Nanoparticle-Enhanced Fish Feed: Benefits and Challenges
Abstract
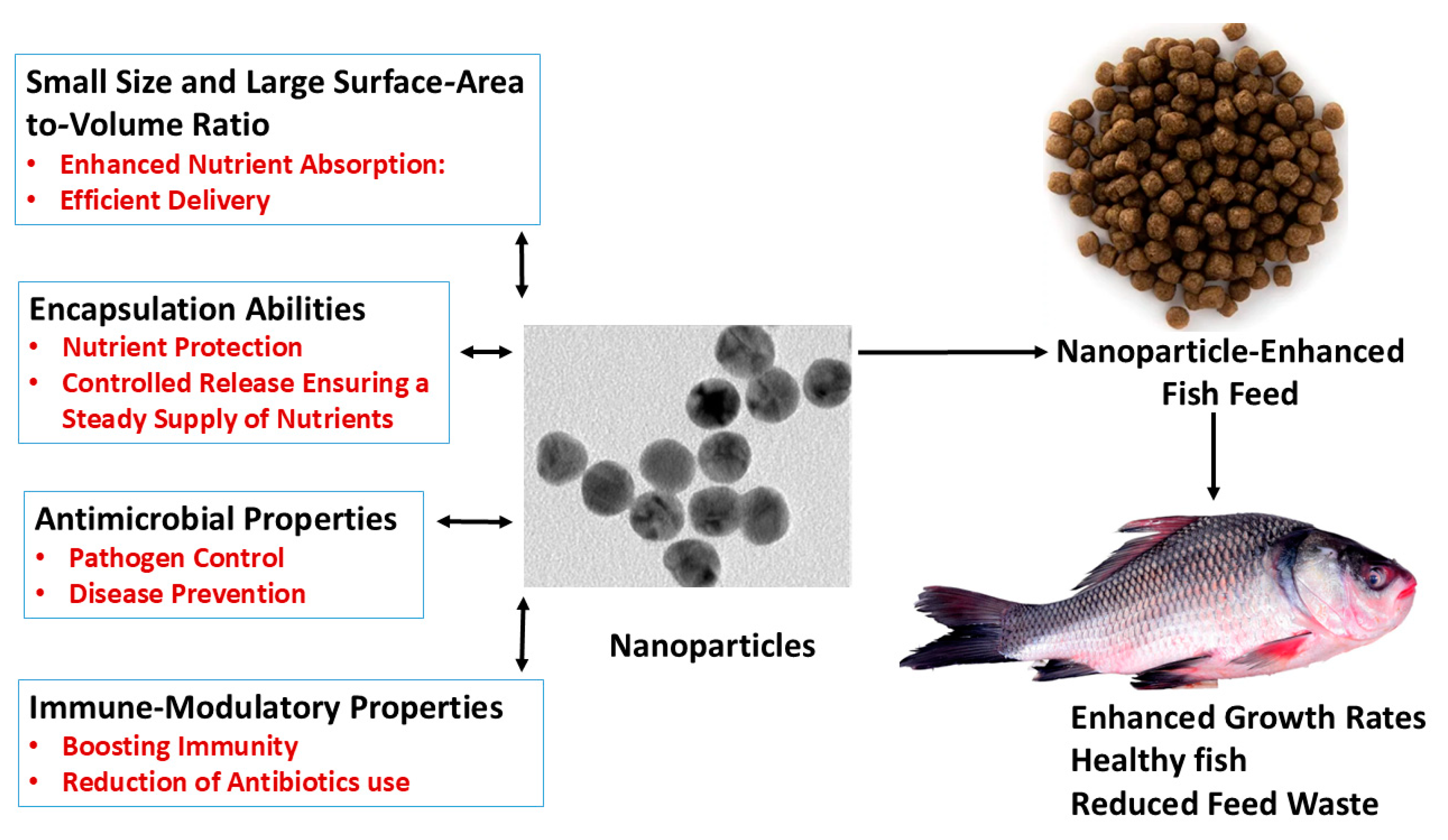
1. Introduction
2. Nanoparticles Used in Fish Feed
2.1. Metal-Based Nanoparticles
2.1.1. Zinc-Based Nanoparticles
2.1.2. Iron Nanoparticles
2.1.3. Magnesium Nanoparticles
2.1.4. Manganese Nanoparticles
2.1.5. Copper Nanoparticles
2.2. Non-Metal-Based Nanoparticles
2.2.1. Selenium Nanoparticles
2.2.2. Protein-Based Nanoparticles
2.2.3. Lipid-Based Nanoparticles
2.2.4. Chitosan-Based Nanoparticles
2.3. Composite and Hybrid Nanoparticles
2.4. Toxicity, Safety, and Environmental Impact of Nanoparticle-Enhanced Fish Feed
2.4.1. Toxicity and Safety Issues
2.4.2. Bioaccumulation and Environmental Impact
3. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Hossain, M.S.; Small, B.C.; Kumar, V.; Hardy, R. Utilization of functional feed additives to produce cost-effective, ecofriendly aquafeeds high in plant-based ingredients. Rev. Aquac. 2024, 16, 121–153. [Google Scholar] [CrossRef]
- Zlaugotne, B.; Pubule, J.; Blumberga, D. Advantages and disadvantages of using more sustainable ingredients in fish feed. Heliyon 2022, 8, e10527. [Google Scholar] [CrossRef]
- Molina-Poveda, C. Nutrient requirements. In Aquafeed Formulation; Nates, S.F., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 75–216. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Pulido Rodriguez, L.F.; Randazzo, B.; Cardinaletti, G.; Giorgini, E.; Belloni, A.; Secci, G.; Faccenda, F.; Pulcini, D.; Parisi, G.; et al. Conventional feed additives or red claw crayfish meal and dried microbial biomass as feed supplement in fish meal-free diets for rainbow trout (Oncorhynchus mykiss): Possible ameliorative effects on growth and gut health status. Aquaculture 2022, 554, 738137. [Google Scholar] [CrossRef]
- Hardy, R.W.; Kaushik, S.J.; Mai, K.; Bai, S.C. Fish nutrition—History and perspectives. In Fish Nutrition; Hardy, R.W., Kaushik, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- FAO. Fish as Feed Inputs for Aquaculture: Practices, Sustainability and Implications; FAO: Rome, Italy, 2009. [Google Scholar]
- Bogmans, C.W.J.; van Soest, D. Can global aquaculture growth help to conserve wild fish stocks? Theory and empirical analysis. Nat. Resour. Model. 2022, 35, e12323. [Google Scholar] [CrossRef]
- Filipe, D.; Gonçalves, M.; Fernandes, H.; Oliva-Teles, A.; Peres, H.; Belo, I.; Salgado, J.M. Shelf-Life Performance of Fish Feed Supplemented with Bioactive Extracts from Fermented Olive Mill and Winery By-Products. Foods 2023, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Nunes AJ, P.; Dalen, L.L.; Leonardi, G.; Burri, L. Developing sustainable, cost-effective and high-performance shrimp feed formulations containing low fish meal levels. Aquac. Rep. 2022, 27, 101422. [Google Scholar] [CrossRef]
- Uzochukwu, I.E.; Aba, P.E.; Ossai, N.I.; Ugwuoke, H.C.; Nyeste, K.; Machebe, N.S. Optimizing feed utilization and reducing deterioration of African catfish feed with sodium propionate supplementation. Aquac. Rep. 2023, 33, 101820. [Google Scholar] [CrossRef]
- Thangapandiyan, S.; Monika, S. Green Synthesized Zinc Oxide Nanoparticles as Feed Additives to Improve Growth, Biochemical, and Hematological Parameters in Freshwater Fish Labeo rohita. Biol. Trace Elem. Res. 2020, 195, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Rahman, M.M.I.A.; El-ghany, A.M.A.; Hassanen, E.A.A.; Al-jabr, O.A.; El-Wahab, R.A.A.; Zayed, S.; Salem, M.A.E.K.; El_Tahawy, S.N.; Youssef, W.; et al. Chlorella vulgaris extract conjugated magnetic iron nanoparticles in nile tilapia (Oreochromis niloticus): Growth promoting, immunostimulant and antioxidant role and combating against the synergistic infection with Ichthyophthirius multifiliis and Aeromonas hydrophila. Fish Shellfish Immunol. 2024, 145, 109352. [Google Scholar] [CrossRef]
- Adel, M.; Sakhaie, F.; Shekarabi, S.P.H.; Gholamhosseini, A.; Impellitteri, F.; Faggio, C. Dietary Mentha piperita essential oil loaded in chitosan nanoparticles mediated the growth performance and humoral immune responses in Siberian sturgeon (Acipenser baerii). Fish Shellfish Immunol. 2024, 145, 109321. [Google Scholar] [CrossRef]
- Asad, F.; Batool, N.; Nadeem, A.; Bano, S.; Anwar, N.; Jamal, R.; Ali, S. Fe-NPs and Zn-NPs: Advancing Aquaculture Performance Through Nanotechnology. Biol. Trace Elem. Res. 2023, 202, 2828–2842. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.T.; Ali, M.S.; Ahamed, T.; Suraiya, S.; Haq, M. Exploring the aspects of the application of nanotechnology system in aquaculture: A systematic review. Aquac. Int. 2024, 32, 4177–4206. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Vreeland, E.C.; Watt, J.; Schober, G.B.; Hance, B.G.; Austin, M.J.; Price, A.D.; Fellows, B.D.; Monson, T.C.; Hudak, N.S.; Maldonado-Camargo, L.; et al. Enhanced Nanoparticle Size Control by Extending LaMer’s Mechanism. Chem. Mater. 2015, 27, 6059–6066. [Google Scholar] [CrossRef]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A. Influence of Processing Parameters to Control Morphology and Optical Properties of Sol-Gel Synthesized ZnO Nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Fajardo, C.; Martinez-rodriguez, G.; Blasco, J.; Miguel, J.; Thomas, B.; De Donato, M. Nanotechnology in aquaculture: Applications, perspectives and regulatory challenges. Aquac. Fish. 2022, 7, 185–200. [Google Scholar] [CrossRef]
- Dumlu, B. Importance of Nano-Sized Feed Additives in Animal Nutrition. J. Agric. Prod. 2024, 5, 55–72. [Google Scholar] [CrossRef]
- Hussain, S.M.; Naeem, E.; Ali, S.; Paray, B.A.; Adrees, M.; Naeem, A. Evaluation of growth, nutrient absorption, body composition and blood indices under dietary exposure of iron oxide nanoparticles in Common carp (Cyprinus carpio). J. Anim. Physiol. Anim. Nutr. 2024, 108, 366–373. [Google Scholar] [CrossRef]
- Lawson, Z.R.; Xu, K.; Boukouvala, C.; Hughes, R.A.; Rosenberger, M.R.; Neretina, S. Large-area arrays of epitaxially aligned silver nanotriangles seeded by gold nanostructures. Mater. Chem. Front. 2024, 8, 2149–2160. [Google Scholar] [CrossRef]
- Dubey, P.; Pathan, S.; Pusplata, P.; Tadge, P.; Ray, S. Recent Advances In Template Assisted Growth Engineering of Inorganic Nanocrystals. ECS Trans. 2022, 107, 20085. [Google Scholar] [CrossRef]
- Ding, J.; Chen, J.; Gao, L.; Jiang, Z.; Zhang, Y.; Li, M.; Xiao, Q.; Lee, S.S.; Chen, X. Engineered nanomedicines with enhanced tumor penetration. Nano Today 2019, 29, 100800. [Google Scholar] [CrossRef]
- Hadji, H.; Bouchemal, K. Effect of micro- and nanoparticle shape on biological processes. J. Control. Release 2022, 342, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Taheri-ledari, R.; Ganjali, F.; Mirmohammadi, S.S.; Qazi, F.S.; Saeidirad, M.; KashtiAray, A.; Zarei-Shokat, S.; Tian, Y.; Maleki, A. Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: A review. R. Soc. Chem. Adv. 2023, 13, 80–114. [Google Scholar] [CrossRef] [PubMed]
- El-shafai, N.M.; El-mehasseb, I.M.; Shukry, M.; Farrag, F.; Ibrahim, M.; Ramadan, M.S.; Dawood , M.A.O.; Moustafa, E.M.; El-Kemary, M.A. Influence nanohybrid of (GO@Se.ZnO) for enhancing the fish production wealth and economical return via the improvement dietary, immunity, physiological and antioxidant activity on Nile Tilapia. Mater. Chem. Phys. 2021, 266, 124536. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Z.; Ma, Y. Preparation and growth-promoting effect of selenium nanoparticles capped by polysaccharide-protein complexes on tilapia. J. Sci. Food. Agric. 2021, 101, 476–485. [Google Scholar] [CrossRef]
- Ni, J.; Ren, L.; Ma, Y.; Xiong, H.; Jian, W. Selenium nanoparticles coated with polysaccharide-protein complexes from abalone viscera improve growth and enhance resistance to diseases and hypoxic stress in juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 134, 108624. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liang, Y.; Liu, L.; Yin, M.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Research on the fate of polymeric nanoparticles in the process of the intestinal absorption based on model nanoparticles with various characteristics: Size, surface charge and pro-hydrophobics. J. Nanobiotechnol. 2021, 19, 32. [Google Scholar] [CrossRef]
- Vibhute, P.; Jaabir, M.; Sivakamavalli, J. Applications of nanoparticles in aquaculture. In Nanotechnological Approaches to the Advancement of Innovations in Aquaculture; Kirthi, A., Loganathan, K., Karunasagar, I., Eds.; Springer: Cham, Switzerland, 2023; pp. 127–155. [Google Scholar] [CrossRef]
- Dasari, R.; Prasanna, A.; Khateef, R. Role of nanoparticles in fish disease management: A review. Biocatal. Agric. Biotechnol. 2024, 58, 103218. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ghafarifarsani, H.; Vuppala, S.; Nedaei, S.; Mahendran, K. Selenium Nanoparticles: Revolutionizing Nutrient Enhancement in Aquaculture—A Review. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Ahmed, J.; Kumaraguru, V.K.P.; Ramalingam, K. Nanoencapsulated Aquafeeds and Current Uses in Fisheries/Shrimps: A Review. Appl. Biochem. Biotechnol. 2023, 195, 7110–7131. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.F.; Faria, M.; Carvalho, J.; Pinho, E. Contribution of nanotechnology to greater efficiency in animal nutrition and production. J. Anim. Physiol. Anim. Nutr. 2024, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Singh, A.; Eren, A.; Sultan, H.; Sharma, M.; Djalovic, I.; Trivan, G. Small molecule, big impacts: Nano-nutrients for sustainable agriculture and food security. J. Plant Physiol. 2024, 301, 154305. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Tsigkou, K.; Zuorro, A.; Sun, Y.; Rabetafika, H.; Razafindralambo, H. Inorganic nanoparticles for use in aquaculture. Rev. Aquac. 2023, 15, 1600–1617. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; Fouda, M.M.S.; Younis, E.M.; Abdelwarith, A.A.; Salem, G.A.; Elkady, A.A.; Ismail, S.H.; Davies, S.J.; Rahman, A.N.A. The anti-bacterial efficacy of zinc oxide nanoparticles synthesized by Nelumbo nucifera leaves against Clostridium perfringes challenge in Oreochromis niloticus. Aquaculture 2024, 578, 740030. [Google Scholar] [CrossRef]
- Sherif, A.H.; Abdelsalam, M.; Ali, N.G.; Mahrous, K.F. Zinc Oxide Nanoparticles Boost the Immune Responses in Oreochromis niloticus and Improve Disease Resistance to Aeromonas hydrophila Infection. Biol. Trace Elem. Res. 2023, 201, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S.; Diab, A.M.; Khalafalla, M.M.; Mohamed, R.A. Synergistic Effects of Selenium and Zinc Oxide Nanoparticles on Growth Performance, Hemato-biochemical Profile, Immune and Oxidative Stress Responses, and Intestinal Morphometry of Nile Tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2022, 200, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; El-gendi, G.M.I.; Ahmed, A.I.; El-haroun, E.R.; Hassaan, M.S. Nano Zinc versus Bulk Zinc Form as Dietary Supplied: Effects on Growth, Intestinal Enzymes and Topography, and Hemato-biochemical and Oxidative Stress Biomarker in Nile Tilapia (Oreochromis niloticus Linnaeus, 1758). Biol. Trace Elem. Res. 2022, 200, 1347–1360. [Google Scholar] [CrossRef]
- Silva, V.F.; Tedesco, M.; Fontes, S.T.; Owatari, M.S.; Gatto, Y.M.G.; Ferreira, M.B.; dos Santos, P.C.; Costa, G.A.C.; Palmieri, A.F.; dos Santos, G.G.; et al. Effects of supplementation with different zinc-based products on the growth and health of Nile tilapia. Fish Shellfish Immunol. 2024, 149, 109534. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, A.; Nasir, M.; Kamran, M.; Majeed, I.; Arif, A. Immunomodulation, Fish Health and Resistance to Staphylococcus aureus of Nile Tilapia (Oreochromis niloticus) Fed Diet Supplemented with Zinc Oxide Nanoparticles and Zinc Acetate. Biol. Trace Elem. Res. 2023, 201, 4912–4925. [Google Scholar] [CrossRef]
- Diab, A.M.; Shokr, B.T.; Shukry, M.; Farrag, F.A.; Mohamed, R.A. Effects of Dietary Supplementation with Green-Synthesized Zinc Oxide Nanoparticles for Candidiasis Control in Oreochromis niloticus. Biol. Trace Elem. Res. 2022, 200, 4126–4141. [Google Scholar] [CrossRef]
- Ahmed, S.A.A.; Ibrahim, R.E.; Younis, E.M.; Abdelwarith, A.A.; Faroh, K.Y.; El Gamal, S.A.; Badr, S.; Khamis, T.; Mansour, A.T.; Davies, S.J.; et al. Antagonistic Effect of Zinc Oxide Nanoparticles Dietary Supplementation Against Chronic Copper Waterborne Exposure on Growth, Behavioral, Biochemical, and Gene Expression Alterations of African Catfish, Clarias gariepinus (Burchell, 1822). Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Changizi, R.; Ghobadi, S.; Shohreh, P.; Vatandoust, S. Effect of Nano-Fe as Feed Supplement on Growth Performance, Survival Rate, Blood Parameters and Immune Functions of the Stellate Sturgeon (Acipenser stellatus). Russ. J. Mar. Biol. 2020, 46, 493–500. [Google Scholar] [CrossRef]
- Mohammady, E.Y.; Elashry, M.A.; Ibrahim, M.S.; Elarian, M.; Salem, S.M.R.; El-Haroun, E.R.; Hassaan, M.S. Nano Iron versus Bulk Iron Forms as Functional Feed Additives: Growth, Body Indices, Hematological Assay, Plasma Metabolites, Immune, Anti-oxidative Ability, and Intestinal Morphometric Measurements of Nile tilapia, Oreochromis niloticus. Biol. Trace Elem. Res. 2024, 202, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Elabd, H.; Youssuf, H.; Mahboub, H.H.; Salem, S.M.R.; Husseiny, A.; Khalid, A.; El-Desouky, H.S.; Faggio, C. Growth, hemato-biochemical, immune-antioxidant response, and gene expression in Nile tilapia (Oreochromis niloticus) received nano iron oxide-incorporated diets. Fish Shellfish Immunol. 2022, 128, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Paulpandian, P.; Sulaikal, I.; Beena, B.; Ramesh, S.; Kamatchi, K.; Paulraj, B. Impact of Camellia sinensis Iron Oxide Nanoparticle on Growth, Hemato-biochemical and Antioxidant Capacity of Blue Gourami (Trichogaster trichopterus) Fingerlings. Biol. Trace Elem. Res. 2023, 201, 412–424. [Google Scholar] [CrossRef]
- Nirmalkar, R.; Suresh, E.; Felix, N.; Kathirvelpandian, A.; Nazir, M.I.; Ranjan, A. Synthesis of Iron Nanoparticles Using Sargassum wightii Extract and Its Impact on Serum Biochemical Profile and Growth Response of Etroplus suratensis Juveniles. Biol. Trace Elem. Res. 2023, 201, 1451–1458. [Google Scholar] [CrossRef]
- Thangapandiyan, S.; Alisha, A.S.A.; Anidha, K. Growth performance, hematological and biochemical effects of iron oxide nanoparticles in Labeo rohita. Biocatal. Agric. Biotechnol. 2020, 25, 101582. [Google Scholar] [CrossRef]
- Radwan, M.; Moussa, M.A.; Manaa, E.A.; El-sharkawy, M.A.; Darweesh, K.F.; Elraey, S.M.A.; Saleh, N.A.; Mohammadein, A.; Al-Otaibi, W.M.; Albadrani, G.M.; et al. Synergistic effect of green synthesis magnesium oxide nanoparticles and seaweed extract on improving water quality, health benefits, and disease resistance in Nile tilapia. Ecotoxicol. Environ. Saf. 2024, 280, 116522. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Deng, Y.; He, C.; Liu, W.; Li, X. The Benefits of Nanosized Magnesium Oxide in Fish Megalobrama amblycephala: Evidence in Growth Performance, Redox Defense, Glucose Metabolism, and Magnesium Homeostasis. Antioxidants 2023, 12, 1350. [Google Scholar] [CrossRef]
- Thabet, S.R.; Abdel-Galil, M.A.; Osman, A.S.; Ahmed, N.S.; Mousa, M.A. Biochemical effects of magnesium oxide nanoparticles in clarias gariepinus. Sohag J. Jr. Sci. Res. 2021, 1, 55–64. [Google Scholar]
- Xu, J.; Zhao, T.; Luo, Z.; Zhong, C.; Zheng, H.; Tan, X. Effects of dietary supplementation with manganese dioxide nanoparticles on growth, Mn metabolism and kidney health of yellow catfish Pelteobagrus fulvidraco. Aquac. Rep. 2023, 33, 101815. [Google Scholar] [CrossRef]
- Soundhariya, N. Different quantities of manganese oxide nanoparticles incorporated feed on the growth and haematological traits of common carp Cyprinus carpio var. communis. J. Appl. Nat. Sci. 2023, 9411, 1587–1594. [Google Scholar] [CrossRef]
- Ramdas, R.; Tukaram, S.; Chandramore, K.; Sammi, K.; Kumar, N. Dietary manganese nano-particles improves gene regulation and biochemical attributes for mitigation of lead and ammonia toxicity in fish. Comp. Biochem. Physiol. Part C 2024, 276, 109818. [Google Scholar] [CrossRef]
- Rajan, M.R.; Sudhabose, S.; Barween, S.R. Multifarious Magnitude of Magnesium Oxide Nanoparticles Integrated Feed on Growth and Hematological Characteristics of Mrigal Cirrhinus mrigala. J. Toxicol. 2023, 13, 1–9. [Google Scholar]
- Kumar, N.; Thorat, S.T.; Singh, A.K. Manganese nanoparticles control the gene regulations against multiple stresses in Pangasianodon hypophthalmus. Sci. Rep. 2023, 13, 15900. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; El-Sharawy, M.E.; Soliman, A.A.; Amer, A.A.; Atia, M.H. Copper Nanoparticles Mitigate the Growth, Immunity, and Oxidation Resistance in Common Carp (Cyprinus carpio). Biol. Trace Elem. Res. 2020, 198, 283–292. [Google Scholar] [CrossRef]
- Delavari, N.M.; Gharaei, A.; Mirdar, H.J.; Davari, A.; Rastiannasab, A. Modulatory effect of dietary copper nanoparticles and vitamin C supplementations on growth performance, hematological and immune parameters, oxidative status, histology, and disease resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2022, 48, 33–51. [Google Scholar] [CrossRef]
- Kumar, N.; Thorat, S.T.; Gite, A.; Patole, P.B. Nano-copper Enhances Gene Regulation of Non-specific Immunity and Antioxidative Status of Fish Reared Under Multiple Stresses. Biol. Trace Elem. Res. 2023, 201, 4926–4950. [Google Scholar] [CrossRef]
- Kaleeswaran, C.; Senthamarai, M.D.; Rajan, M.R. Evaluation of disparate multiplicities of copper oxide nanoparticles integrated feed on the growth and hematology of koi carp. J. Toxicol. Stud. 2024, 2, 497. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Alagawany, M.; Sewilam, H. The Role of Zinc Microelement in Aquaculture: A Review. Biol. Trace Elem. Res. 2022, 200, 3841–3853. [Google Scholar] [CrossRef] [PubMed]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Thwaite, R.; Ji, J.; Torrealba, D.; Coll, J.; Sabés, M.; Villaverde, A.; Roher, N. Protein Nanoparticles Made of Recombinant Viral Antigens: A Promising Biomaterial for Oral Delivery of Fish Prophylactics. Front. Immunol. 2018, 9, 1652. [Google Scholar] [CrossRef]
- Kumaran, S.; Anahas, A.M.P.; Prasannabalaji, N.; Karthiga, M.; Bharathi, S.; Rajasekar, T.; Joseph, J.; Prasad, S.G.; Pandian, S.K.; Pugazhvendan, S.R.; et al. Chitin derivatives of NAG and chitosan nanoparticles from marine disposal yards and their use for economically feasible fish feed development. Chemosphere 2021, 281, 130746. [Google Scholar] [CrossRef] [PubMed]
- Summer, M.; Ali, S.; Muhammad, H.; Rimsha, T.; Umaima, A. Mode of Action of Biogenic Silver, Zinc, Copper, Titanium and Cobalt Nanoparticles against Antibiotics Resistant Pathogens; Springer: Berlin/Heidelberg, Germany, 2024; Volume 34. [Google Scholar] [CrossRef]
- Metryka, O.; Wasilkowski, D.; Mrozik, A. Undesirable consequences of the metallic nanoparticles action on the properties and functioning of Escherichia coli, Bacillus cereus and Staphylococcus epidermidis membranes. J. Hazard. Mater. 2023, 446, 130728. [Google Scholar] [CrossRef] [PubMed]
- Al-Wrafy, F.A.; Al-Gheethi, A.A.; Ponnusamy, S.K.; Noman, E.A.; Fattah, S.A. Nanoparticles approach to eradicate bacterial biofilm-related infections: A critical review. Chemosphere 2022, 288, 132603. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024. [Google Scholar] [CrossRef]
- Mokhtari, H.; Abdulrazaq, T.; Somayeh, A.; Tooba, A.; Salehzadeh, A. Synergic Antibiofilm Effect of Thymol and Zinc Oxide Nanoparticles Conjugated with Thiosemicarbazone on Pathogenic Pseudomonas aeruginosa Strains. Arab. J. Sci. Eng. 2024, 49, 9089–9097. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Hossain, M.M.M.; Khan, M.; Ali, M.S.; Aktar, S.; Moniruzzaman, M. Influence of nanoparticle-based nano-nutrients on the growth performance and physiological parameters in tilapia (Oreochromis niloticus). RSC Adv. 2020, 10, 29918–29922. [Google Scholar] [CrossRef]
- Mukherjee, K.; Saha, A.; Bhattacharjee, S. Nanoscale iron for sustainable aquaculture and beyond. Biocatal. Agric. Biotechnol. 2022, 44, 102440. [Google Scholar] [CrossRef]
- Chandrapalan, T.; Kwong, R.W.M. Functional significance and physiological regulation of essential trace metals in fish. J. Exp. Biol. 2021, 224, jeb238790. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, A. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: A review. J. Food Sci. Technol. 2022, 59, 3319–3335. [Google Scholar] [CrossRef] [PubMed]
- Abdel, A.N.; Masoud, S.R.; Fouda, M.M.S.; Abdelwarith, A.A.; Younis, E.M.; Khalil, S.S.; Zaki, H.T.; Mohammed, E.; Davies, S.J.; Ibrahim, R.E. Antimicrobial efficacy of magnetite nanoparticles against Aeromonas sobria challenge in African catfish: Biochemical, protein profile, and immuno-antioxidant indices. Aquac. Rep. 2023, 32, 101692. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. BioMetals 2021, 34, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, L.; Yu, H.; Yuan, Z.; Ma, C.; Wu, X.; Kong, W. Effects of dietary manganese supplementation on the growth performance, tissue manganese content and antioxidant capacity of coho salmon (Oncorhynchus kisutch) post-smolts. Aquac. Rep. 2024, 34, 101924. [Google Scholar] [CrossRef]
- Gatou, M.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium Oxide (MgO) Nanoparticles: Synthetic Strategies and Biomedical Applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Sudhabose, S.; Sooryakanth, B.; Rajan, M.R. Impact of acute and sub-acute exposure of magnesium oxide nanoparticles on mrigal Cirrhinus mrigala. Heliyon 2023, 9, e15605. [Google Scholar] [CrossRef]
- Liu, D.; Li, L.; Zhang, Q.; Yu, H. Effect of Dietary Manganese on the Growth Performance, Lipid Metabolism, and Antioxidant Capacity in the Post-Larval Coho Salmon (Oncorhynchus kisutch). Animals 2023, 13, 1310. [Google Scholar] [CrossRef]
- EL-Erian, M.A.; Ibrahim, M.S.; Salem, S.M.R.; Mohammady, E.Y.; El-Haroun, E.R.; Hassaan, M.S. Evaluation of Different Copper Sources in Nile Tilapia Diets: Growth, Body Indices, Hematological Assay, Plasma Metabolites, Immune, Anti-Oxidative Ability, and Intestinal Morphometric Measurements. Biol. Trace Elem. Res. 2023, 201, 4900–4911. [Google Scholar] [CrossRef]
- Zhong, C.C.; Ke, J.; Song, C.C.; Tan, X.Y.; Xu, Y.C.; Lv, W.H.; Song, Y.-F.; Luo, Z. Effects of dietary copper (Cu) on growth performance, body composition, mineral content, hepatic histology and Cu transport of the GIFT strain of Nile tilapia (Oreochromis niloticus). Aquaculture 2023, 574, 739638. [Google Scholar] [CrossRef]
- Kumar, N.; Thorat, S.T.; Chavhan, S.R. Multifunctional role of dietary copper to regulate stress-responsive gene for mitigation of multiple stresses in Pangasianodon hypophthalmus. Sci. Rep. 2024, 14, 2252. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Gao, C.; Wang, C.; Yan, Y.; Zhou, F. Dietary copper for fish: Homeostasis, nutritional functions, toxicity, and affecting factors. Aquaculture 2024, 587, 740875. [Google Scholar] [CrossRef]
- Scott, A.; Vadalasetty, K.P.; Chwalibog, A.; Sawosz, E. Copper nanoparticles as an alternative feed additive in poultry diet: A review. Nanotechnol. Rev. 2018, 7, 69–93. [Google Scholar] [CrossRef]
- Afshari, A.; Sourinejad, I.; Gharaei, A.; Johari, S.A.; Ghasemi, Z. The effects of diet supplementation with inorganic and nanoparticulate iron and copper on growth performance, blood biochemical parameters, antioxidant response and immune function of snow trout Schizothorax zarudnyi (Nikolskii, 1897). Aquaculture 2021, 539, 736638. [Google Scholar] [CrossRef]
- Eissa, E.H.; Bazina, W.K.; El-Aziz, Y.M.A.; Elghany, N.A.A.; Tawfik, W.A.; Mossa, M.I.; El Megeed, O.H.A.; El-Hamed, N.N.B.A.; El-Saeed, A.F.; El-Haroun, E.; et al. Nano-selenium impacts on growth performance, digestive enzymes, antioxidant, immune resistance and histopathological scores of Nile tilapia, Oreochromis niloticus against Aspergillus flavus infection. Aquac. Int. 2024, 32, 1587–1611. [Google Scholar] [CrossRef]
- Sheikh, S.; Ghojoghi, F.; Ghelichi, A.; Jorjani, S. Dietary Effects of Selenium Nanoparticles on Growth Performance, Survival Rate, Chemical Composition, and Muscle Bioaccumulation of Nile Tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2024, 202, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.H.; Zommara, M.A. Selenium Nanoparticles Ameliorate Adverse Impacts of Aflatoxin in Nile Tilapia with Special Reference to Streptococcus agalactiae Infection. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, Z.; Liu, Q.; Wang, X.; Zheng, L.; He, S.; Yang, F.; Ji, H.; Dong, W. Selenium nanoparticles in aquaculture: Unique advantages in the production of Se-enriched grass carp (Ctenopharyngodon idella). Anim. Nutr. 2024, 16, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Mohtashemipour, H.; Mohammadian, T.; Mozanzadeh, M.T.; Mesbah, M.; Nejad, A.J. Dietary Selenium Nanoparticles Improved Growth and Health Indices in Asian Seabass (Lates calcarifer) Juveniles Reared in High Saline Water. Aquac. Nutr. 2024, 2024, 7480824. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi-mousavi, S.E.; Keyvanshokooh, S.; Mozanzadeh, M.T. Exploring the effects and possible mechanisms of nutritional selenium nanoparticles on production performance and stress recovery in the Arabian yellowfin seabream (Acanthopagrus arabicus) model. Aquac. Rep. 2024, 36, 102051. [Google Scholar] [CrossRef]
- Abdollahi-mousavi, S.E.; Keyvanshokooh, S.; Mozanzadeh, M.T. Efficacy of nutritional selenium nanoparticles on growth performance, immune response, antioxidant capacity, expression of growth and immune-related genes, and post-stress recovery in juvenile Sobaity seabream (Sparidentex hasta). Fish Shellfish Immunol. 2024, 147, 109452. [Google Scholar] [CrossRef]
- Santillan, E.; Yasumaru, F.; Vethathirri, R.S.; Thi, S.S.; Hoon, H.Y.; Chan, D.; Sian, D.C.P.; Wuertz, S. Microbial community-based protein from soybean-processing wastewater as a sustainable alternative fish feed ingredient. Sci. Rep. 2024, 14, 2620. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhou, F.; Shen, P.; Zhang, Y.; Lin, L. Self-assembled soy protein nanoparticles by partial enzymatic hydrolysis for pH-Driven Encapsulation and Delivery of Hydrophobic Cargo Curcumin. Food Hydrocoll. 2021, 120, 106759. [Google Scholar] [CrossRef]
- Yostawonkul, J.; Kitiyodom, S.; Supchukun, K.; Thumrongsiri, N.; Saengkrit, N.; Pinpimai, K.; Hajitou, A.; Thompson, K.D.; Rattanapinyopituk, K.; Maita, M.; et al. Masculinization of Red Tilapia (Oreochromis spp.) Using 17 α-Methyltestosterone-Loaded Alkyl Polyglucosides Integrated into Nanostructured Lipid Carriers. Animals 2023, 13, 1364. [Google Scholar] [CrossRef]
- Trapani, A.; Esteban, M.Á.; Curci, F.; Manno, D.E.; Serra, A.; Fracchiolla, G.; Espinosa-Ruiz, C.; Castellani, S.; Conese, M. Solid Lipid Nanoparticles Administering Antioxidant Grape Seed-Derived Polyphenol Compounds: A Potential Application in Aquaculture. Molecules 2022, 27, 344. [Google Scholar] [CrossRef] [PubMed]
- Bunnoy, A.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Klongklaew, N.; Pirarat, N.; Espinosa-Ruiz, C.; Castellani, S.; Conese, M. Mucoadhesive cationic lipid-based Flavobacterium oreochromis nanoencapsulation enhanced the efficacy of mucoadhesive immersion vaccination against columnaris disease and strengthened immunity in Asian sea bass (Lates calcarifer). Fish Shellfish Immunol. 2022, 127, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Nuzaiba, P.M.; Gupta, S.; Gupta, S.; Jadhao, S.B. Synthesis of L-methionine-loaded chitosan nanoparticles for controlled release and their in vitro and in vivo evaluation. Sci. Rep. 2023, 13, 7606. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Al-Sagheer, A.A.; Negm, S.S.; Naiel, M.A.E. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Ismael, N.E.M.; Abd El-hameed, S.A.A.; Amer, M.S. The antioxidative and immunity roles of chitosan nanoparticle and vitamin C-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture 2020, 523, 735219. [Google Scholar] [CrossRef]
- Sabra, M.S.; El-Aal, M.A.; Idriss, S.K.A.; Soliman, H.A.M.; Salaah, S.M.; Sayed, A.E.D.H. Possible beneficial effects of nano chitosan against doxycycline toxicity in Nile tilapia (Oreochromis niloticus). Aquaculture 2024, 587, 740855. [Google Scholar] [CrossRef]
- Saleh, M.; Essawy, E.; Shaalan, M.; Osman, S.; Ahmed, F.; El-matbouli, M. Therapeutic Intervention with Dietary Chitosan Nanoparticles Alleviates Fish Pathological and Molecular Systemic Inflammatory Responses against Infections. Mar. Drugs 2022, 20, 425. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.U.; Zuberi, A. An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol. Biochem. 2017, 43, 1689–1705. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.S.; Abdel-tawwab, M. Embracing nanotechnology for selenium application in aquafeeds. Rev. Aquac. 2023, 15, 112–129. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Fu, H.; Zhang, J.; Zhang, Q.; Wang, Y.; Wang, D.; Cai, L.; Chen, J.; Yu, H.; Lyu, B. Preparation of soybean protein-based nanoparticles and its application as encapsulation carriers of bioactive substances. LWT 2024, 191, 115680. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Cheng, J.; Guo, M. Stability, Digestion, and Cellular Transport of Soy Isoflavones Nanoparticles Stabilized by Polymerized Goat Milk Whey Protein. Antioxidants 2024, 13, 567. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D. Curcumin loaded chitosan nanoparticles fortify shrimp feed pellets with enhanced antioxidant activity. Mater. Sci. Eng. C 2021, 120, 111737. [Google Scholar] [CrossRef]
- Harshitha, M.; Nayak, A.; Disha, S.; Akshath, U.S.; Dubey, S.; Mweemba, H.; Chakraborty, A.; Karunasagar, I.; Maiti, B. Nanovaccines to Combat Aeromonas hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges. Vaccines 2023, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kov, A.; Berk, S. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, N.N.; Habte-Tsion, H.-M.; Haulofu, M. Perspectives of nanotechnology in aquaculture: Fish nutrition, disease, and water treatment. In Emerging Nanomaterials for Advanced Technologies. Nanotechnology in the Life Sciences; Krishnan, A., Ravindran, B., Balasubramanian, B., Swart, H., Panchu, S., Prasad, R., Eds.; Springer: Cham, Switzerland, 2022; pp. 463–485. [Google Scholar] [CrossRef]
- Araujo, J.M.; Fortes-silva, R.; Pola, C.C.; Yamamoto, F.Y.; Gatlin, D.M.; Gomes, C.L. Delivery of selenium using chitosan nanoparticles: Synthesis, characterization, and antioxidant and growth effects in Nile tilapia (Orechromis niloticus). PLoS ONE 2021, 16, e0251786. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.G.; Ali, T.E.S.; Aboyadak, I.M.; Elbakry, M.A. Controlling Pseudomonas aeruginosa infection in Oreochromis niloticus spawners by cefotaxime sodium. Aquaculture 2021, 544, 737107. [Google Scholar] [CrossRef]
- Oniha, M.I.; Oyesola, O.L.; Taiwo, O.S.; Oyejide, S.O.; Akindana, S.A.; Christiana Oluwatoyin Ajanaku, P.O.I. Nanochitosan-Based Fish Disease Prevention and Control. In Nanochitosan-Based Enhancement of Fisheries and Aquaculture; Isibor, P.O., Adeogun, A.O., Enuneku, A.A., Eds.; Springer: Cham, Switzerland, 2024; pp. 113–138. [Google Scholar] [CrossRef]
- Olaniyan, O.T.; Adetunji, C.O.; Dare, A.; Adeniyi, M.J.; Ajayi, O.O. Application of nanochitosan and polymeric chitosan as antibacterial, antivirus and antifungal activities when incorporated into aquatic and animal-based food materials. In Next Generation Nanochitosan; Adetunji, C., Hefft, D., Jeevanandam, J., Danquah, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 401–420. [Google Scholar] [CrossRef]
- El-gazzar, N.; Elez, R.M.M.A.; Attia, A.S.A.; Darwish, M.M.; Younis, E.M.; Eltahlawi, R.A.; Mohamed, K.I.; Davies, S.J.; Elsohaby, I. Antifungal and antibiofilm effects of probiotic Lactobacillus salivarius, zinc nanoparticles, and zinc nanocomposites against Candida albicans from Nile tilapia (Oreochromis niloticus), water and humans. Front. Cell. Infect. Microbiol. 2024, 14, 1358270. [Google Scholar] [CrossRef]
- Elabd, H.; Mahboub, H.H.; Salem, S.M.R.; Abdelwahab, A.M.; Alwutayd, K.M.; Shaalan, M.; Ismail, S.H.; Abdelfattah, A.M.; Khalid, A.; Mansour, A.T.; et al. Nano-Curcumin/Chitosan Modulates Growth, Biochemical, Immune, and Antioxidative Profiles, and the Expression of Related Genes in Nile tilapia, Oreochromis niloticus. Fishes 2023, 8, 333. [Google Scholar] [CrossRef]
- Abinaya, M.; Shanthi, S.; Palmy, J.; Al-ghanim, K.A.; Govindarajan, M.; Vaseeharan, B. Exopolysaccharides-Mediated ZnO Nanoparticles for the Treatment of Aquatic Diseases in Freshwater Fish Oreochromis mossambicus. Toxics 2023, 11, 313. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-madawy, K.M.; Moussa, S.H.; Assas, M.A. Application of Cystoseira myrica phycosynthesized selenium nanoparticles incorporated with nano-chitosan to control a fl atoxigenic fungi in fish feed. J. Sci. Food Agric. 2024, 104, 7678–7687. [Google Scholar] [CrossRef]
- Hashem, N.M.; Hosny, N.S.; El-Desoky, N.; Soltan, Y.A.; Elolimy, A.A.; Sallam, S.M.A.; Abu-Tor, E.-S.M. Alginate Nanoencapsulated Synbiotic Composite of Pomegranate Peel Phytogenics and Multi-Probiotic Species as a Potential Feed Additive: Physicochemical, Antioxidant, and Antimicrobial Activitie. Animals 2023, 13, 2432. [Google Scholar] [CrossRef]
- Latham, R.L.; Boyle, J.T.; Barbano, A.; Loveman, W.G.; Brown, N.A. Diverse mycotoxin threats to safe food and feed cereals. Essays Biochem. 2023, 67, 797–809. [Google Scholar] [PubMed]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Mir, S.A.; Bashir, M. Nanoencapsulation of Food Ingredients; IGI Global: Hershey, PA, USA, 2018. [Google Scholar]
- Dube, E.; Okuthe, G.E. Engineered nanoparticles in aquatic systems: Toxicity and mechanism of toxicity in fish. Emerg. Contam. 2023, 9, 100212. [Google Scholar] [CrossRef]
- Paolino, D.; Mancuso, A.; Cristiano, M.C.; Froiio, F.; Lammari, N.; Celia, C.; Fresta, M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials 2021, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural Parameters of Nanoparticles Affecting Their Toxicity for Biomedical Applications: A Review; Springer: Berlin/Heidelberg, Germany, 2023; Volume 25. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hedayati, S.A.; Yousefi, M.; Hoseinifar, S.H.; Yarahmadi, P.; Mahmoudi, S.S.; Van Doan, H. Toxic and bioaccumulative effects of zinc nanoparticle exposure to goldfish, Carassius auratus (Linnaeus, 1758). Drug Chem. Toxicol. 2022, 46, 984–994. [Google Scholar] [CrossRef]
- Qin, C.; Du, Q.; Luo, H.; Lin, J.; Li, M.; Xu, Y. Mitigation effects of humic acid on toxicity of zinc oxide nanoparticles (ZnO-NPs) on zebrafish (Danio rerio). Asian J. Ecotoxicol. 2022, 17, 201–209. [Google Scholar]
- Deepa, S.; Murugananthkumar, R.; Gupta, Y.R.; K.S, M.G.; Senthilkumaran, B. Effects of zinc oxide nanoparticles and zinc sulfate on the testis of common carp, Cyprinus carpio. Nanotoxicology 2019, 13, 240–257. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Gendy, G.M.E.; Ahmed, A.I.; Elharoun, E.R.; Hassaan, M.S. Nanoselenium versus bulk selenium as a dietary supplement: Effects on growth, feed efficiency, intestinal histology, biochemical and oxidative stress biomarkers in Nile tilapia (Oreochromis niloticus Linnaeus, 1758) fingerlings. Aquac. Res. 2021, 52, 5642–5655. [Google Scholar] [CrossRef]
- Seyedi, J.; Reza, M.; Esmaeilbeigi, M.; Tayemeh, M.B.; Moghadam, J.A. Toxicity and deleterious impacts of selenium nanoparticles at supranutritional and imbalance levels on male goldfish (Carassius auratus) sperm. J. Trace Elem. Med. Biol. 2021, 66, 126758. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Erratum: Guillermo M.; et al. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2021, 14, 1710. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L. Environmental Fate of Metal Nanoparticles in Estuarine Environments. Water 2022, 14, 1297. [Google Scholar] [CrossRef]
- Petrovic, S.; Bita, B. Nanoformulations in Pharmaceutical and Biomedical Applications: Green Perspectives. Int. J. Mol. Sci. 2024, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Fang, J.; Lou, C.; Yang, L.; Shan, S.; Wang, Z.; Chen, Y.; Li, H.; Li, X. Engineered nanoparticles for precise targeted drug delivery and enhanced therapeutic efficacy in cancer immunotherapy. Acta Pharm. Sin. B 2024, 14, 3432–3456. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; El-Aty, A.M.A.; Su, W.; Tan, M. Advancements in precision nutrition: Steady-state targeted delivery of food functional factors for nutrition intervention of chronic diseases. Food Saf. Health 2023, 1, 22–40. [Google Scholar] [CrossRef]
- Amin, N.; Vedi, F.; Wais, M.N.; Zulkifli, S.Z.; Azmai, M.N.A.; Ismail, A. Developmental Toxicity of Zinc Oxide Nanoparticles on the Early Life Stage of Java Medaka (Oryzias javanicus Bleeker, 1856). Trop. Agric. Sci. 2024, 47, 575–590. [Google Scholar] [CrossRef]
- Casiano-muñiz, I.M.; Ortiz-rom, M.I. Synthesis, Characterization, and Ecotoxicology Assessment of Zinc Oxide Nanoparticles by In Vivo Models. Nanomaterials 2024, 14, 255. [Google Scholar] [CrossRef]
- Shanmugam, K.; Jeyaraman, P. Nanoparticles in Aquatic Ecosystems: Origins, Destiny and Ecological Consequences. Environ. Sci. Arch. 2024, 3, 111–124. [Google Scholar] [CrossRef]
- Singh, S.; Mohan, S.; Gausiya, P. Fate and toxicity of nanoparticles in aquatic systems. Acta Geochim 2023, 42, 63–76. [Google Scholar] [CrossRef]
- Tran, T.; Nguyen, M.; Lin, C.; Hoang, T. Review on fate, transport, toxicity and health risk of nanoparticles in natural ecosystems: Emerging challenges in the modern age and solutions toward a sustainable environment. Sci. Total Environ. 2024, 912, 169331. [Google Scholar] [CrossRef]
- Yamini, V.; Shanmugam, V.; Rameshpathy, M.; Venkatraman, G.; Ramanathan, G.; Al, H.; AL Garalleh, H.; Hashmi, A.; Brindhadevi, K.; Rajeswari, V.D. Environmental effects and interaction of nanoparticles on beneficial soil and aquatic microorganisms. Environ. Res. 2023, 236, 116776. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sundaram, B. Fate, transport, and toxicity of nanoparticles: An emerging pollutant on biotic factors. Process Saf. Environ. Prot. 2023, 174, 595–607. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.M.R. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2023, 202, 360–386. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Gulshad, L.; Haq, U.; Farooq, M.A.; Al, A.; Siddique, F.R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Handy, R.D.; Poxton, M.G. Nitrogen pollution in mariculture: Toxicity and excretion of nitrogenous compounds by marine fish. Rev. Fish Biol. Fish. 1993, 3, 205–241. [Google Scholar] [CrossRef]
| NP | Fish | Dosage | Effects | Ref. |
|---|---|---|---|---|
| Zinc oxide NPs (ZnONPs) | Labeo rohita | 10 mg/kg for 45 days | Higher improvement of growth and metabolic functions | [12] |
| Oreochromis niloticus (Nile tilapia) | 1.5 mg/L | Antibacterial activity against Clostridium perfringes | [40] | |
| 60 µg/g for 8 weeks | Boost immune responses and improve disease resistance to Aeromonas hydrophila infection | [41] | ||
| 10 mg/kg for 60 days | Improved growth performance, blood health, intestinal histomorphology, and activated the oxidative stress response | [42] | ||
| 60 mg/kg for 84 days | Best growth, digestive enzyme activity, intestinal health, and improved antioxidant capacity. | [43] | ||
| 15 mg/kg for 60 days | Innate defense increased during Streptococcus agalactiae infection | [44] | ||
| 40 mg/kg for 8 weeks | Enhanced health parameters, antioxidant capacity, immune response, and disease resistance | [45] | ||
| 40 mg/kg for 8 weeks | Significant improvement in the growth performance, survival, serum lysozyme activity, phagocytic activity, phagocytic index, respiratory burst activity, expression of immune-related genes, digestive enzyme activity, and histopathological findings in Candida albicans-infected fish | [46] | ||
| Clarias gariepinus (African catfish) | 30 mg/kg for 60 days | Alleviated the negative impacts of chronic waterborne Cu exposure on growth performance, physiological changes, gene expression, and tissue architecture | [47] | |
| Iron oxide NPs (Fe2O3-NPs) | Cyprinus carpio (common carp) | 40 mg/kg for 70 days | Elevated growth, nutrient absorption, body composition and blood indices. | [23] |
| Acipenser stellatus (stellate sturgeon) | 50 mg/kg for 60 days | Improved the welfare and survival of stellate sturgeon juveniles | [48] | |
| O. niloticus | 0.4 mg/kg for 12 weeks | Improves immunological performance, filet composition, and the healthiness of the intestinal structure | [49] | |
| 1 g/kg for 30 days | Enhanced properties of growth, hemato-biochemical, immune, and antioxidative profiles, and related genes’ expression of O. niloticus | [50] | ||
| Trichogaster trichopterus (blue gourami) | 40 mg/kg for 60 days | Enhanced growth performance, improved biochemical constituents, better hematological parameters, and increased antioxidant activity | [51] | |
| Etroplus suratensis | 20 mg/kg for 60 days | Improved growth performance, nutrient utilization, and the health of E. suratensis. | [52] | |
| L. rohita | 10 mg/kg for 45 days | Improved growth performance | [53] | |
| Magnesium oxide NPs (MgO NPs) | O. niloticus | 7.5 μg/mL for 60 days | Improved growth, health status, and increased the resistance of O. niloticus against bacterial and parasitic infection | [54] |
| Megalobrama amblycephala (blunt snout bream) | 120 mg/kg for 12 weeks | Enhanced growth performance and feed utilization | [55] | |
| C. gariepinus | 0.04 mg/kg for 60 days | Significant improvement in body weight and antioxidant capacity | [56] | |
| Manganese dioxide NPs (MnO2NPs) | Pelteobagrus fulvidraco (yellow catfish) | 13.6 mg/kg for 8 weeks | Better growth performance and feed utilization | [57] |
| C. carpio | 6 mg/100 g for 21 days | Enhanced growth and hematological traits | [58] | |
| O. niloticus | 2 mg/kg for 38 days | Improved growth performance in under multiple stresses | [59] | |
| Cirrhinus cirrhosus (mrigal carp) | 75 mg/kg for 21 days | Enhanced Mrigal’s growth and decreased hematological criterion | [60] | |
| Pangasianodon hypophthalmus | 3 mg/kg for 105 days | Controlled gene regulations against multiple stresses | [61] | |
| Copper NPs (CuNPs) | C. carpio | 2.19 to 2.91 mg/kg for 8 weeks | Improved growth performance, feed utilization, immune parameters, and oxidation resistance | [62] |
| Oncorhynchus mykiss (rainbow trout) | 2 mg/kg for 60 days | Elevated the growth performance, antioxidant capacity, and health of rainbow trout. | [63] | |
| Pangasianodon hypophthalmus | 1.5 mg/kg | Promoted fish immunity, growth performance, and controlled gene regulation | [64] | |
| Copper oxide NPs (CuONPs) | C. carpio | 200 mg/100 g for 21 days | Improved the growth performance of the Koi carp | [65] |
| NP | Fish | Dosage | Effects | Ref. |
|---|---|---|---|---|
| Selenium NPs (SeNPs) | O. niloticus | 1 mg/kg | Significant improvements in growth, feed efficiency, and survival rates. | [91] |
| 2 mg/kg for 56 days | Improved growth performance, survival, and chemical composition (protein). | [92] | ||
| 1 mg/kg for 4 weeks | SeNPs could mitigate the oxidative stress induced by feeding the aflatoxin diet and could boost the immunity of stressed O. niloticus (Nile tilapia). | [93] | ||
| 0.1–1.2 mg/kg | Improved growth performance and antioxidant capacity, stabilized the intestinal structure, and enhanced resistance to hypoxic stress and Streptococcus agalactiae-based infection of juvenile O. niloticus (Nile tilapia). | [31] | ||
| Ctenopharyngodon idella (grass carp) | 0.3 mg/kg for 60 days | Promoted the growth and antioxidant capacity of grass carp, with excellent bioavailability of Se. | [94] | |
| Lates calcarifer (Asian seabass) | 4 mg/kg for 60 days | Improved growth and health indices in L. calcarifer juveniles. | [95] | |
| Acanthopagrus arabicus (Arabian yellowfin seabream) | 1 mg/kg for 60 days | Enhanced growth, immunity, and stress resistance. | [96] | |
| Sparidentex hasta (sobaity seabream) | 2 mg /kg for 60 days | Promoted growth performance and enhanced antioxidant and immune parameters in sobaity juveniles. | [97] | |
| Soy protein NPs (SPNPs) | Lates calcarifer (Asian sea bass) | 50% of fishmeal protein replaced with single-cell protein (SCP) from soybean processing wastewater for 24 days | The replacement of fishmeal protein with microbial community-based SCP did not affect Asian seabass growth or survival. | [98] |
| - | Dispersion gave 155.7 mg/L solubilized curcumin | Protected curcumin from degradation or precipitation during simulated gastric-intestinal digestion, showing a significantly enhanced bioaccessibility. | [99] | |
| Protein NPs | D. rerio (Zebrafish) and O. mykiss (rainbow trout) | 20 μg/fish | Nanoparticles are taken up in vitro by zebrafish ZFL cells and in vivo by intubating zebrafish. NPs evoke an antiviral innate immune response in ZFL cells and in rainbow trout head kidney macrophages. | [68] |
| Lipid-based NPs (LNPs) | Oreochromis spp. (Red tilapia) | 30 ppm for 21 days | Alkyl polyglucoside nanostructured lipid carriers achieved high encapsulation efficiency, thermal stability, and cost-effectiveness for the masculinization of tilapia with 17 alpha-methyltestosterone when delivered orally at 30 ppm for 21 days. | [100] |
| Gilthead sea bream and European sea bass | 20 µg/mL for 24 h | Grape seed extract (GSE) mixture containing several antioxidant compounds loaded on solid lipid nanoparticles (SLNPs) enhanced the viability and antioxidant properties of fish cells. | [101] | |
| Lates calcarifer (Asian sea bass) | Mucoadhesive nano-encapsulated vaccine: water dilution of 1:100 (v/v) for 8 weeks | Mucoadhesive nano-encapsulated vaccine (cationic lipid-based nanoparticles combined with an antigen obtained from F. oreochromis) improved the efficiency of mucoadhesive vaccination against columnaris disease and strengthened immunity in Asian sea bass by increasing serum antibody levels, upregulating immune-related genes, and increasing survival. | [102] | |
| Chitosan-based NPs (CTNPs) | L. rohita | 0.6% L-methionine in in total feed for 60 days | L-methionine-loaded chitosan nanoparticles (M-CTNPs) exhibited the sustained and slow release of methionine over a prolonged period resulting in higher growth rates, improved protein efficiency, and enhanced sero-immunological test scores. | [103] |
| O. niloticus (Nile tilapia) | 1.0 g/kg of N-acetyl-D-glucosamine (NAG)-loaded CTNPs | NAG-loaded CTNPs significantly enhanced the immune-modulatory properties, growth performance, and disease resistance of O. niloticus (Nile tilapia). | [69] | |
| 5 g/kg for 10 weeks | Improved growth and some health indications. | [104] | ||
| 5 g/kg thymol and CTNPs mixture for 10 weeks | The synergistic effect of thymol combined with CTNPs enhanced feed utilization, protein utilization, the hematological profile, antioxidant enzymes, and intestine morphology. | |||
| 5 g/kg CTNPs mixed with 250 or 500 mg vitamin C for 72 days | Significant enhancement in all growth performance parameters, body indices, and survival rates. | [105] | ||
| 0.5% CTNPs in fish diet | Alleviated doxycycline-induced toxicity in fish by controlling oxidative stress and inflammatory cytokines. | [106] | ||
| Oncorhynchus mykiss (rainbow trout) | 5 g/kg for 21 days | Enhanced resistance and inflammatory responses to infections. | [107] |
| NP | Fish | Dosage | Effects | Ref. |
|---|---|---|---|---|
| Se/ZnONPs | Oreochromis niloticus (Nile tilapia) O. niloticus | 10 mg/kg for 60 days | Synergistic effects that improve growth performance, blood health, and intestinal histomorphology. | [42] |
| ZnNPs/probiotic Lactobacillus salivarius composite | - | Antifungal and antibiofilm activities against Candida albicans from O. niloticus. | [125] | |
| Graphene oxide (GO) nanosheets/ Se-ZnONPs | 1 mg/kg for 8 weeks | Enhanced antioxidant activity and physiological functions; had efficient protein synthesis and increased fish weight. | [29] | |
| GO/ZnONPs nanocomposite | ||||
| GO/SeNPs nanocomposite | Enhanced antioxidant capacity and physiological functions. | |||
| Nano-curcumin/chitosan blend (NCur/CT) | 0.00625 + 0.5 g/kg diet for 4 weeks | Promoted growth, digestion, immune status, liver function, antioxidant status, and related gene expression in O. niloticus. | [126] | |
| Exopolysaccharides/ZnONPs | O. mossambicus | 10 mg/g for 30 days | Improved growth performance, lowered the death rate and improved the disease resistance of O. mossambicus on exposure to A. hydrophila and V. parahaemolyticus. | [127] |
| Chitosan nanoparticles (CTNPs)/ SeNPs nanocomposite | In vitro study | 50 μL of 1 g L−1 | Significantly reduced Aspergillus flavus count and growth. | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, E. Nanoparticle-Enhanced Fish Feed: Benefits and Challenges. Fishes 2024, 9, 322. https://doi.org/10.3390/fishes9080322
Dube E. Nanoparticle-Enhanced Fish Feed: Benefits and Challenges. Fishes. 2024; 9(8):322. https://doi.org/10.3390/fishes9080322
Chicago/Turabian StyleDube, Edith. 2024. "Nanoparticle-Enhanced Fish Feed: Benefits and Challenges" Fishes 9, no. 8: 322. https://doi.org/10.3390/fishes9080322
APA StyleDube, E. (2024). Nanoparticle-Enhanced Fish Feed: Benefits and Challenges. Fishes, 9(8), 322. https://doi.org/10.3390/fishes9080322






