Design Principles for Laser-Printed Macrofluidics
Abstract
1. Introduction
Applications
- Biomedical Research: The ability to control fluid flow, temperature, and agitation makes laser-printed macrofluidics ideal for culturing cells, conducting drug screening assays, and studying biochemical reactions.
- Chemical Engineering: Macrofluidic devices can be used for process monitoring and optimization in chemical manufacturing processes, including mixing, reaction kinetics, and separation techniques.
- Environmental Monitoring: Laser-printed macrofluidics enable on-site analysis of environmental samples, such as water quality testing and air pollution monitoring.
2. Materials and Methods
2.1. Fabrication Method
2.2. Fabrication and Production Process
- Design on CAD software.
- Visual inspection of the PAPE film.
- Introduction of the material into the laser cutter.
- The fluidic circuit design is transferred to the thermoadhesive film using a laser printing system by welding and cutting.
- The laser selectively heats the film, causing it to adhere to the substrate and create fluidic channels, ports, chambers, and other fluidic elements for the process it was designed for.
- Fitting the printed fluidic on a vertical holder or other vertical arrangement.
- Introducing liquids and gasses, applying actuation if needed, and monitoring the experiment with or without sensors.
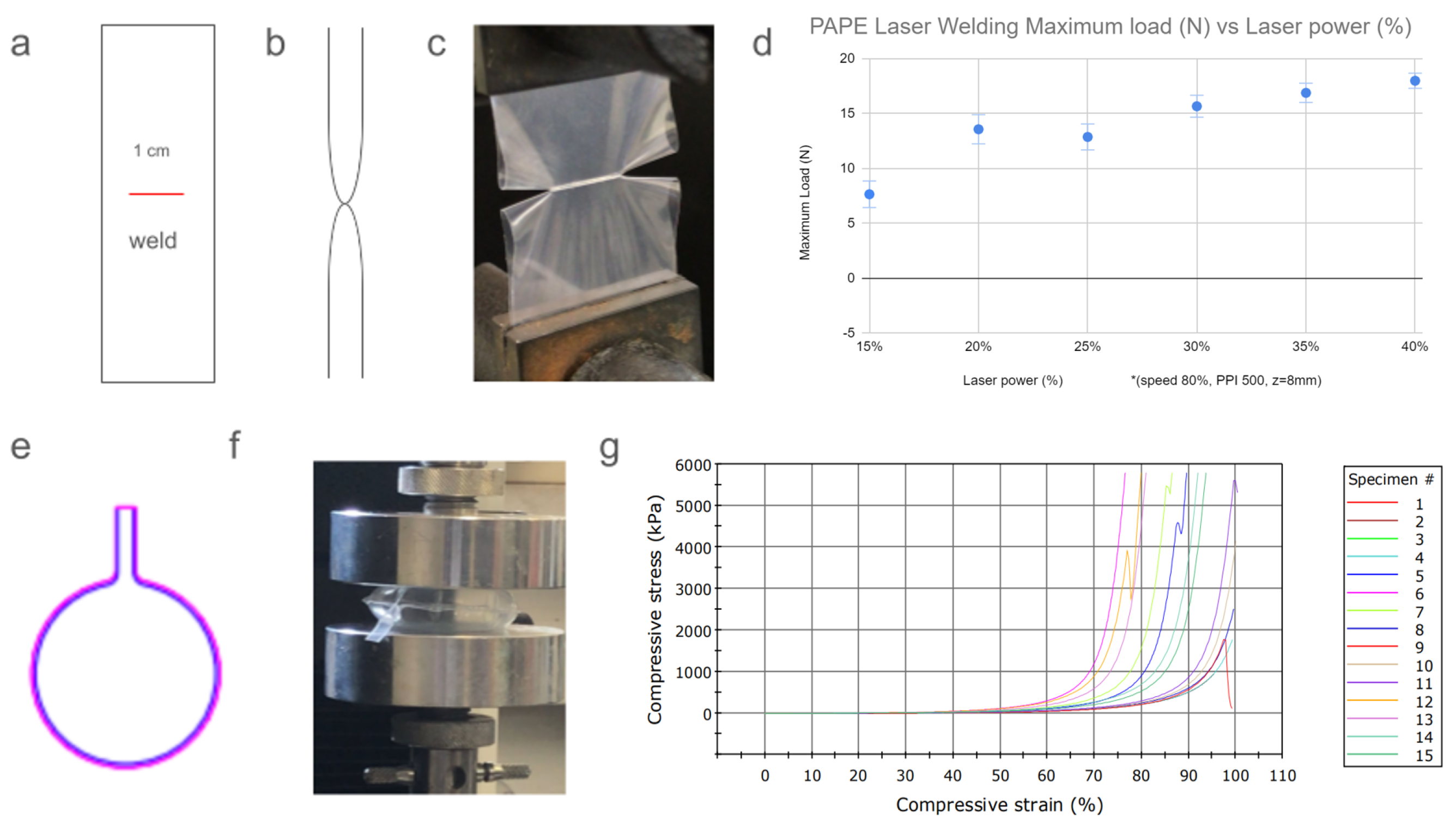
2.3. Laser Welding Validation Assay
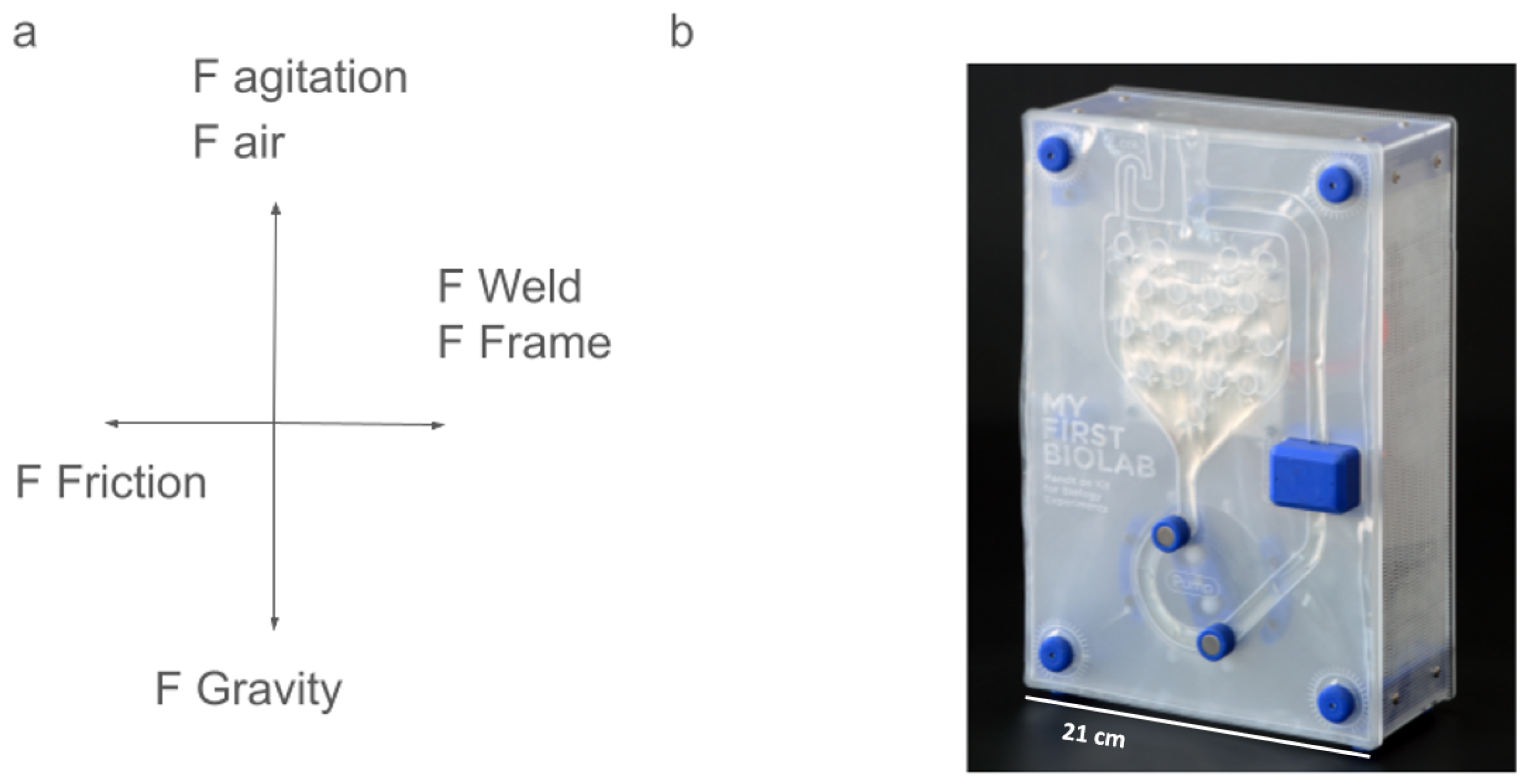
2.4. Design Principles
2.4.1. Fluidic Channel Layout
2.4.2. Introduction of Gasses, Liquids, or Solids
2.4.3. Integration of Sensors and Actuators
2.4.4. Control and Monitoring
3. Results
3.1. Laser Welding Validation Assay
3.2. Sterility
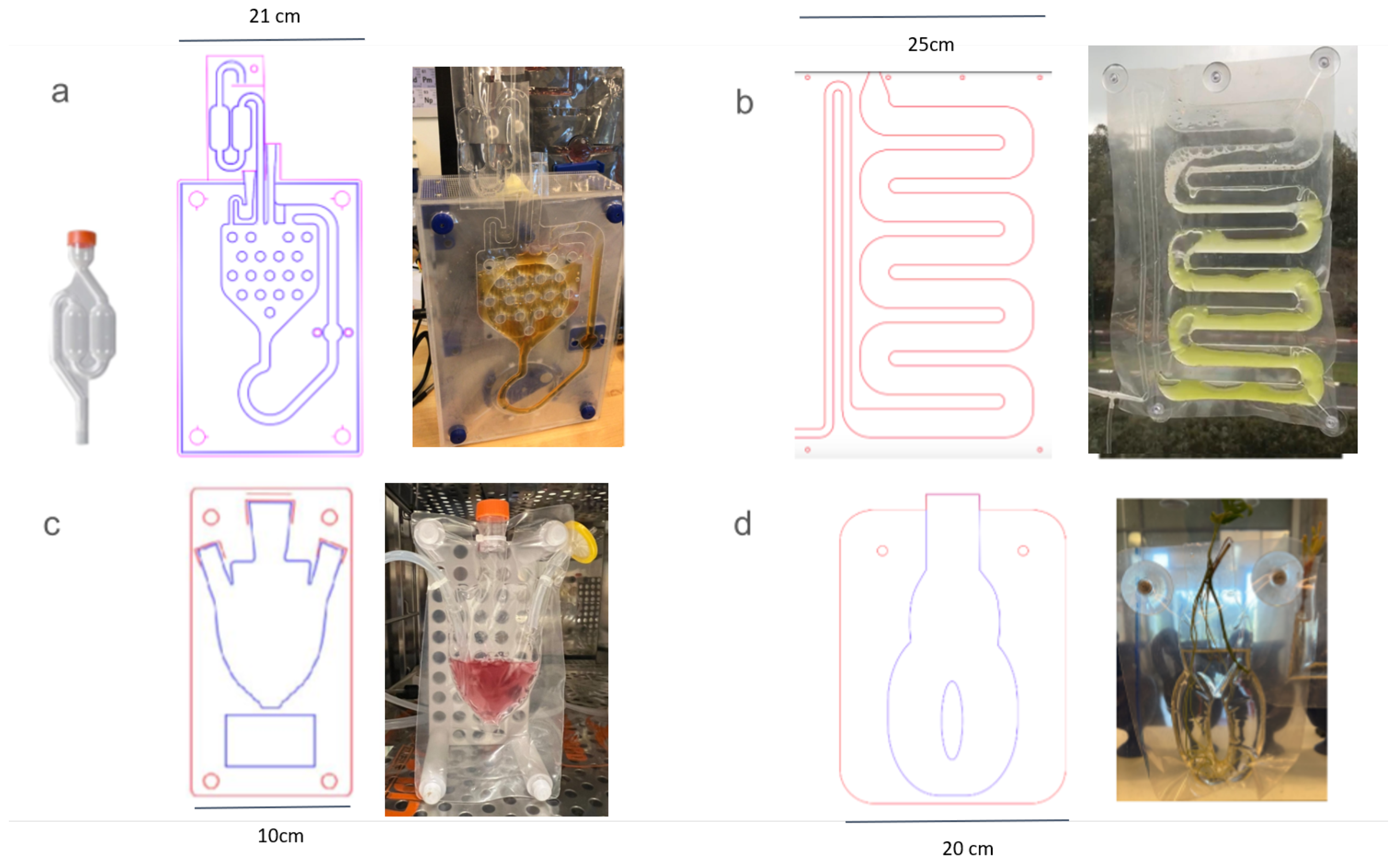
3.3. Designs for Cultivation of All Kingdoms of Life
3.4. Microorganisms in Suspension
