Nanocomposites Based on Cerium, Lanthanum, and Titanium Oxides Doped with Silver for Biomedical Application
Abstract
:1. Introduction
2. Results
2.1. The Physicochemical Characteristics of Metal Oxide Nanopowders Doped with Silver
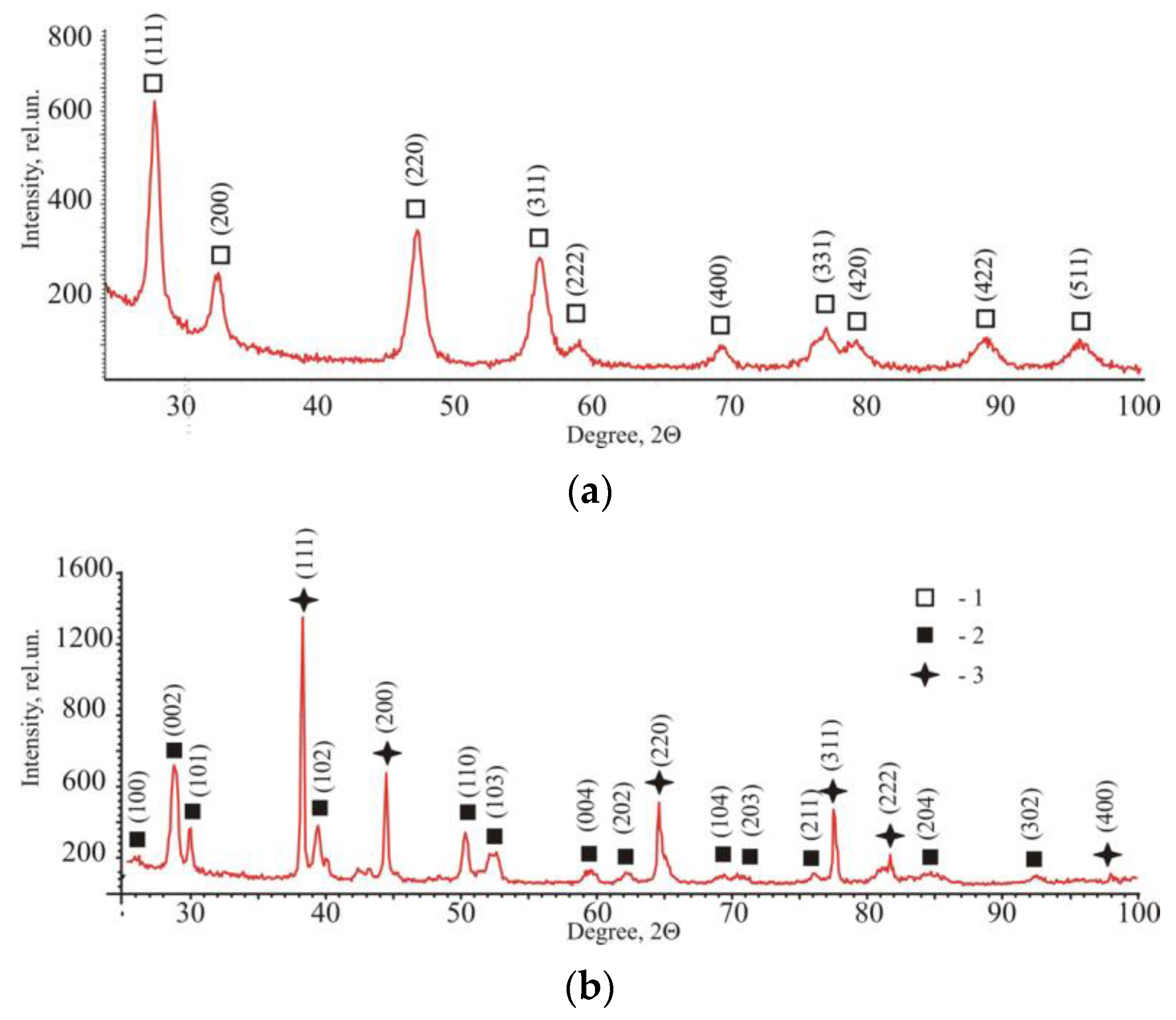
2.1.1. X-ray Diffraction Data
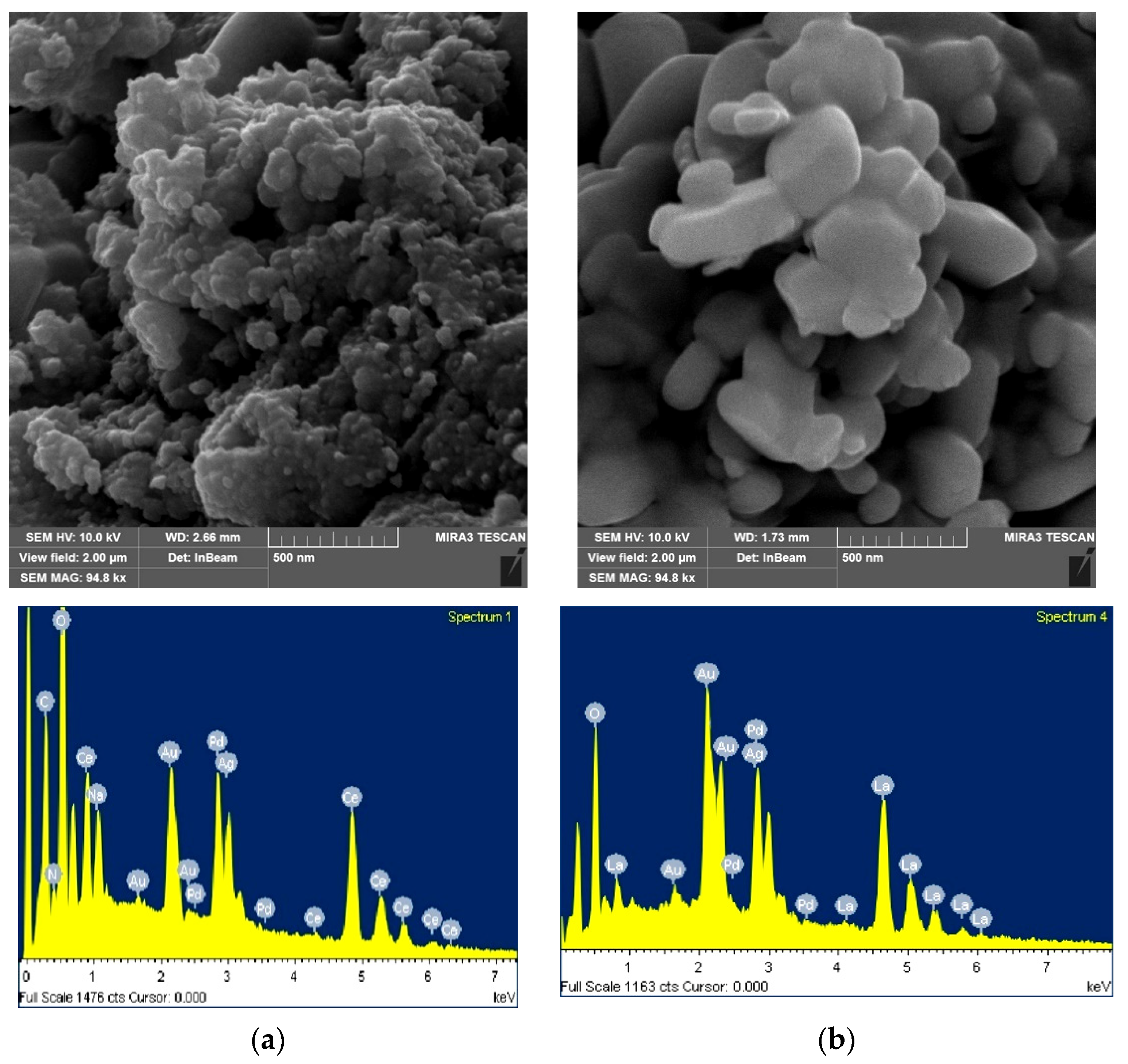
2.1.2. SEM and EDS Analysis
2.1.3. Surface Area Study
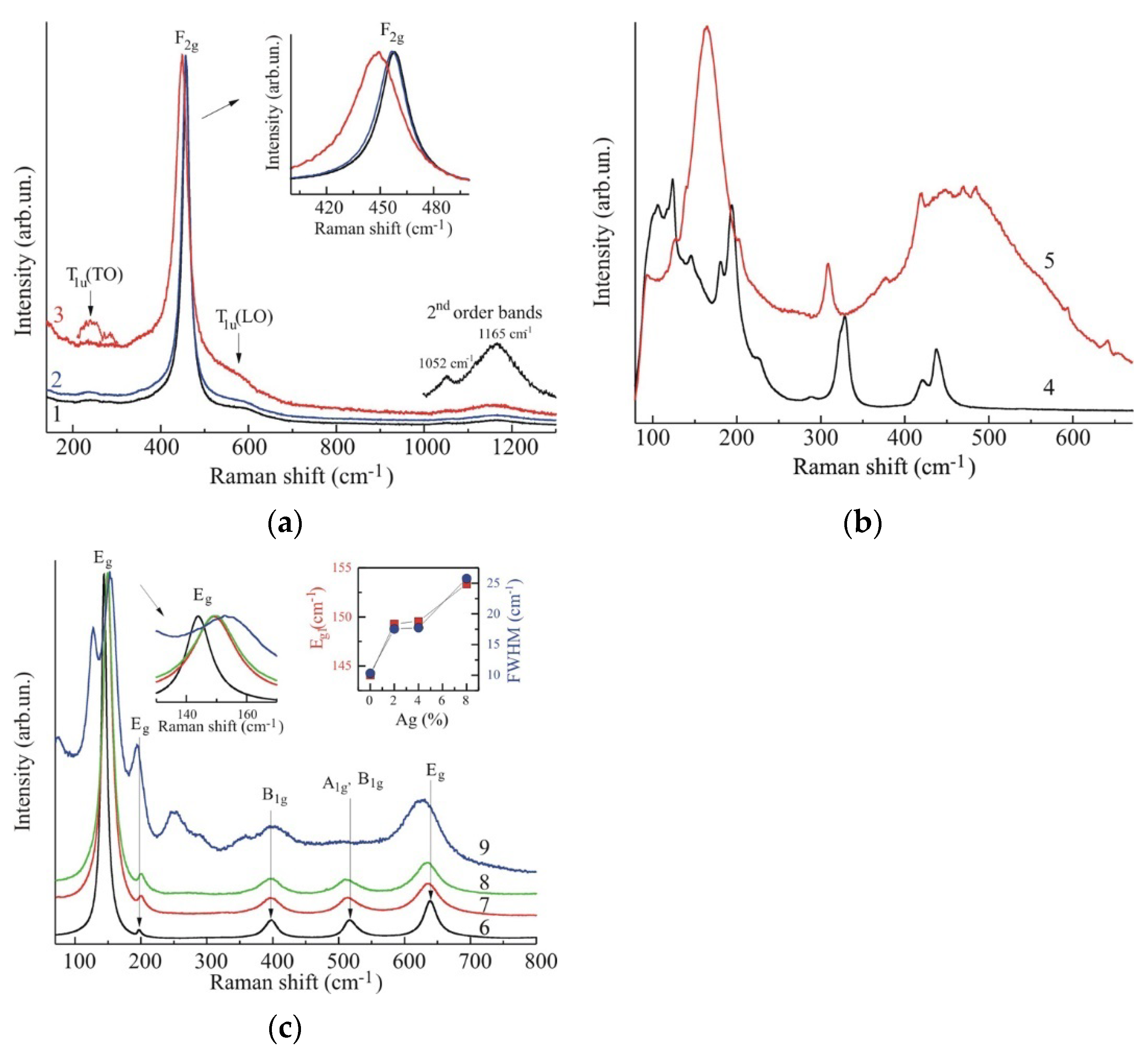
2.1.4. Raman Spectroscopy Study
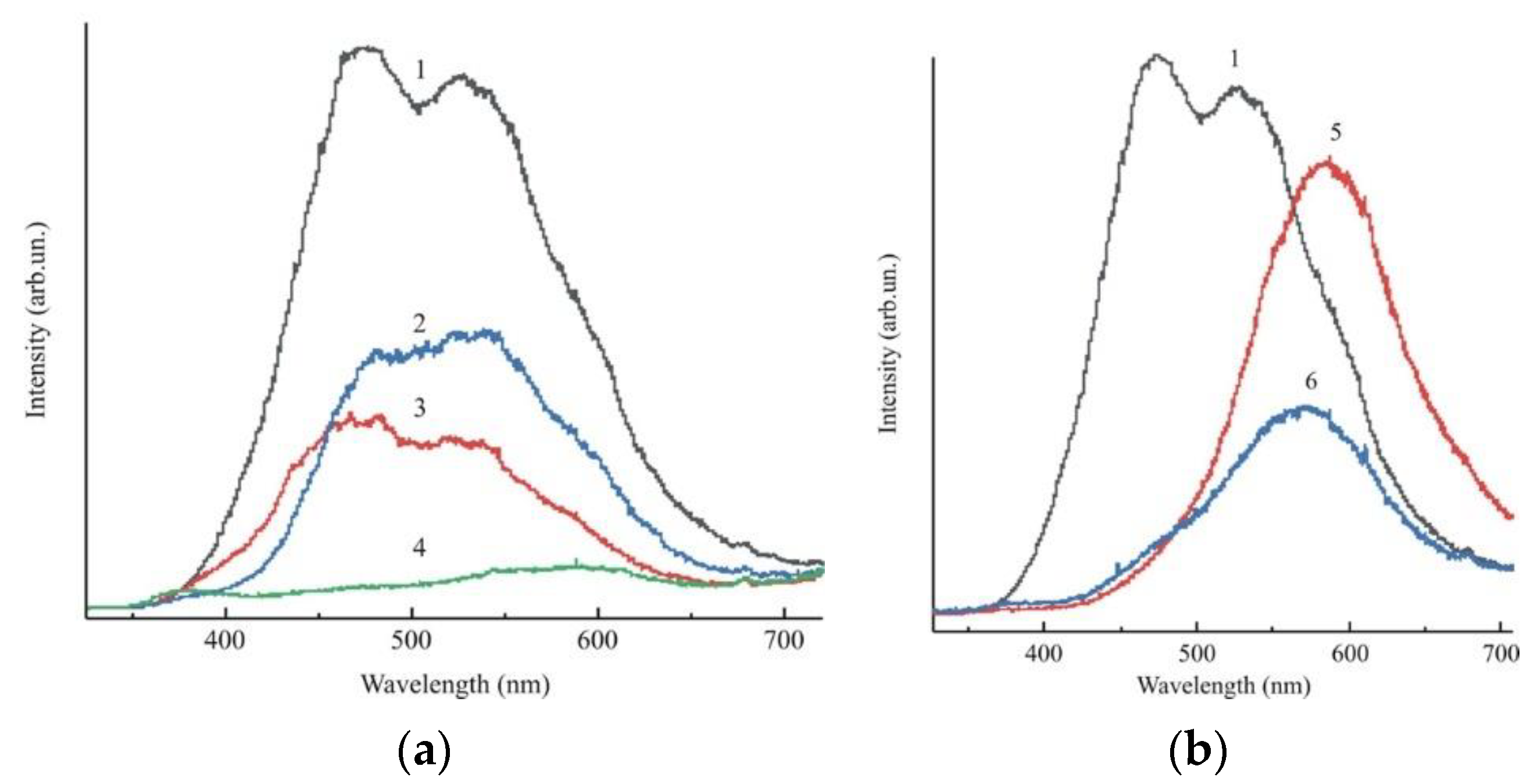
2.1.5. Photoluminescence
2.2. Study of the Biological Activity of CeO2, La2O3, TiO2 and Silver-Doped Nanocomposites
2.2.1. The Effect of CeO2, La2O3, TiO2 and Silver-Doped Nanocomposites on Microorganisms of Different Systematic Groups
2.2.2. The Cytotoxicity Study of Nanocomposites Based on CeO2 and La2O3
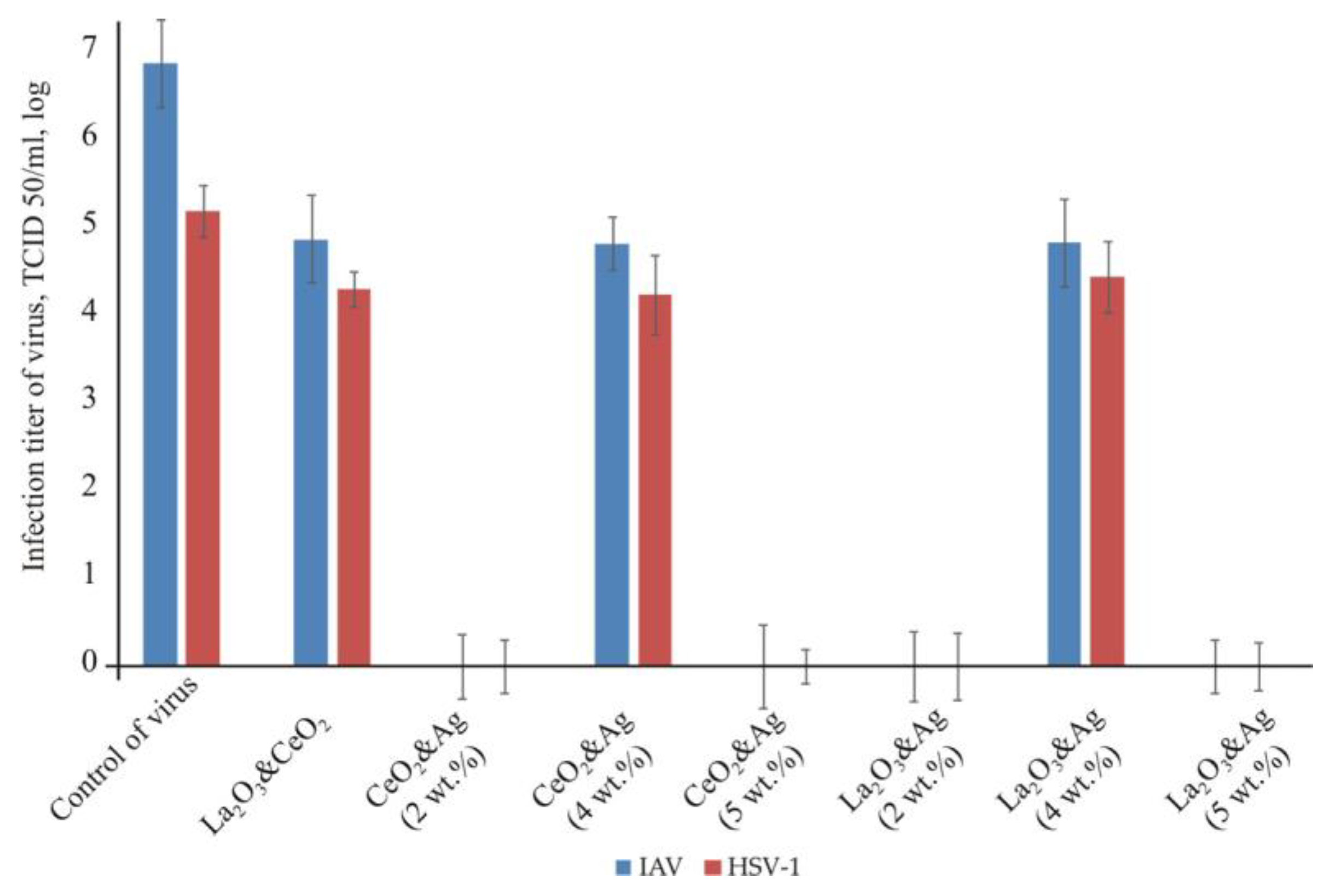
2.2.3. Influence of Silver-Doped Nanocomposites CeO2 and La2O3 on Human Virus Reproduction
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Pang, Y.; Liu, Z.; Vovk, E.I.; Van Bavel, A.P.; Li, S.; Yang, Y. Active Oxygen center in Oxidative Coupling of Methane on La2O3 Catalyst. J. Energ. Chem. 2021, 60, 649–659. [Google Scholar] [CrossRef]
- Kafadaryan, Y.A.; Petrosyan, S.I.; Badalyan, G.R.; Lazaryan, V.G.; Shirinyan, G.H.; Aghamalyan, N.R.; Hovsepyan, R.K.; Semerjian, H.S.; Igityan, A.S.; Kuzanyan, A.M. Structural characteristics of La2O3 thin film grown on LaB6. Int. J. Mod. Phys. Conf. Ser. 2012, 15, 61–66. [Google Scholar] [CrossRef]
