Lactiplantibacillus plantarum I Induces Gonad Growth in the Queen Scallop Aequipecten opercularis (Linnaeus, 1758) under Conditions of Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scallop Culture
2.2. Supplementation with the Probiotic Strain Lactiplantibacillus plantarum I
2.3. Experimental Design
2.4. Morphological Measurements
2.5. Statistical Analyses
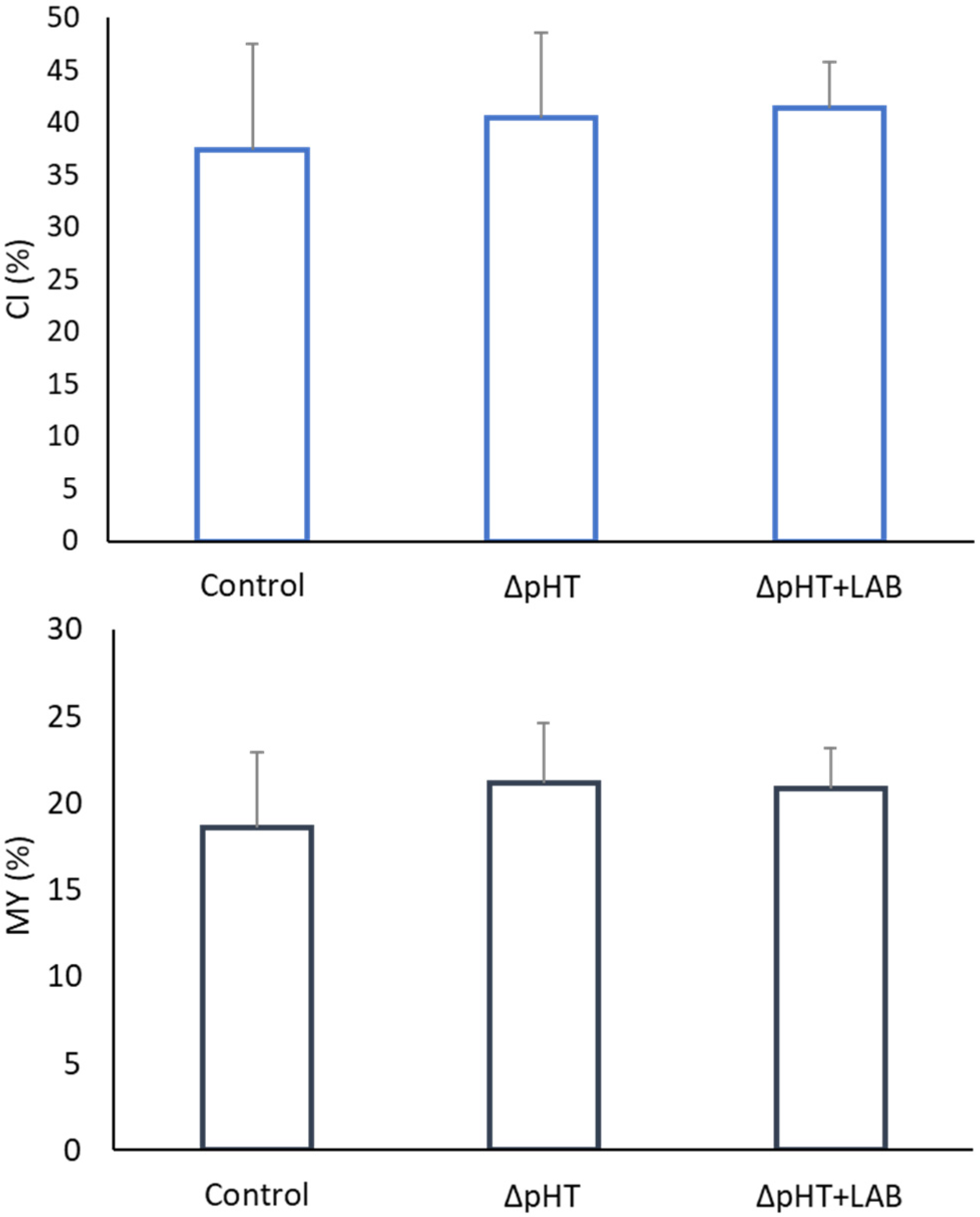
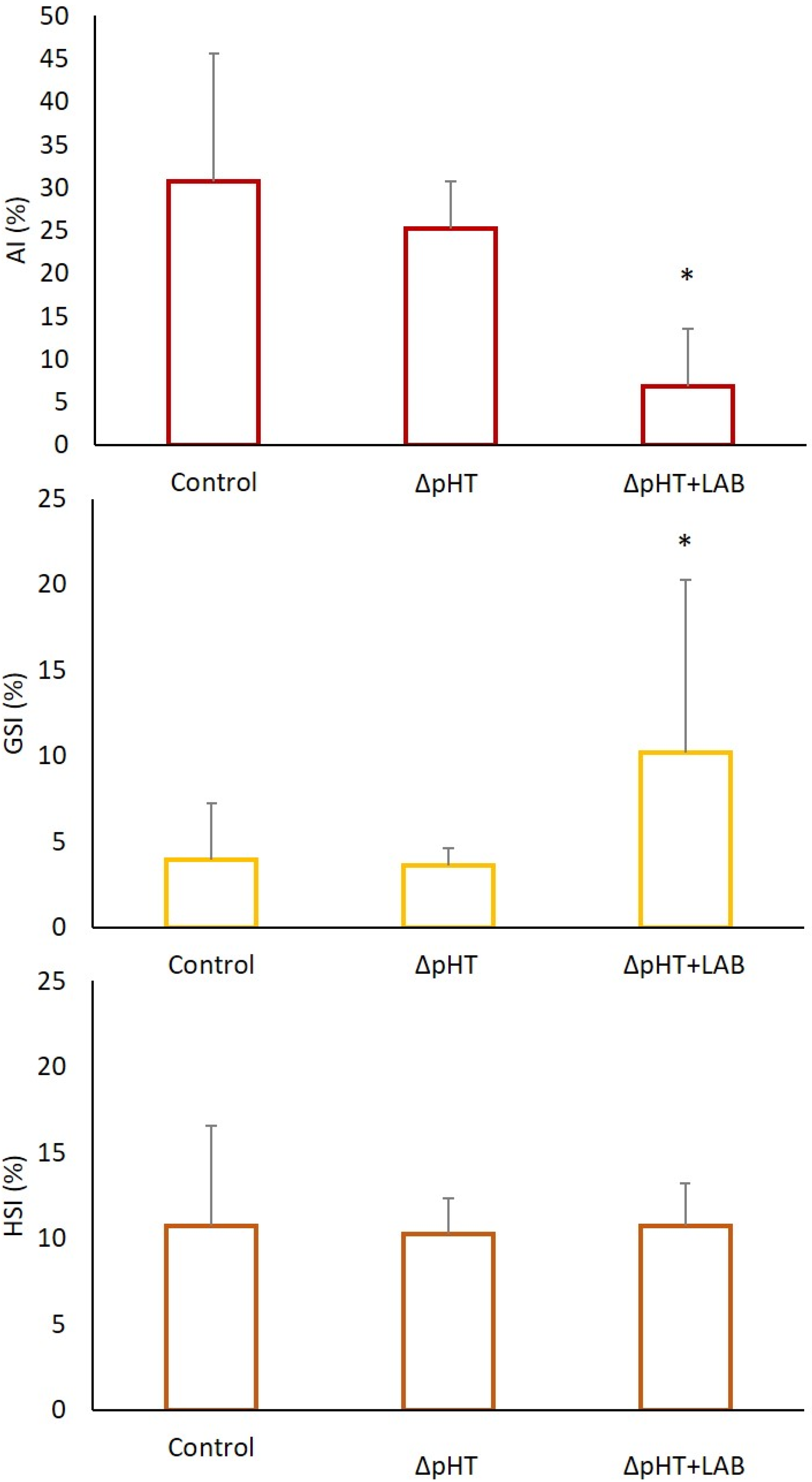
3. Results
3.1. Seawater Conditions
3.2. Morphometric Indices, Allometry, and Somatic Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Salgado-García, R.L.; Kraffe, E.; Maytorena-Verdugo, C.I.; Rivera-Camacho, A.R.; Sicard, M.T.; Arellano-Martínez, M.; Racotta, I.S. Metabolic responses of adult lion’s paw scallops Nodipecten subnodosus exposed to acute hyperthermia in relation to seasonal reproductive effort. Sci. Rep. 2020, 10, 2449. [Google Scholar] [CrossRef]
- Soon, T.K.; Zheng, H. Climate Change and Bivalve Mass Mortality in Temperate Regions. Rev. Environ. Contam. Toxicol. 2019, 251, 109–129. [Google Scholar] [CrossRef]
- Mahato, A. Climate change and its impact on agriculture. Int. J. Sci. Res. 2014, 4, 1–6. [Google Scholar]
- Gentilucci, M.; Parisi, C.; Coppola, M.R.; Majdoubi, F.-Z.; Madonna, A.; Guerriero, G. Influence of Mediterranean Sea Temperature Increase on Gaeta Gulf (Tyrrhenian Sea) Biodiversity. Proc. Zool. Soc. 2021, 74, 91–103. [Google Scholar] [CrossRef]
- Grbec, B.; Matić, F.; Beg Paklar, G.; Morović, M.; Popović, R.; Vilibić, I. Long-Term Trends, Variability and Extremes of In Situ Sea Surface Temperature Measured Along the Eastern Adriatic Coast and Its Relationship to Hemispheric Processes. Pure Appl. Geophys. 2018, 175, 4031–4046. [Google Scholar] [CrossRef]
- Bonacci, O.; Vrsalović, A. Differences in Air and Sea Surface Temperatures in the Northern and Southern Part of the Adriatic Sea. Atmosphere 2022, 13, 1158. [Google Scholar] [CrossRef]
- Vilibić, I.; Dunić, N.; Peharda, M. Near-Surface Ocean Temperature Variations across Temporal Scales in the Coastal Eastern Adriatic. Cont. Shelf Res. 2022, 245, 104786. [Google Scholar] [CrossRef]
- Raicich, F.; Colucci, R.R. A near-surface sea temperature time series from Trieste, northern Adriatic Sea (1899–2015). Earth Syst. Sci. Data 2019, 11, 761–768. [Google Scholar] [CrossRef]
- Rizzi, J.; Torresan, S.; Critto, A.; Zabeo, A.; Brigolin, D.; Carniel, S.; Pastres, R.; Marcomini, A. Climate change impacts on marine water quality: The case study of the Northern Adriatic sea. Mar. Pollut. Bull. 2016, 102, 271–282. [Google Scholar] [CrossRef]
- Kružić, P.; Popijač, A. Mass mortality events of the coral Balanophyllia europaea (Scleractinia, Dendrophylliidae) in the Mljet National Park (eastern Adriatic Sea) caused by sea temperature anomalies. Coral Reefs 2015, 34, 109–118. [Google Scholar] [CrossRef]
- Pavičić, M.; Vilibić, I.; Šepić, J.; Vrdoljak, D.; Stagličić, N.; Šegvić Bubić, T.; Vujević, A.; Matić-Skoko, S. Temperature-driven abundance change of European lobster (Homarus gammarus) in the Adriatic Sea. In 13. Croatian Biological Congress with International Participation—Collection of Abstracts; Croatian Biological Society: Zagreb, Croatia, 2018; pp. 308–309. Available online: https://www.croris.hr/crosbi/publikacija/prilog-skup/693298 (accessed on 24 April 2024).
- Dimarchopoulou, D.; Tsikliras, A.C. Linking growth patterns to sea temperature and oxygen levels across European sardine (Sardina pilchardus) populations. Environ. Biol. Fish 2022, 105, 1335–1345. [Google Scholar] [CrossRef]
- Chatzimentor, A.; Doxa, A.; Katsanevakis, S.; Mazaris, A.D. Are Mediterranean marine threatened species at high risk by climate change? Glob. Chang. Biol. 2023, 29, 1809–1821. [Google Scholar] [CrossRef]
- Scanes, E.; Byrne, M. Warming and hypoxia threaten a valuable scallop fishery: A warning for commercial bivalve ventures in climate change hotspots. Glob. Chang. Biol. 2023, 29, 2043–2045. [Google Scholar] [CrossRef]
- Mohamed, K.S. Probiotics and its application in mariculture. In U: Winter School on Recent Advances in Mariculture Genetics and Biotechnology, 4–24 November 2003; Central Marine Fisheries Research Institute: Kochi, India, 2003. [Google Scholar]
- Prado, S.; Romalde, J.L.; Barja, J.L. Review of probiotics for use in bivalve hatcheries. Vet. Microbiol. 2010, 145, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kanduč, T.; Šlejkovec, Z.; Falnoga, I.; Mori, N.; Budič, B.; Kovačić, I.; Pavičić-Hamer, D.; Hamer, B. Environmental status of the NE Adriatic Sea, Istria, Croatia: Insights from mussel Mytilus galloprovincialis condition indices, stable isotopes and metal(loid)s. Mar. Pollut. Bullet. 2018, 126, 525–534. [Google Scholar] [CrossRef]
- Topić Popović, N.; Beer Ljubić, B.; Strunjak-Perović, I.; Babić, S.; Lorencin, V.; Jadan, M.; Čižmek, L.; Matulić, D.; Bojanić, K.; Čož-Rakovac, R. Seasonal antioxidant and biochemical properties of the Northern Adriatic Pecten jacobaeus. PLoS ONE 2020, 15, e0230539. [Google Scholar] [CrossRef]
- Kovačić, I.; Pavičić-Hamer, D.; Kanduč, T.; Hamer, B. Adaptation of cultured mussel Mytilus galloprovincialis Lamarck, 1819 from the northern Adriatic Sea to nearby aquaculture sites and translocation. Acta Adriat. 2017, 58, 285–296. [Google Scholar] [CrossRef]
- Kovačić, I.; Žunec, A.; Matešković, M.; Burić, P.; Iveša, N.; Štifanić, M.; Frece, J. Commercial Quality, Biological Indices and Biochemical Composition of Queen Scallop Aequipecten opercularis in Culture. Fishes 2023, 8, 48. [Google Scholar] [CrossRef]
- Filgueira, R.; Guyondet, T.; Comeau, L.A.; Tremblay, R. Bivalve aquaculture-environment interactions in the context of climate change. Glob. Chang. Biol. 2016, 22, 3901–3913. [Google Scholar] [CrossRef]
- Melaku Canu, D.; Solidoro, C.; Cossarini, G.; Giorgi, F. Effect of global change on bivalve rearing activity and the need for adaptive management. Clim. Res. 2010, 42, 13–26. [Google Scholar] [CrossRef]
- Gosling, E. Bivalve Molluscs: Biology, Ecology and Culture; John Wiley and Sons: Hoboken, NJ, USA, 2008; Volume 456. [Google Scholar]
- Čanak, I.; Kovačić, I.; Žunec, A.; Jakopović, Ž.; Kostelac, D.; Markov, K.; Štifanić, M.; Burić, P.; Iveša, N.; Frece, J. Study of the Impact of Lactiplantibacillus plantarum I on the Health Status of Queen Scallop Aequipecten opercularis. Appl. Sci. 2023, 13, 7723. [Google Scholar] [CrossRef]
- Čanak, I.; Kovačić, I.; Žunec, A.; Jakopović, Ž.; Kostelac, D.; Markov, K.; Štifanić, M.; Burić, P.; Iveša, N.; Frece, J. Effect of dietary supplementation with Lactiplantibacillus plantarum i on queen scallop Aequipecten opercularis under simulated climate change conditions. Croat. J. Fish. 2024, 82, 1–8. [Google Scholar] [CrossRef]
- Duncan, P.F.; Brand, A.R.; Strand, Ø.; Foucher, E. The European scallop fisheries for Pecten maximus, Aequipecten opercularis, Chlamys islandica, and Mimachlamys varia. Dev. Aquac. Fish. Sci. 2016, 40, 781–858. [Google Scholar] [CrossRef]
- Čanak, J.; Kostelac, D.; Jakopović, Ž.; Markov, K.; Frece, J. Lactic Acid Bacteria of Marine Origin as a Tool for Successful Shellfish Farming and Adaptation to Climate Change Conditions. Foods 2024, 13, 1042. [Google Scholar] [CrossRef]
- Marčeta, T.; Marin, M.G.; Codognotto, V.F.; Bressan, M. Settlement of Bivalve Spat on Artificial Collectors (Net Bags) in Two Commercial Mussel Parks in the North-Western Adriatic Sea. J. Mar. Sci. Eng. 2022, 10, 210. [Google Scholar] [CrossRef]
- Šimunović, A.; Piccinetti, C.; Despalatović, M.; Grubelić, I. Experimental catches and distribution of Queen scallop Aequipecten opercularis (LINNAEUS, 1758) (Pectinidae, Mollusca Bivalvia) in the Adriatic Sea. Acta Adriat. 2002, 43, 49–57. [Google Scholar]
- Rathman, M.; Bolotin, J.; Glavić, N.; Barišić, J. Influence of water depth on growth and mortality of Chlamys varia (Linnaeus, 1758): Implications for cage culture in Mali Ston Bay, Croatia. Aquac. Int. 2017, 25, 135–146. [Google Scholar] [CrossRef]
- Prato, E.; Biandolino, F.; Parlapiano, I.; Papa, L.; Denti, G.; Fanelli, G. Estimation of growth parameters of the black scallop Mimachlamys varia in the gulf of Taranto (Ionian Sea, Southern Italy). Water 2020, 12, 3342. [Google Scholar] [CrossRef]
- Strand, Ø.; Louro, A.; Duncan, P.F. European aquaculture. In Developments in Aquaculture and Fisheries Science. Elsevier 2016, 40, 859–890. [Google Scholar]
- Kovačić, I.; Burić, P.; Žunec, A.; Bilić, J.; Prgić, A.; Čanak, I.; Iveša, N.; Štifanić, M.; Frece, J. The Effect of Lactiplantibacillus plantarum I-Enriched Diet on the Phenolic Content and Antioxidant Capacity of Queen Scallop (Aequipecten opercularis Linnaeus, 1758) Extracts. Microorganisms 2023, 11, 2723. [Google Scholar] [CrossRef]
- Kovačić, I.; Žunec, A.; Matešković, M.; Burić, P.; Iveša, N.; Štifanić, M. Seasonal changes in morphometric and physiological parameters of the queen scallop Aequipecten opercularis (Linnaeus, 1758) cultured in captivity. In Croatian Biology Congress Book of Abstracts; Croatian Scientific Bibliography: Pula, Croatia, 2022; Available online: https://www.bib.irb.hr:8443/1225620 (accessed on 24 April 2024).
- Cataldo, D.H.; Boltovskoy, D.; Stripeikis, J.; Pose, M. Condition index and growth rates of field caged Corbicula fluminea (Bivalvia) as biomarkers of pollution gradients in the Paraná river delta (Argentina). Aquat. Ecosyst. Health Manag. 2001, 4, 187–201. [Google Scholar] [CrossRef]
- Blaise, C.; Gagné, F.; Burgeot, T. Three simple biomarkers useful in conducting water quality assessments with bivalve mollusks. Environ. Sci. Pollut. Res. 2017, 24, 27662–27669. [Google Scholar] [CrossRef]
- Johnson, A.L.A.; Hickson, J.A.; Swan, J.; Brown, M.R.; Heaton, T.H.E.; Chenery, S.; Balson, P.S. The Queen Scallop Aequipecten opercularis: A new source of information on late Cenozoic marine environments in Europe. Geol. Soc. Lond. Spec. Publ. 2000, 177, 425–439. [Google Scholar] [CrossRef]
- Fernández-Reiriz, M.J.; Range, P.; Álvarez-Salgado, X.A.; Espinosa, J.; Labarta, U. Tolerance of juvenile Mytilus galloprovincialis to experimental seawater acidification. Mar. Ecol. Prog. Ser. 2012, 454, 65–74. [Google Scholar] [CrossRef]
- Mackenzie, C.L.; Ormondroyd, G.A.; Curling, S.F.; Ball, R.J.; Whiteley, N.M.; Malham, S.K. Ocean warming, more than acidification, reduces shell strength in a commercial shellfish species during food limitation. PLoS ONE 2014, 9, e86764. [Google Scholar] [CrossRef]
- Ong, E.Z.; Briffa, M.; Moens, T.; Van Colen, C. Physiological responses to ocean acidification and warming synergistically reduce condition of the common cockle Cerastoderma edule. Mar. Environ. Res. 2017, 130, 38–47. [Google Scholar] [CrossRef]
- Byrne, M.; Foo, S.A.; Ross, P.M.; Putnam, H.M. Limitations of cross- and ultigenerational plasticity for marine invertebrates faced with global climate change. Glob. Chang. Biol. 2020, 26, 80–102. [Google Scholar] [CrossRef]
- Kamermans, P.; Saurel, C. Interacting climate change effects on mussels (Mytilus edulis and M. galloprovincialis) and oysters (Crassostrea gigas and Ostrea edulis): Experiments for bivalve individual growth models. Aquat. Living Resour. 2022, 35, 1. [Google Scholar] [CrossRef]
- Schmidt, M.; Philipp, E.E.; Abele, D. Size and age-dependent changes of escape response to predator attack in the Queen scallop Aequipecten opercularis. Mar. Biol. Res. 2008, 4, 442–450. [Google Scholar] [CrossRef]
- Çolakoğlu, S.; Çolakoğlu, F.; Künili, İ.E. Length–Weight Relationships, Meat Yield and Morphometric Indices of Five Commercial Bivalve Species Collected from the Çanakkale Strait (Türkiye). Aquat. Sci. Eng. 2023, 39, 36–42. [Google Scholar] [CrossRef]
- Vereycken, J.E.; Aldridge, D.C. Bivalve molluscs as biosensors of water quality: State of the art and future directions. Hydrobiologia 2022, 850, 231–256. [Google Scholar] [CrossRef]
- Gazeau, F.; Alliouane, S.; Bock, C.; Bramanti, L.; López, C.M.; Gentile, M.; Hirse, T.; Pörtner, H.; Ziveri, P. Impact of ocean acidification and warming on the Mediterranean mussel (Mytilus galloprovincialis). Front. Mar. Sci. 2014, 1, 62–74. [Google Scholar] [CrossRef]
- Watson, S.; Southgate, P.C.; Miller, G.M.; Moorhead, J.A.; Knauer, J. Ocean acidification and warming reduce juvenile survival of the fluted giant clam, Tridacna squamosa. Molluscan Res. 2012, 32, 177–180. [Google Scholar] [CrossRef]
- Gooding, R.A.; Harley, C.D.G.; Tang, E. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc. Natl. Acad. Sci. USA 2009, 106, 9316–9321. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, O.; Reuben, O.; Olatoye, O. Global climate change. J. Geosci. Environ. Prot. 2014, 2, 114. [Google Scholar] [CrossRef]
- Dash, G.; Raman, R.P.; Prasad, K.P.; Makesh, M.; Pradeep, M.A.; Sen, S. Evaluation of Lactobacillus plantarum as feed supplement on host associated microflora, growth, feed efficiency, carcass biochemical composition and immune response of giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquaculture 2014, 432, 225–236. [Google Scholar] [CrossRef]
- Dash, G.; Raman, R.P.; Prasad, K.P.; Makesh, M.; Pradeep, M.A.; Sen, S. Evaluation of paraprobiotic applicability of Lactobacillus plantarum in improving the immune response and disease protection in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Fish Shellfish Immun. 2015, 43, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Dash, G.; Raman, R.P.; Prasad, K.P.; Marappan, M.; Pradeep, M.A.; Sen, S. Evaluation of Lactobacillus plantarum as a water additive on host associated microflora, growth, feed efficiency and immune response of giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquac. Res. 2016, 47, 804–818. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Wang, C.; Zhang, L.; Tu, K.; Zheng, Z. Insights into the intestinal microbiota of several aquatic organisms and association with the surrounding environment. Aquaculture 2019, 507, 196–202. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Moura, P.; Pereira, F.; Pereira, A.M.; Gaspar, M.B. Morphometric relationships and relative growth of 20 uncommon bivalve species from the Algarve coast (southern Portugal). J. Mar. Biol. Assoc. U. K. 2018, 98, 463–474. [Google Scholar] [CrossRef]
- Caill-Milly, N.; Bru, N.; Mahé, K.; Borie, C.; d’Amico, F. Shell shape analysis and spatial allometry patterns of Manila clam (Ruditapes philippinarum) in a mesotidal coastal lagoon. J. Mar. Biol. 2012, 2012, 281206. [Google Scholar] [CrossRef]
- Caill-Milly, N.; Bru, N.; Barranger, M.; Gallon, L.; D’amico, F. Morphological trends of four Manila clam populations (Venerupis philippinarum) on the French Atlantic coast: Identified spatial patterns and their relationship to environmental variability. J. Shellfish Res. 2014, 33, 355–372. [Google Scholar] [CrossRef]
- Ricker, W.E. Linear regressions in fishery research. J. Fish. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Gaspar, M.B.; Santos, M.N.; Vasconcelos, P.; Monteiro, C.C. Shell morphometric relationships of the most common bivalve species (Mollusca: Bivalvia) of the Algarve coast (southern Portugal). Hydrobiologia 2002, 477, 73–80. [Google Scholar] [CrossRef]
- Huxley, J.S.; Teissier, G. Terminology of relative growth. Nature 1936, 137, 780–781. [Google Scholar] [CrossRef]
- Nascimento-Schulze, J.C.; Bean, T.P.; Houston, R.D.; Santos, E.M.; Sanders, M.B.; Lewis, C.; Ellis, R.P. Optimizing hatchery practices for genetic improvement of marine bivalves. Rev. Aquac. 2021, 13, 2289–2304. [Google Scholar] [CrossRef]
- Smaal, A.C.; Ferreira, J.G.; Grant, J.; Petersen, J.K.; Strand, Ø. Goods and Services of Marine Bivalves; Springer Nature: Berlin, Germany, 2019; Volume 591. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, H.; Zheng, H. Selective breeding of edible bivalves and its implication of global climate change. Rev. Aquac. 2020, 12, 2559–2572. [Google Scholar] [CrossRef]
- Pagano, G.; Castello, G.; Gallo, M.; Borriello, I.; Guida, M. Complex Mixture-Associated Hormesis and Toxicity: The Case of Leather Tanning Industry. Dose-Response 2008, 6, 383–396. [Google Scholar] [CrossRef]
- Range, P.; Piló, D.; Ben-Hamadou, R.; Chícharo, M.A.; Matias, D.; Joaquim, S.; Oliveira, A.P.; Chícharo, L. Seawater acidification by CO2 in a coastal lagoon environment: Effects on life history traits of juvenile mussels Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 2012, 424, 89–98. [Google Scholar] [CrossRef]
- Ozvarol, Y.; Gokoglu, M. Some Biological Aspects of Scallop Chlamys varia (Linnaeus, 1758), Bivalvia: Pectinida from Aegean Sea coast of Turkey. J. Appl. Biol. Sci. 2013, 7, 68–70. [Google Scholar]
- Tate, R.D.; Benkendorff, K.; Ab Lah, R.; Kelaher, B.P. Ocean acidification and warming impacts the nutritional properties of the predatory whelk, Dicathais orbita. J. Exp. Mar. Biol. Ecol. 2017, 493, 7–13. [Google Scholar] [CrossRef]
- Enríquez-Díaz, M.; Pouvreau, S.; Chávez-Villalba, J.; Le Pennec, M. Gametogenesis, reproductive investment, and spawning behavior of the Pacific giant oyster Crassostrea gigas: Evidence of an environment-dependent strategy. Aquac. Int. 2009, 17, 491–506. [Google Scholar] [CrossRef]
- Sreedevi, P.R.; Uthayakumar, V.; Jayakumar, R.; Ramasubramanian, V. Influence of rearing water temperature on induced gonadal development and spawning behaviour of tropical green mussel, Perna viridis. Asian Pac. J. Reprod. 2014, 3, 204–209. [Google Scholar] [CrossRef]
- Suja, N.; Muthiah, P. Effect of starvation and temperature on gonad development of baby clam, Marcia opima (Gmelin). J. Mar. Biol. Assoc. India 2009, 51, 21–25. [Google Scholar]
- Delgado, M.; Pérez-Camacho, A. Comparative study of gonadal development of Ruditapes philippinarum (Adams and Reeve) and Ruditapes decussatus (L.) (Mollusca: Bivalvia): Influence of temperature. Sci. Mar. 2007, 71, 471–484. [Google Scholar] [CrossRef]
- Beltrán-Lugo, A.I.; Maeda-Martínez, A.N.; Pacheco-Aguilar, R.; Nolasco-Soria, H.G. Seasonal variations in chemical, physical, textural, and microstructural properties of adductor muscles of Pacific lions-paw scallop (Nodipecten subnodosus). Aquaculture 2006, 258, 619–632. [Google Scholar] [CrossRef]
- Pichaud, N.; Briatte, S.; Desrosiers, V.; Pellerin, J.; Fournier, M.; Blier, P.U. Metabolic capacities and immunocompetence of sea scallops (Placopecten magellanicus, Gmelin) at different ages and life stages. J. Shellfish Res. 2009, 28, 865–876. [Google Scholar] [CrossRef]
- Pilditch, C.A.; Grant, J. Effect of temperature fluctuations and food supply on the growth and metabolism of juvenile sea scallops (Placopecten magellanicus). Mar. Biol. 1999, 134, 235–248. [Google Scholar] [CrossRef]

| Control | ΔpHT | ΔpHT + LAB | |
|---|---|---|---|
| pH | 7.88 ± 0.07 | 7.64 ± 0.08 | 7.67 ± 0.08 |
| Temperature (°C) | 15.67 ± 15.65 | 16.88 ± 0.46 | 16.83 ± 0.49 |
| Dissolved oxygen (%) | 89.94 ± 1.59 | 89.92 ± 1.54 | 89.94 ± 1.50 |
| Control | ΔpHT | ΔpHT + LAB | |
|---|---|---|---|
| Elongation index | |||
| 1st day | 0.987 ± 0.019 | 0.988 ± 0.017 | 0.98 ± 0.017 |
| 30th day | 0.987 ± 0.019 | 1.012 ± 0.083 | 0.98 ± 0.014 |
| Compactness index | |||
| 1st day | 0.335 ± 0.023 | 0.321 ± 0.018 | 0.330 ± 0.018 |
| 30th day | 0.338 ± 0.020 | 0.337 ± 0.027 | 0.337 ± 0.023 |
| Convexity index | |||
| 1st day | 0.339 ± 0.024 | 0.325 ± 0.019 | 0.332 ± 0.020 |
| 30th day | 0.343 ± 0.022 | 0.334 ± 0.018 | 0.341 ± 0.024 |
| Density index | |||
| 1st day | 0.309 ± 0.038 | 0.288 ± 0.032 | 0.290 ± 0.032 |
| 30th day | 0.292 ± 0.040 | 0.256 ± 0.030 | 0.283 ± 0.043 |
| Control | ΔpHT | ΔpHT + LAB | |
|---|---|---|---|
| a | |||
| 1st day | 2.302 | 2.433 | 2.462 |
| 30th day | 2.532 | 2.942 | 2.925 |
| b | |||
| 1st day | 2.707 | 2.221 | 2.991 |
| 30th day | 3.125 | 0.943 | 3.798 |
| Allometry | |||
| 1st day | A− | A− | A− |
| 30th day | A+ | A− | A+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačić, I.; Burić, P.; Čanak, I.; Žunec, A.; Panić, A.; Kolić, V.; Iveša, N.; Frece, J.; Štifanić, M. Lactiplantibacillus plantarum I Induces Gonad Growth in the Queen Scallop Aequipecten opercularis (Linnaeus, 1758) under Conditions of Climate Change. Fishes 2024, 9, 326. https://doi.org/10.3390/fishes9080326
Kovačić I, Burić P, Čanak I, Žunec A, Panić A, Kolić V, Iveša N, Frece J, Štifanić M. Lactiplantibacillus plantarum I Induces Gonad Growth in the Queen Scallop Aequipecten opercularis (Linnaeus, 1758) under Conditions of Climate Change. Fishes. 2024; 9(8):326. https://doi.org/10.3390/fishes9080326
Chicago/Turabian StyleKovačić, Ines, Petra Burić, Iva Čanak, Ante Žunec, Anamarija Panić, Valentina Kolić, Neven Iveša, Jadranka Frece, and Mauro Štifanić. 2024. "Lactiplantibacillus plantarum I Induces Gonad Growth in the Queen Scallop Aequipecten opercularis (Linnaeus, 1758) under Conditions of Climate Change" Fishes 9, no. 8: 326. https://doi.org/10.3390/fishes9080326
APA StyleKovačić, I., Burić, P., Čanak, I., Žunec, A., Panić, A., Kolić, V., Iveša, N., Frece, J., & Štifanić, M. (2024). Lactiplantibacillus plantarum I Induces Gonad Growth in the Queen Scallop Aequipecten opercularis (Linnaeus, 1758) under Conditions of Climate Change. Fishes, 9(8), 326. https://doi.org/10.3390/fishes9080326








