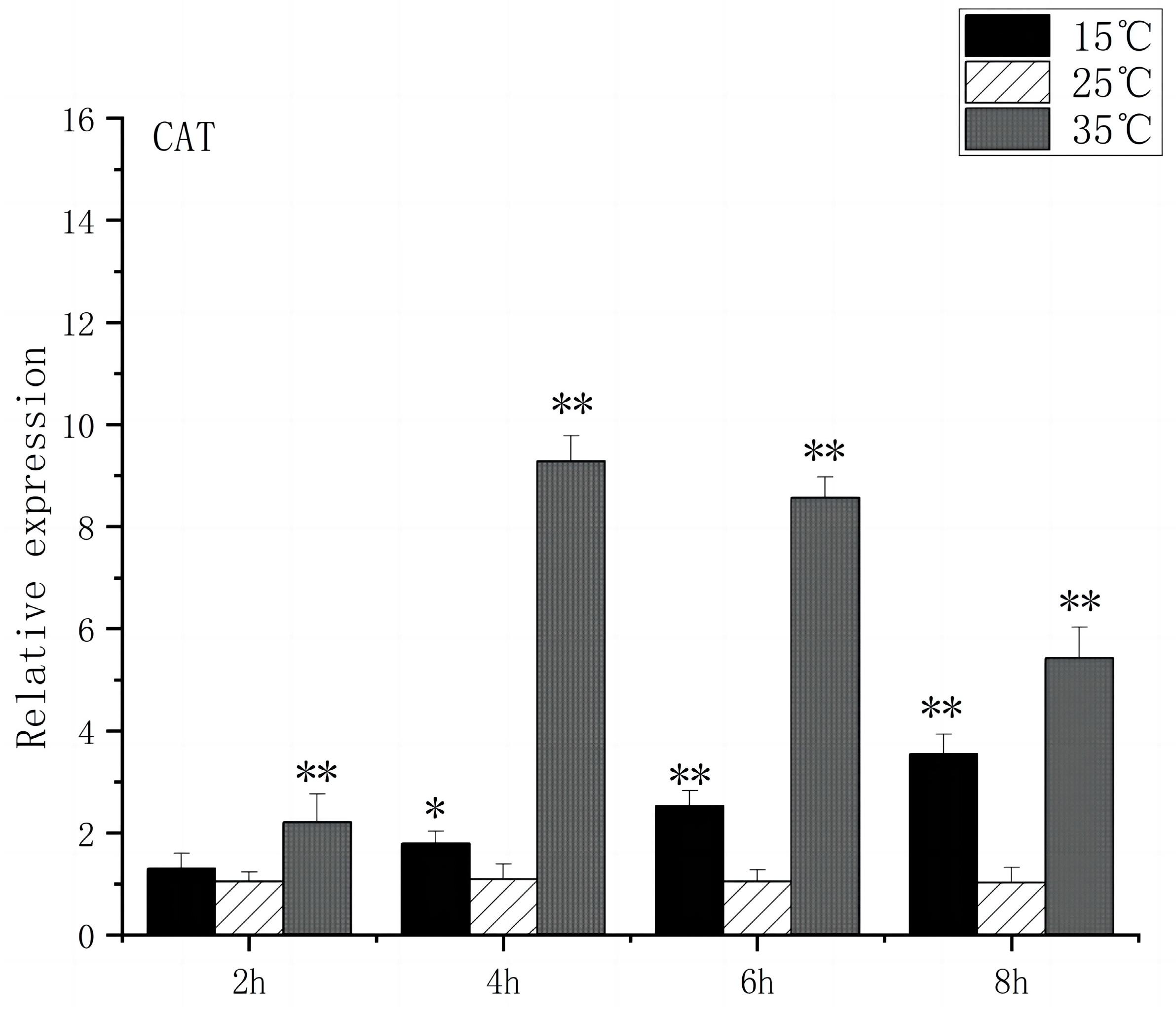
3.4. Expression of Oxidative Stress Gene in the Brain of O. bidens
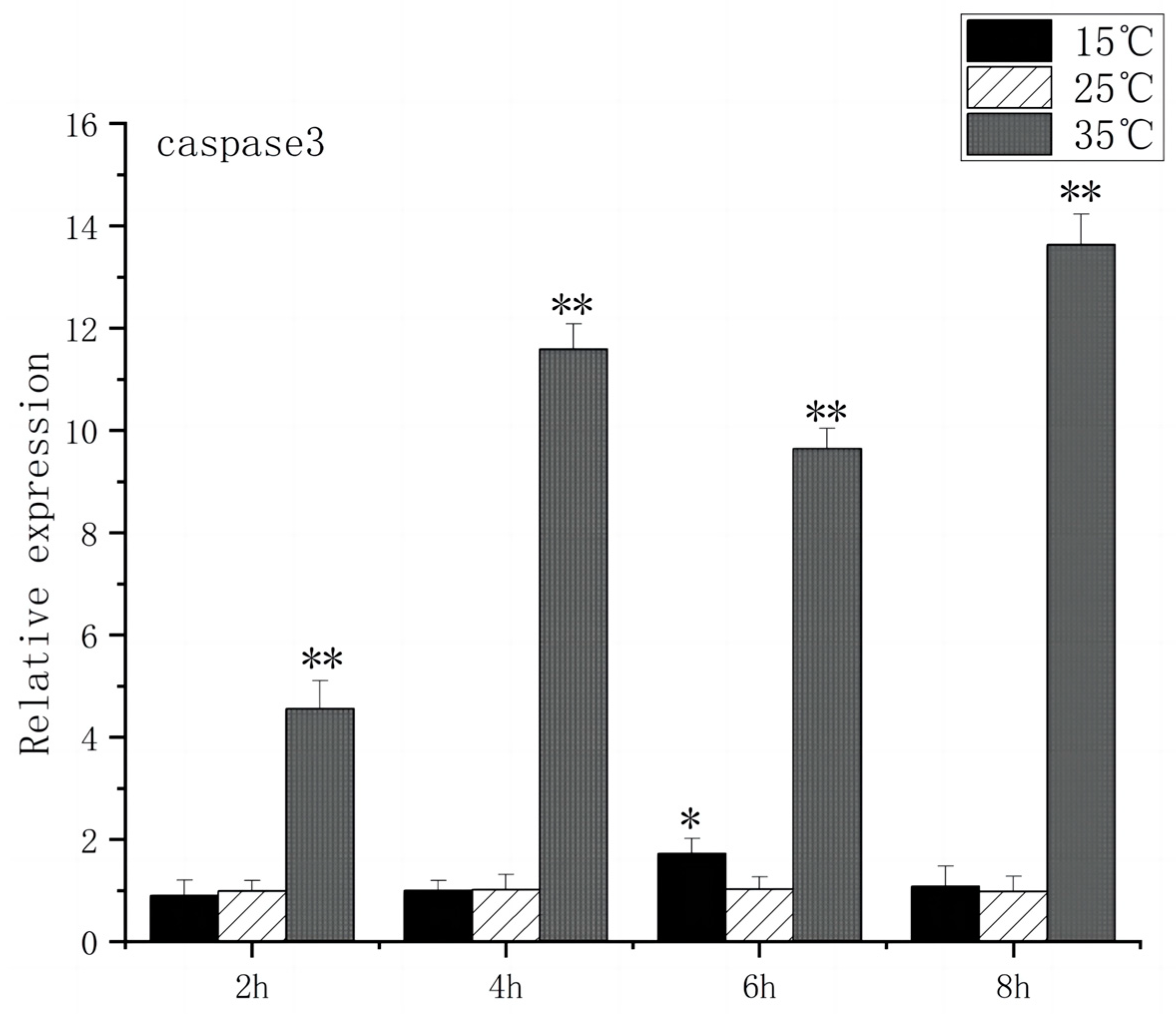
3.5. Expression of Apoptosis Gene in Brain Cells of O. bidens
4. Discussion
4.1. Effects of Acute High-Temperature Stress on the Expression of Brain-Related Genes in the O. bidens
4.2. Effects of Acute Low-Temperature Stress on the Expression of Brain-Related Genes in the O. bidens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toni, M.; Angiulli, E.; Miccoli, G.; Cioni, C.; Alleva, E.; Frabetti, F.; Pizzetti, F.; Grassi Scalvini, F.; Nonnis, S.; Negri, A.; et al. Environmental temperature variation affects brain protein expression and cognitive abilities in adult zebrafish (Danio rerio): A proteomic and behavioural study. J. Proteom. 2019, 204, 103396. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.M.; Castro, V.; Krasnov, A.; Torgersen, J.; Timmerhaus, G.; Hevrøy, E.M.; Hansen, T.J.; Susort, S.; Breck, O.; Takle, H. Cardiac responses to elevated seawater temperature in Atlantic salmon. BMC Physiol. 2014, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Stitt, B.C.; Burness, G.; Burgomaster, K.A.; Currie, S.; McDermid, J.L.; Wilson, C.C. Intraspecific variation in thermal tolerance and acclimation capacity in brook trout (Salvelinus fontinalis): Physiological implications for climate change. Physiol. Biochem. Zool. 2014, 87, 15–29. [Google Scholar] [CrossRef]
- Carney Almroth, B.; Asker, N.; Wassmur, B.; Rosengren, M.; Jutfelt, F.; Gräns, A.; Sundell, K.; Axelsson, M.; Sturve, J. Warmer water temperature results in oxidative damage in an Antarctic fish, the bald notothen. J. Exp. Mar. Biol. Ecol. 2015, 468, 130–137. [Google Scholar] [CrossRef]
- Bowyer, J.; Booth, M.; Qin, J.; D’Antignana, T.; Thomson, M.; Stone, D. Temperature and dissolved oxygen influence growth and digestive enzyme activities of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquac. Res. 2014, 45, 2010–2020. [Google Scholar] [CrossRef]
- Donelson, J.; Munday, P.; McCormick, M.; Pankhurst, N.; Pankhurst, P. Effects of elevated water temperature and food on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–243. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yang, F.-F.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L.; Tan, J.-W.; Chen, X.-Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Shahjahan, M.; Uddin, M.H.; Bain, V.; Haque, M.M. Increased water temperature altered hemato-biochemical parameters and structure of peripheral erythrocytes in striped catfish Pangasianodon hypophthalmus. Fish Physiol. Biochem. 2018, 44, 1309–1318. [Google Scholar] [CrossRef]
- Qi, Z.-H.; Liu, Y.-F.; Luo, S.-W.; Chen, C.-X.; Liu, Y.; Wang, W.-N. Molecular cloning, characterization and expression analysis of tumor suppressor protein p53 from orange-spotted grouper, Epinephelus coioides in response to temperature stress. Fish Shellfish Immunol. 2013, 35, 1466–1476. [Google Scholar] [CrossRef]
- Cacialli, P.; Gueguen, M.M.; Coumailleau, P.; D’Angelo, L.; Kah, O.; Lucini, C.; Pellegrini, E. BDNF Expression in Larval and Adult Zebrafish Brain: Distribution and Cell Identification. PLoS ONE 2016, 11, e0158057. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Wurzelmann, M.; Romeika, J.; Sun, D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen. Res. 2017, 12, 7–12. [Google Scholar] [PubMed]
- Çomakli, S.; Köktürk, M.; Topal, A.; Özkaraca, M.; Ceyhun, S.B. Immunofluorescence/fluorescence assessment of brain-derived neurotrophic factor, c-Fos activation, and apoptosis in the brain of zebrafish (Danio rerio) larvae exposed to glufosinate. NeuroToxicology 2018, 69, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Effects of Acute Temperature Stress on the Expression of c-Fos and 5-HT1A Receptors in the Brain of Batrachuperus pinchoni. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2008. [Google Scholar]