3.5. Animal Cell Lines
3.6. Plants
3.7. Design Mimicry for Existing Macrofluidic Infrastructure
3.8. Design for Bioprocess—Media Replacement and Perfusion through a Scaffold
4. STEM Education
4.1. Algae Oxygen Monitoring
4.2. Inquiry-Based Learning for Bacterial Fermentation
4.3. Experiment in a Bag
5. Discussion
6. Conclusions
7. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAPE | Polyethylene–Polyamide |
| MSUB | Macrofluidic Single-Use Bioreactor |
References
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Mohan, J.M.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Emerging trends in miniaturized and microfluidic electrochemical sensing platforms. Curr. Opin. Electrochem. 2022, 33, 100930. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Wu, M.; Ren, W.; Jensen, M.A.; Davis, R.W.; Hesselink, L. Controlled Transport of Individual Microparticles Using Dielectrophoresis. Langmuir 2023, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, M.; Kothandan, R.; Govindarajan, D.K.; Meganathan, Y.; Kandaswamy, K. Active microfluidic systems for cell sorting and separation. Curr. Opin. Biomed. Eng. 2020, 13, 60–68. [Google Scholar] [CrossRef]
- Truskey, G.A.; Fu, J. The future of Biomedical Engineering: Bioengineering of Organoids and Tissue Development. Curr. Opin. Biomed. Eng. 2020, 13, A1–A2. [Google Scholar] [CrossRef]
- Li, J. Current commercialization status of electrowetting-on-dielectric (EWOD) digital microfluidics. Lab Chip 2020, 20, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Goller, C. Library Preparation 1–Miro and VolTRAX; Portable Genome Sequencing; Automation for Library Prep: San Diego, CA, USA, 2024. [Google Scholar]
- Matos, R.S.; Maselli, D.; McVey, J.H.; Heiss, C.; Campagnolo, P. 3D printed bioreactor enabling the pulsatile culture of native and angioplastied large arteries. Front. Cardiovasc. Med. 2022, 9, 864580. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, B.M.; Dikshit, V.; Zhang, Y. 3D-printed bioreactors for in vitro modeling and analysis. Int. J. Bioprint. 2020, 6, 267. [Google Scholar] [CrossRef]
- Hoyle, H.; Smith, L.; Williams, R.; Przyborski, S. Applications of novel bioreactor technology to enhance the viability and function of cultured cells and tissues. Interface Focus 2020, 10, 20190090. [Google Scholar] [CrossRef]
- Saunders, S.K.; Cole, S.Y.; Acuna Sierra, V.; Bracamonte, J.H.; Toldo, S.; Soares, J.S. Evaluation of perfusion-driven cell seeding of small diameter engineered tissue vascular grafts with a custom-designed seed-and-culture bioreactor. PLoS ONE 2022, 17, e0269499. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.F.; Thanarak, J.; Pontin, M.; Green, N.H.; Damian, D.D. Design and development of a robotic bioreactor for in vitro tissue engineering. In Proceedings of the 2021 IEEE International Conference on Robotics and Automation (ICRA), Xi’an, China, 30 May–5 June 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 12428–12434. [Google Scholar]
- Hoyle, H.; Stenger, C.; Przyborski, S. Design considerations of benchtop fluid flow bioreactors for bio-engineered tissue equivalents in vitro. Biomater. Biosyst. 2022, 8, 100063. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Kreidl, E.; Bertschinger, M.; Vetsch, P. Benchtop bioreactors in mammalian cell culture: Overview and guidelines. In Bioreactors in Stem Cell Biology: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–15. [Google Scholar]
- Reina-Mahecha, A.; Beers, M.J.; van der Veen, H.C.; Zuhorn, I.S.; van Kooten, T.G.; Sharma, P.K. A Review of the Role of Bioreactors for iPSCs-Based Tissue-Engineered Articular Cartilage. Tissue Eng. Regen. Med. 2023, 20, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Todros, S.; Spadoni, S.; Maghin, E.; Piccoli, M.; Pavan, P.G. A novel bioreactor for the mechanical stimulation of clinically relevant scaffolds for muscle tissue engineering purposes. Processes 2021, 9, 474. [Google Scholar] [CrossRef]
- Bender, R.J.; Askinas, C.; Vernice, N.A.; Dong, X.; Harris, J.; Shih, S.; Spector, J.A. Perfuse and Reuse: A Low-Cost Three-Dimensional-Printed Perfusion Bioreactor for Tissue Engineering. Tissue Eng. Part C Methods 2022, 28, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.C.; Lynch, T.J.; Ahlers, B.A.; Ievlev, V.; Engelhardt, J.F.; Parekh, K.R. A novel bioreactor for reconstitution of the epithelium and submucosal glands in decellularized ferret tracheas. Cells 2022, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, C.; Maschke, R.W.; Pörtner, R.; Eibl, D. An overview of drive systems and sealing types in stirred bioreactors used in biotechnological processes. Appl. Microbiol. Biotechnol. 2021, 105, 2225–2242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harrison, S.T.; Tai, S.L. Advances in bioreactor systems for the production of biologicals in mammalian cells. ChemBioEng Rev. 2022, 9, 42–62. [Google Scholar] [CrossRef]
- Nogueira, D.E.; Cabral, J.M.; Rodrigues, C.A. Single-use bioreactors for human pluripotent and adult stem cells: Towards regenerative medicine applications. Bioengineering 2021, 8, 68. [Google Scholar] [CrossRef]
- Nikita, S.; Mishra, S.; Gupta, K.; Runkana, V.; Gomes, J.; Rathore, A.S. Advances in bioreactor control for production of biotherapeutic products. Biotechnol. Bioeng. 2023, 120, 1189–1214. [Google Scholar] [CrossRef]
- Nielsen, M.; Meyer, A.; Arnau, J. The Next Food Revolution Is Here: Recombinant Microbial Production of Milk and Egg Proteins by Precision Fermentation. Annu. Rev. Food Sci. Technol. 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Maguire, D.; Coughlan, N.E.; Jansen, M.A.; Byrne, E.P.; Kavousi, F. Where engineering meets biology: The Computational Fluid Dynamic analysis of a stacked duckweed bioreactor. Aquac. Eng. 2024, 104, 102375. [Google Scholar] [CrossRef]
- Garcia-Aponte, O.F.; Herwig, C.; Kozma, B. Lymphocyte expansion in bioreactors: Upgrading adoptive cell therapy. J. Biol. Eng. 2021, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB—Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S. How DIY technologies are democratizing science. Nature 2020, 587, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Aidelberg, G.; Aronoff, R.; Eliseeva, T.; Quero, F.J.; Vielfaure, H.; Codyre, M.; Hadasch, K.; Lindner, A.B. Corona Detective: A simple, scalable, and robust SARS-CoV-2 detection method based on reverse transcription loop-mediated isothermal amplification. J. Biomol. Tech. JBT 2021, 32, 89. [Google Scholar] [CrossRef] [PubMed]
- Cerda Rojas, A.; Aravena, A.; Arce, A.; Zapata, V.; Araya, W.; Gallardo, D.; Aviles, J.; Quero, F.; Nunez, I.; Matute, T.; et al. Open Educational Resources for distributed hands-on teaching in molecular biology. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wenzel, T. Open hardware: From DIY trend to global transformation in access to laboratory equipment. PLoS Biol. 2023, 21, e3001931. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Sicher, E.; Ayala-Garcia, C.; Sanchez-Fayos, I.M.; Conterno, L.; Ugur Yavuz, S. Innocell Bioreactor: An Open-Source Development to Produce Biomaterials for Food and Packaging Based on Fermentation Processes. Fermentation 2023, 9, 915. [Google Scholar] [CrossRef]
- Cohen, N.; Sicher, E.; Merino, I.; Yavuz, S.U. An Open-Source Bioreactor Enhancing Microbial Cellulose Production and Novel Sustainable substances. In Sustainable Design and Manufacturing, Proceedings of the 8th International Conference on Sustainable Design and Manufacturing (KES-SDM 2021), