Two Distinct Maternal Lineages of Threespine Stickleback (Gasterosteus aculeatus) in a Small Norwegian Subarctic Lake
Abstract
1. Introduction
2. Materials and Methods
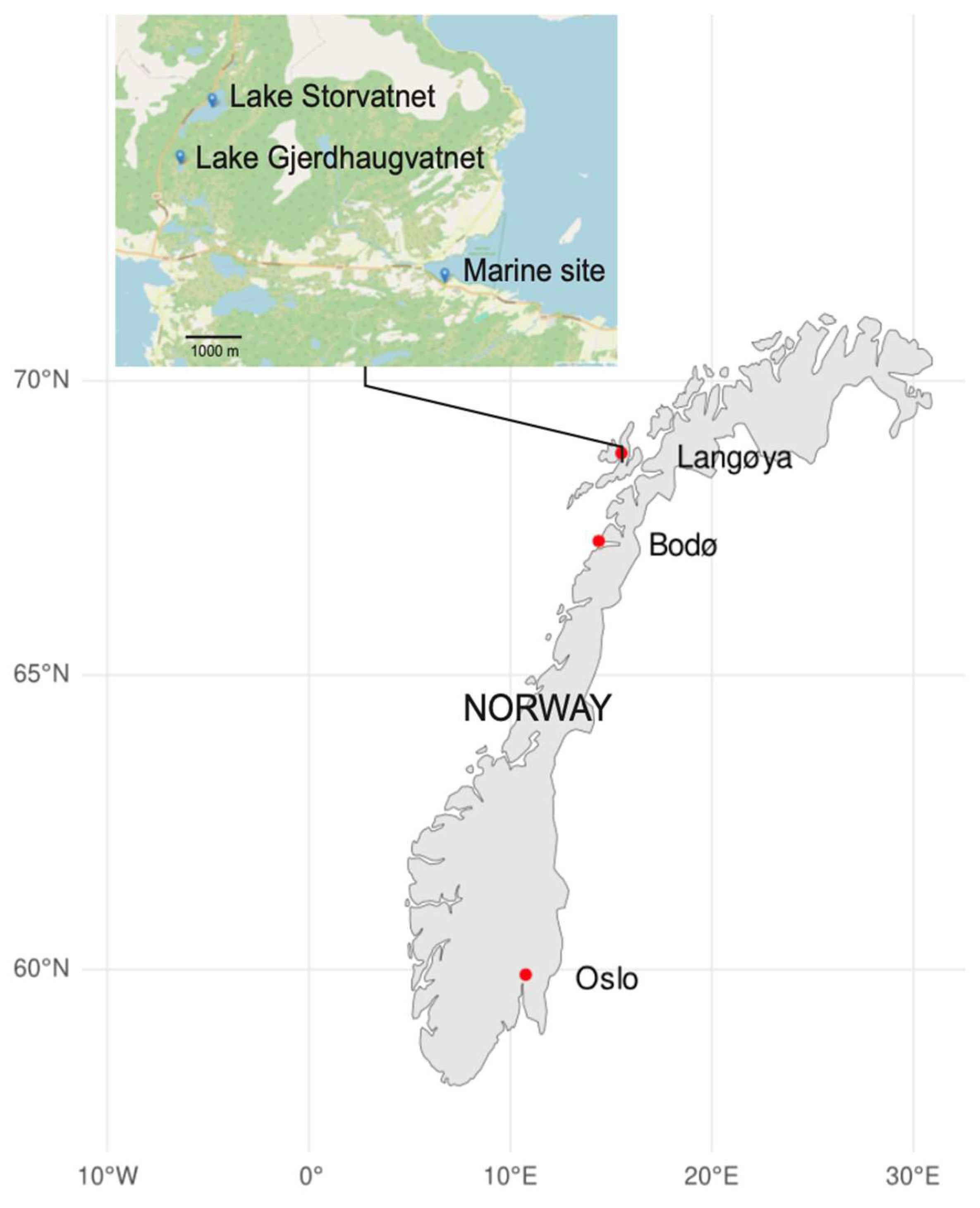
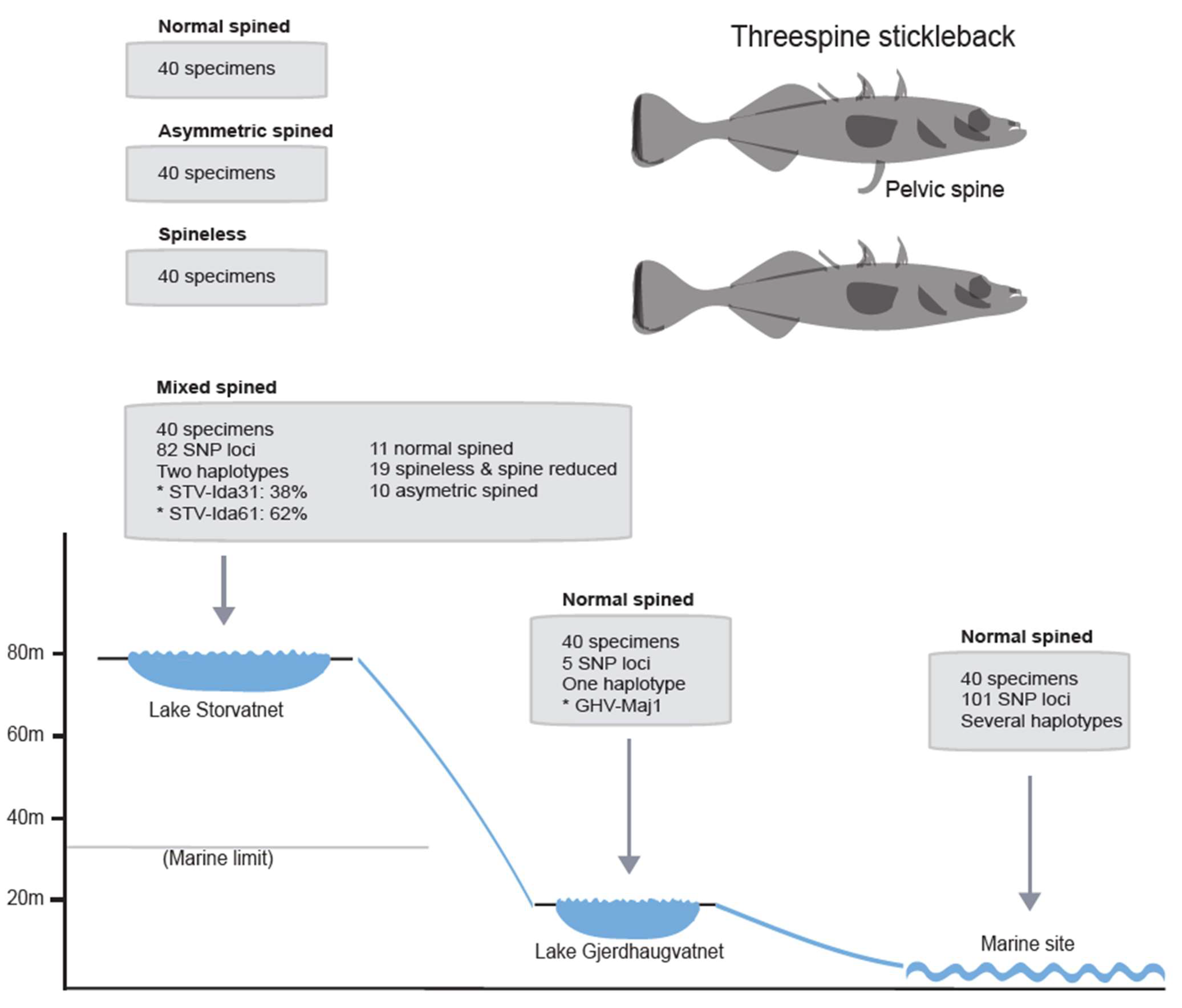
2.1. Fish Sampling and Morphology Scores
2.2. DNA Isolation and DNA Pooling
2.3. DNA Sequencing
2.4. Mitogenome Assembly and Analysis
2.5. Molecular Phylogeny
2.6. Genetic Differentiation Statistics
3. Results
3.1. Mitogenome Sequence of the Reference Specimen STV-Ida31
3.2. Mitogenome Comparison between STV-Ida31 and STV-Ida61
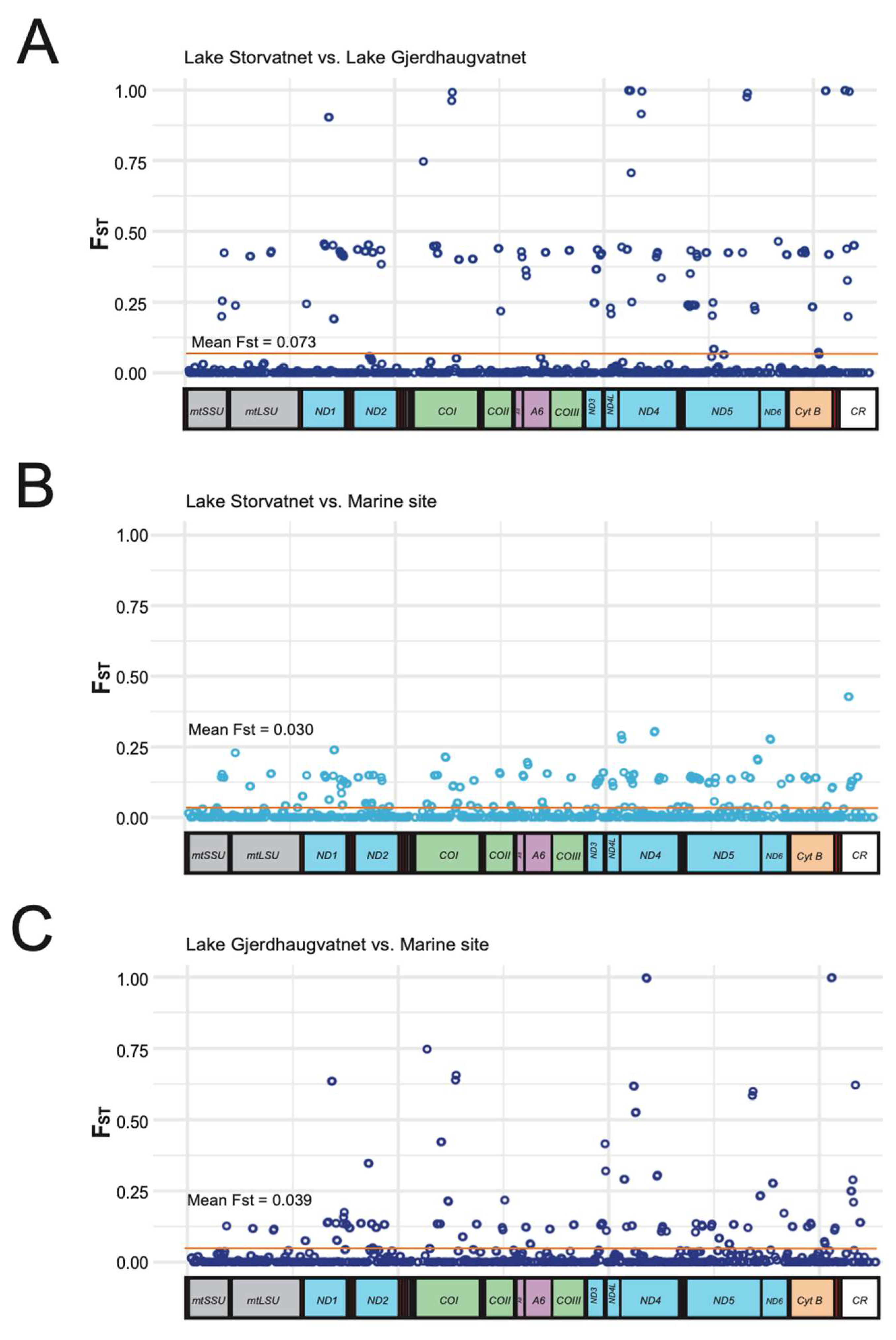
3.3. Pairwise Comparisons of Populations Based on Pooled DNA
3.4. Mitogenome Genetic Differentiation between Sample Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reid, K.; Bell, M.A.; Veeramah, K.R. Treespine stickleback: A model system for evolutionary genomics. Ann. Rev. Genom. 2021, 22, 357–383. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.F.; Marks, M.E.; Jones, F.C.; Villarreal, G., Jr.; Shapiro, M.D.; Brady, S.D.; Southwick, A.M.; Absher, D.M.; Grimwood, J.; Schmutz, J.; et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science 2010, 327, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Capellini, T.D.; Guenther, C.A.; Chan, Y.F.; Infante, C.R.; Menke, D.B.; Kingsley, D.M. A novel enhancer near the Pitx1 gene influences development and evolution of pelvic appendages in vertebrates. eLife 2018, 7, e38555. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.T.; Wang, G.; Thompson, A.C.; Wucherpfennig, J.I.; Reimchen, T.E.; MacColl, A.D.C.; Schluter, D.; Bell, M.A.; Vasquez, K.M.; Kingsley, D.M. DNA fragility in the parallel evolution of pelvic reduction in stickleback fish. Science 2019, 363, 81–84. [Google Scholar] [CrossRef]
- Klepaker, T.O.; Østbye, K. Pelvic anti-predator armour reduction in Norwegian populations of the threespine stickleback: A rare phenomenon with adaptive implications? J. Zool. 2008, 276, 81–88. [Google Scholar] [CrossRef]
- Klepaker, T.; Ostbye, K.; Bernatchez, L.; Vollestad, L.A. Spatio-temporal patterns in pelvic reduction in threespine stickleback (Gasterosteus aculeatus L.) in Lake Storvatnet. Evol. Ecol. Res. 2012, 14, 169–191. [Google Scholar]
- Klepaker, T.; Østbye KBell, M.A. Regressive evolution of the pelvic complex in stickleback fishes: A study of convergent evolution. Evol. Ecol. Res. 2013, 15, 413–435. [Google Scholar]
- Adhikari, D.; Hanssen, I.; Johansen, S.D.; Moum, T.; Nordeide, J.T. Pitx1 enhancer variants in spined and spine-reduced European sticklebacks. Fishes 2023, 8, 164. [Google Scholar] [CrossRef]
- Rackham, O.; Filipovska, A. Organization and expression of the mammalian mitochondrial genome. Nat. Rev. Genet. 2022, 23, 606–623. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Satoh, T.P.; Miya, M.; Mabuchi, K.; Nishida, M. Structure and variation of the mitochondrial genome of fishes. BMC Genom. 2016, 17, 719. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.E.; Johansen, S.D. Expanding the coding potential of vertebrate mitochondrial genomes: Lesson learned from the Atlantic cod. In Mirochondrial DNA–New Insights; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Formenti, G.; Rhie, A.; Balacco, J.; Haase, B.; Mountcasle, J.; Fedrigo, O.; Brown, S.; Capodiferro, M.R.; Al-Ajli, F.O.; Ambrosini, R.; et