- Topal, A.; Atamanalp, M.; Oruç, E.; Halıcı, M.B.; Şişecioğlu, M.; Erol, H.S.; Gergit, A.; Yılmaz, B. Neurotoxic effects of nickel chloride in the rainbow trout brain: Assessment of c-Fos activity, antioxidant responses, acetylcholinesterase activity, and histopathological changes. Fish Physiol. Biochem. 2015, 41, 625–634. [Google Scholar] [CrossRef]
- Varani, A.P.; Moutinho Machado, L.; Balerio, G.N. Baclofen prevented the changes in c-Fos and brain-derived neutrophic factor expressions during mecamylamine-precipitated nicotine withdrawal in mice. Synapse 2014, 68, 508–517. [Google Scholar] [CrossRef]
- Huang, L. Transcriptional Expression of Immune-Related Genes and Heat Shock Protein Genes of Japanese Flounder (Paralichthys olivaceus). Ph.D. Thesis, Graduate School of Chinese Academy of Sciences (Institute of Oceanology), Beijing, China, 2015. [Google Scholar]
- Wang, Q.; Liu, Y.; Zheng, Z.; Deng, Y.; Jiao, Y.; Du, X. Adaptive response of pearl oyster Pinctada fucata martensii to low water temperature stress. Fish Shellfish Immunol. 2018, 78, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Kennedy, C.J.; Iwama, G.K. The effects of stress on the association between hsp70 and the glucocorticoid receptor in rainbow trout. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 134, 655–663. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Yang, L.; Yang, E.; Xie, R.; Chen, G.; Huang, J. Transcription level of immune related genes of juvenile cobia (Rachycentron canadum) under hypoxia stress. Haiyang Xuebao 2021, 43, 92–101. [Google Scholar]
- Tian, X.R.; Wu, H.; Li, J. Accumulation effect of fresh heavy metals and antioxidant system response of Cd, Pb composite stress in wetland. J. Agro-Environ. Sci. 2015, 34, 844–851. [Google Scholar]
- Chen, K.; Ma, P.; Huang, J.; Huang, D.; Wang, T.; Huang, X. Effects of hypoxic stress on superoxide dismutase SOD content in juvenile pearl grouper. Jiangxi Aquat. Sci. Technol. 2021, 4, 16–18. [Google Scholar]
- Shi, Z.-H.; Zhang, C.-J.; Peng, S.-M.; Wang, J.-G.; Gao, Q.-X. Effect of salinity on serum osmolality, catalase and gill ion-regulatory enzyme activities in silver pomfret. J. Fish. 2013, 37, 1697–1705. [Google Scholar] [CrossRef]
- Buckley, B.A.; Gracey, A.Y.; Somero, G.N. The cellular response to heat stress in the goby Gillichthys mirabilis: A cDNA microarray and protein-level analysis. J. Exp. Biol. 2006, 209, 2660–2677. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Xu, M.; Wang, T. Endoplasmic reticulum stress response and associated molecular chaperones. Prog. Anat. Sci. 2014, 20, 381–384. [Google Scholar]
- Rebl, A.; Verleih, M.; Nipkow, M.; Altmann, S.; Bochert, R.; Goldammer, T. Gradual and Acute Temperature Rise Induces Crossing Endocrine, Metabolic, and Immunological Pathways in Maraena Whitefish (Coregonus maraena). Front. Genet. 2018, 9, 241. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Dong, M.; Wu, Y.; Fan, Y.; Xu, M.; Zhang, J. c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci. Lett. 2006, 400, 177–180. [Google Scholar] [CrossRef]
- Topal, A.; Özdemir, S.; Arslan, H.; Çomaklı, S. How does elevated water temperature affect fish brain? (A neurophysiological and experimental study: Assessment of brain derived neurotrophic factor, cFOS, apoptotic genes, heat shock genes, ER-stress genes and oxidative stress genes). Fish Shellfish Immunol. 2021, 115, 198–204. [Google Scholar] [CrossRef]
- Kim, B.-S.; Jung, S.J.; Choi, Y.J.; Kim, N.N.; Choi, C.Y.; Kim, J.-W. Effects of different light wavelengths from LEDs on oxidative stress and apoptosis in olive flounder (Paralichthys olivaceus) at high water temperatures. Fish Shellfish Immunol. 2016, 55, 460–468. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Tian, Y.-S.; Ma, W.-H.; Zhai, J.-M. Gene expression changes in response to low temperatures in embryos of the kelp grouper, Epinephelus moara. Cryobiology 2020, 97, 159–167. [Google Scholar] [CrossRef]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Chen, Y. Effects of low-temperature stress on serum biochemical, antioxidant enzymes activity and apoptosis-related gene expression in liver of juvenile Cobia (Rachycentron canadum). J. Guangdong Ocean. Univ. 2022, 42, 18–26. [Google Scholar]


| Aim Gene | Primer (5′–3′) |
|---|---|
| HSP70F | 5′-TGTGAGCGAGCCAAGAGAACCC-3′ |
| HSP70R | 5′-AAGCGAGCTCTGGTGATGGACG-3′ |
| HSP90F | 5′-CAGTACGGATGGTCTGGCAA-3′ |
| HSP90R | 5′-ATACCCTGACCGAAGCGTTG-3′ |
| CATF | 5′-TGAGTCTGGGTCGGCAGATA-3′ |
| CATR | 5′-AGAGTGGATGAAGGACGGGA-3′ |
| SODF | 5′-GTGGACCAACCGACAGTGAA-3′ |
| SODR | 5′-GATGGAGTTTGGCCCTGACA-3′ |
| BDNFF | 5′-AGCTCAGCGTTTGTGACAGT-3′ |
| BDNFR | 5′-TGTCCGGCATTGCGAGTTAT-3′ |
| c-FosF | 5′-TCTGCTCACAAACCCCACTG-3′ |
| c-FosR | 5′-AGTCCCCAAAGATCGCAGTG-3′ |
| caspase3F | 5′-TGTTGTTGGGTGTTTGGGGA-3′ |
| caspase3R | 5′-CAAACAGCCTCCCCAAAAGC-3′ |
| baxF | 5′-CGTAATCATTGAGCAGGGTGC-3′ |
| baxR | 5′-TCCCAGATCCTCATGGCTCA-3′ |
| IRE1F | 5′-CACAAGTGCAAACTACCGAACA-3′ |
| IRE1R | 5′-AGCCATCTTCACTCAAGCCA-3′ |
| GRP78F | 5′-AGACGGGCAAAGATGTCAGG-3′ |
| GRP78R | 5′-TCCAACACTTTCTGGACGGG-3′ |
| actinF | 5′-CCCAAAGCCAACAGAGAGAA-3′ |
| actinR | 5′-ACCAGAAGCGTACAGAGAGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Xiong, L.; Zhu, Y.; Zheng, A.; Zheng, S. Effects of Acute Temperature Stress on the Expression of Related Genes in the Brain of Opsariichthys bidens. Fishes 2024, 9, 248. https://doi.org/10.3390/fishes9070248
Li Q, Xiong L, Zhu Y, Zheng A, Zheng S. Effects of Acute Temperature Stress on the Expression of Related Genes in the Brain of Opsariichthys bidens. Fishes. 2024; 9(7):248. https://doi.org/10.3390/fishes9070248
Chicago/Turabian StyleLi, Qianhui, Luomei Xiong, Yechen Zhu, Anrui Zheng, and Shanjian Zheng. 2024. "Effects of Acute Temperature Stress on the Expression of Related Genes in the Brain of Opsariichthys bidens" Fishes 9, no. 7: 248. https://doi.org/10.3390/fishes9070248
APA StyleLi, Q., Xiong, L., Zhu, Y., Zheng, A., & Zheng, S. (2024). Effects of Acute Temperature Stress on the Expression of Related Genes in the Brain of Opsariichthys bidens. Fishes, 9(7), 248. https://doi.org/10.3390/fishes9070248