- Zhou, X.; Vovk, E.I.; Liu, Y.; Guan, C.; Yang, Y. An in situ Temperature-Dependent Study of La2O3 Reactivation Process. Front. Chem. 2021, 9, 694559. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, B.; Deng, S.; Wang, Y.; Wang, Y. Lanthanum Dioxide Carbonate La2O2CO3 Nanorods as a Sensing Material for Chemoresistive CO2 Gas Sensor. Electrochim. Acta 2014, 127, 355–361. [Google Scholar] [CrossRef]
- Evcin, A.; Arli, E.; Baz, Z.; Esen, R.; Sever, E.G. Characterization of Ag-TiO2 Powders Prepared by Sol-Gel Process. ACTA Phys. Pol. A 2017, 132, 608–611. [Google Scholar] [CrossRef]
- Fudala, A.S.; Salih, W.M.; Alkazaz, F.F. Synthesis different sizes of cerium oxide CeO2 nanoparticles by using different concentrations of precursor via sol–gel method. Mater. Today Proc. 2022, 49, 2786–2792. [Google Scholar] [CrossRef]
- Mazloumi, M.; Zanganeh, S.; Kajbafvala, A.; Shayegh, M.R.; Sadrnezhaad, S.K. Formation of lanthanum hydroxide nanostructures: Effect of NaOH and KOH solvents. IJE Trans. B Appl. 2008, 21, 169–176. [Google Scholar]
- Chang, S.; Li, M.; Hua, Q.; Zhang, L.; Ma, Y.; Ye, B.; Huang, W. Shape-dependent interplay between oxygen vacancies and Ag–CeO2 interaction in Ag/CeO2 catalysts and their influence on the catalytic activity. J. Catal. 2012, 293, 195–204. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.J.; Lee, D.-W.; Choung, J.W.; Kim, C.H.; Lee, K.-Y. Synergistic effect of Cu on a Ag-loaded CeO2 catalyst for soot oxidation with improved generation of active oxygen species and reducibility. Fuel 2020, 275, 117930. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Sanjay, P.; Chinnasamy, E.; Deepa, K.; Madhavan, J.; Senthil, S. Synthesis, Structural, Morphological and Optical Characterization of TiO2 and Nd3+ Doped TiO2 Nanoparticles by Sol Gel Method: A Comparative Study for Photovoltaic Application. IOP Conf. Ser. Mater. Sci. Eng. 2018, 360, 012011. [Google Scholar] [CrossRef] [Green Version]
- Le, T.A.; Kim, Y.; Kim, H.W.; Lee, S.-U.; Kim, J.-R.; Kim, T.-W.; Lee, Y.-J.; Chae, H.-J. RusupportedLanthania-Ceria Composite as an Efficient Catalyst for COx-free H2 Production from Ammonia Decomposition. Appl. Catal. B Environ. 2021, 285, 119831. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360. [Google Scholar] [CrossRef]
- Harada, H.; Onoda, A.; Uematsu, T.; Kuwabata, S.; Hayashi, T. Photocatalytic Properties of TiO2 Composites Immobilized with Gold Nanoparticle Assemblies Using the Streptavidin–Biotin Interaction. Langmuir 2016, 32, 6459–6467. [Google Scholar] [CrossRef] [PubMed]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; el KacemQaiss, A.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviours. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Tavangar, T.; Karimi, M.; Rezakazemi, M.; Reddy, K.R. Textile Waste, Dyes/Inorganic Salts Separation of Cerium Oxide-Loaded Loose Nanofiltration Polyethersulfone Membranes. Chem. Eng. J. 2020, 385, 123787. [Google Scholar]
- Tang, Z.; Zhang, Y.; Xu, Y. A facile and high-yield approach to synthesize one-dimensional CeO2 nanotubes with well-shaped hollow interior as a photocatalyst for degradation of toxic pollutants. RSC Adv. 2011, 1, 1772–1777. [Google Scholar] [CrossRef]
- Ismail, R.A.; Abid, S.A.; Taba, A.A. Preparation and characterization of CeO2@Ag core/shell nanoparticles by pulsed laser ablation in water. Lasers Manuf. Mater. Process. 2019, 6, 126–135. [Google Scholar] [CrossRef]
- Gaiser, B.K.; Fernandes, T.F.; Jepson, M.; Lead, J.R.; Tyler, C.R.; Stone, V. Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ. Health 2009, 8 (Suppl. S1), S2. [Google Scholar] [CrossRef] [Green Version]
- Neal, C.J.; Fox, C.R.; Sakthivel, T.S.; Kumar, U.; Fu, Y.; Drake, C.; Parks, G.D.; Seal, S. Metal-Mediated Nanoscale Cerium Oxide Inactivates Human Coronavirus and Rhinovirus by Surface Disruption. ACS Nano 2021, 15, 14544–14556. [Google Scholar] [CrossRef]
- Lavrynenko, O.M.; Zahornyi, M.M.; Paineau, E.; Pavlenko, O.Y.; Tyshenko, N.I.; Bykov, O.I. Characteristic of TiO2&Ag0 nanocomposites formed via transformation of metatitanic acid and titanium (IV) isopropoxide. Mater. Today Proc. 2022, 62, 7664–7669. [Google Scholar] [CrossRef]
- Abid, S.A. Biological Applications of Cerium Oxide-Silver-Core-Shell Nanoparticles Prepared by Laser Ablation in Liquid. Master’s Thesis, Al-Mustansiriya University, Baghdad, Iraq, 2020. [Google Scholar] [CrossRef]
- Zimou, J.; Nouneh, K.; Hsissou, R.; El-Habib, A.; Gana, L.E.; Talbi, A.; Addou, M. Structural, morphological, optical, and electrochemical properties of Co-doped CeO2 thin films. Mater. Sci. Semicond. Process. 2021, 135, 106049. [Google Scholar] [CrossRef]
- Villa-Aleman, E.; Houk, A.L.; Dick, D.D.; Murph, S.E.H. Hyper Raman spectroscopy of CeO2. J. Raman Spectrosc. 2020, 51, 1260–1263. [Google Scholar] [CrossRef]
- Wang, P.; Meng, F.; Gao, C.; Xie, W.; Wang, J.; Li, A. Structural, morphological and optical characteristics of fusiform Co-doped CeO2 via a facile hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 29, 11482–11488. [Google Scholar] [CrossRef]
- Kumar, P.; Ahmad, B.; Chand, F.; Asokan, K. Magnetic and electronic structures of Co ion implanted CeO2 thin films. Appl. Surf. Sci. 2018, 452, 217–222. [Google Scholar] [CrossRef]
- Zahornyi, M.M.; Tyschenko, N.I.; Lobunets, T.F.; Kolomys, O.F.; Strelchuk, V.V.; Naumenko, K.S.; Biliavska, L.O.; Zahorodnia, S.D.; Lavrynenko, O.M.; Ievtushenko, A.I. The Ag Influence on the Surface States of TiO2, Optical Activity and Its Cytotoxicity. J. Nano-Electron. Phys. 2021, 13, 06009. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178. [Google Scholar] [CrossRef]
- Bilel, C.; Jbeli, R.; Jemaa, I.B.; Boukhachem, A.; Saadallah, F.; Amlouk, M.; Ezzaouïa, H. Physical investigations on annealed structure Cu/La2O3 for photocatalytic application under sunlight. J. Mater. Sci. Mater. Electron. 2020, 31, 7398–7410. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scaterring study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Georgescu, D.; Baia, L.; Ersen, O.; Baia, M.; Simon, S. Experimental assessment of the phonon confinement in TiO2 anatase nanocrystallites by Raman spectroscopy. J. Raman. Spect. 2012, 43, 876–883. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Delogu, L.G. Toward nanotechnology-enabled approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral Potential of Nanoparticles—Can Nanoparticles Fight Against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, K.; Ma, J.; Gao, F. Cerium oxide nanoparticles in cancer. OncoTargetsTher 2014, 7, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110924. [Google Scholar] [CrossRef]
- Malleshappa, J.; Nagabhushana, H.; Sharma, S.C.; Sharma, Y.S.; Surendra, B.S. Leucas aspera mediated multifunctional CeO2 nanoparticles: Structural, photoluminescent, photocatalytic and antibacterial properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 452–462. [Google Scholar] [CrossRef]
- Lavrynenko, O.M.; Pavlenko, O.Y.; Zahornyi, M.N.; Korichev, S.F. Morphology, phase and chemical composition of the nanostructures formed in the systems containing lanthanum, cerium, and silver. Chem. Phys. Technol. Surf. 2021, 12, 382–392. [Google Scholar] [CrossRef]
- Lavrynenko, O.M.; Zahornyi, M.M.; Pavlenko, O.Y.; Tyschenko, N.I.; Bykov, O.I. Comparative Analysis of CeO2&Ag0 and TiO2&Ag0 Nanoparticles Formed under the Co-Precipitation. In Proceedings of the IEEE 11th International Conference on Nanomaterials: Applications & Properties (NAP-2021), Odessa, Ukraine, 5–11 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Khan, S.; Ansari, A.A.; Rolfo, C.; Coelho, A.; Abdulla, M.; Al-Khayal, K.; Ahmad, R. Evaluation of in vitro cytotoxicity, biocompatibility, and changes in the expression of apoptosis regulatory proteins induced by cerium oxide nanocrystals. Sci. Technol. Adv. Mater. 2017, 18, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Lin, T.; Yang, C.; Wang, K.; Lin, L.; Li, C. Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J. Antimicrob. Chem. 2004, 53, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Kusmierek, E. A CeO2 Semiconductor as a Photocatalytic and Photoelectrocatalytic Material for the Remediation of Pollutants in Industrial Wastewater: A Review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- Kohn, L.K.; Foglio, M.A.; Rodrigues, R.A.; Sousa, I.M.; De, O.; Martini, M.C.; Padilla, M.A.; DeLima Neto, D.F.; Arns, C.W. In-Vitro Antiviral Activities of Extracts of Plants of the Brazilian Cerrado against the Avian Metapneumovirus (aMPV). Braz. J. Poult. Sci. 2015, 17, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Dudnikova, Y.N.; Zykov, I.Y.; Fedorova, N.I.; Ismagilov, Z.R. Adsorption method for determining the texture characteristics of Kuzbass fossil coals of the metamorphism series. J. Phys. Conf. Ser. 2021, 1749, 012019. [Google Scholar] [CrossRef]

| Type of Nanoparticles | The Influence of Nanoparticles on the Viability and Growth Processes of Bacterial Cells | |
|---|---|---|
| E. coli | Bacillus sp. | |
| Control (without particles) | +++ | +++ |
| CeO2 | + | + |
| La2O3 | + | ++ |
| TiO2 | ++ | +++ |
| CeO2–Ag (4 wt.%) | – | – |
| La2O3–Ag (4 wt.%) | – | ++ |
| TiO2–Ag (4 wt.%) | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrynenko, O.M.; Zahornyi, M.M.; Vember, V.V.; Pavlenko, O.Y.; Lobunets, T.F.; Kolomys, O.F.; Povnitsa, O.Y.; Artiukh, L.O.; Naumenko, K.S.; Zahorodnia, S.D.; et al. Nanocomposites Based on Cerium, Lanthanum, and Titanium Oxides Doped with Silver for Biomedical Application. Condens. Matter 2022, 7, 45. https://doi.org/10.3390/condmat7030045
Lavrynenko OM, Zahornyi MM, Vember VV, Pavlenko OY, Lobunets TF, Kolomys OF, Povnitsa OY, Artiukh LO, Naumenko KS, Zahorodnia SD, et al. Nanocomposites Based on Cerium, Lanthanum, and Titanium Oxides Doped with Silver for Biomedical Application. Condensed Matter. 2022; 7(3):45. https://doi.org/10.3390/condmat7030045
Chicago/Turabian StyleLavrynenko, Olena Mykolaivna, Maksym Mykytovych Zahornyi, Valeriia Volodymyrivna Vember, Olesia Yuriivna Pavlenko, Tatyana Fedorovna Lobunets, Olexandr Fedorovych Kolomys, Olga Yurievna Povnitsa, Luibov Oleksievna Artiukh, Krystyna Sergiivna Naumenko, Svitlana Dmitrievna Zahorodnia, and et al. 2022. "Nanocomposites Based on Cerium, Lanthanum, and Titanium Oxides Doped with Silver for Biomedical Application" Condensed Matter 7, no. 3: 45. https://doi.org/10.3390/condmat7030045
APA StyleLavrynenko, O. M., Zahornyi, M. M., Vember, V. V., Pavlenko, O. Y., Lobunets, T. F., Kolomys, O. F., Povnitsa, O. Y., Artiukh, L. O., Naumenko, K. S., Zahorodnia, S. D., & Garmasheva, I. L. (2022). Nanocomposites Based on Cerium, Lanthanum, and Titanium Oxides Doped with Silver for Biomedical Application. Condensed Matter, 7(3), 45. https://doi.org/10.3390/condmat7030045