al. Complete vertebrate mitogenomes reveal widespread repeats and gene duplications. Genome Biol. 2021, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Orti, G.; Bell, M.A.; Reimchen, T.E.; Meyer, A. Global survey of mitochondrial DNA sequence in the threespine stickleback: Evidence for recent migrations. Evolution 1994, 48, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Makinen, H.S.; Merila, J. Mitochondrial DNA phylogeography of the three-spined stickleback (Gasterosteus aculeatus) in Europe–Evidence for multiple glacial refugia. Mol. Phylogenet Evol. 2008, 46, 167–182. [Google Scholar] [CrossRef] [PubMed]
- DeFaveri, J.; Zanella, L.N.; Zanella, D.; Markovcic, M.; Merila, J. Phylogeography of isolated freshwater three-spined stickleback Gasterosteus aculeatus populations in the Adriatic Sea basin. J. Fish. Biol. 2012, 80, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Ravinet, M.; Harrod, C.; Eizaguirre, C.; Prodohl, P.A. Unique mitochondrial DNA lineages in Irish stickleback populations: Cryptic refugium or rapid recolonization? Evol. Evol. 2013, 4, 2488–2504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Wang, Q.X.; Liu, J.W.; Liu, C.Z. Characterization of the complete mitochondrial genome of tree-spined stickleback, Gasterosteus aculeatus. Mitochondrial DNA Part B 2018, 3, 1133–1134. [Google Scholar] [CrossRef] [PubMed]
- Kirch, M.; Romundset, A.; Gilbert, M.T.P.; Jones, F.C.; Foote, A.D. Ancient and modern stickleback genomes reveal the demographic constraints on adaptation. Curr. Biol. 2021, 31, 2027–2036. [Google Scholar] [CrossRef]
- Beck, E.A.; Bassham, S.; Cresko, W.A. Extreme intraspecific divergence in mitochondrial haplotypes makes the threespine stickleback fish an emerging evolutionary mutant model for mito-nuclear interactions. Front. Genet. 2022, 13, 925786. [Google Scholar] [CrossRef]
- Dubin, A.; Jørgensen, T.E.; Jakt, L.M.; Johansen, S.D. The mitochondrial transcriptome of the anglerfish Lophius piscatorius. BMC Res. Notes 2019, 12, 800. [Google Scholar] [CrossRef]
- Gregorius, H.R. The relationship between the concepts of genetic diversity and differentiation. Theor. Appl. Genet. 1987, 74, 397–401. [Google Scholar] [CrossRef]
- Holsinger, K.E.; Weir, B.S. Genetics in geographically structures populations: Defining, estimating and interpreting FST. Nat. Rev. Genet. 2009, 10, 639–650. [Google Scholar] [CrossRef]
- Karlsen, B.O.; Emblem, Å.; Jørgensen, T.E.; Klingan, K.A.; Nordeide, J.T.; Moum, T.; Johansen, S.D. Mitogenome sequencing variation in migratory and stationary ecotypes of North-east Atlantic cod. Mar. Genom. 2014, 15, 103–108. [Google Scholar] [CrossRef]
- Kofler, R.; Orozco-terWengel, P.; De Maio, N.; Pandey, R.V.; Nolte, V.; Futschik, A.; Kosiol, C.; Schlotterer, C. Popoolation: A toolbox for population genetic analysis of next generation sequencing data from pooled individuals. PLoS ONE 2011, 6, e15925. [Google Scholar] [CrossRef]
- Starner, H.; Påhlsson, C.; Linden, M. Tandem repeat polymorphism and heteroplasmy in the mitochondrial DNA control region of threespine stickleback (Gasterosteus aculeatus). Behaviour 2004, 141, 1357–1369. [Google Scholar] [CrossRef]
- Lescak, E.A.; Bassham, S.L.; Catchen, J.; Gelmond, O.; Sherbick, M.L.; von Hippel, F.A.; Cresko, W.A. Evolution of stickleback in 50 years on earthquake-uplifted island. Proc. Natl. Acad. Sci. USA 2015, 112, E7204–E7212. [Google Scholar] [CrossRef]
- Aquirre, W.E.; Reid, K.; Rivera, J.; Heins, D.C.; Veeramah, K.R.; Bell, M.A. Freshwater colonization, adaptation, and genomic divergence in threespine stickleback. Integr. Comp. Biol. 2022, 62, 388–405. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsen, B.O.; Adhikari, D.; Jørgensen, T.E.; Hanssen, I.K.; Moum, T.B.; Nordeide, J.T.; Johansen, S.D. Two Distinct Maternal Lineages of Threespine Stickleback (Gasterosteus aculeatus) in a Small Norwegian Subarctic Lake. Fishes 2024, 9, 285. https://doi.org/10.3390/fishes9070285
Karlsen BO, Adhikari D, Jørgensen TE, Hanssen IK, Moum TB, Nordeide JT, Johansen SD. Two Distinct Maternal Lineages of Threespine Stickleback (Gasterosteus aculeatus) in a Small Norwegian Subarctic Lake. Fishes. 2024; 9(7):285. https://doi.org/10.3390/fishes9070285
Chicago/Turabian StyleKarlsen, Bård Ove, Dhurba Adhikari, Tor Erik Jørgensen, Ida Klykken Hanssen, Truls Borg Moum, Jarle Tryti Nordeide, and Steinar Daae Johansen. 2024. "Two Distinct Maternal Lineages of Threespine Stickleback (Gasterosteus aculeatus) in a Small Norwegian Subarctic Lake" Fishes 9, no. 7: 285. https://doi.org/10.3390/fishes9070285
APA StyleKarlsen, B. O., Adhikari, D., Jørgensen, T. E., Hanssen, I. K., Moum, T. B., Nordeide, J. T., & Johansen, S. D. (2024). Two Distinct Maternal Lineages of Threespine Stickleback (Gasterosteus aculeatus) in a Small Norwegian Subarctic Lake. Fishes, 9(7), 285. https://doi.org/10.3390/fishes9070285






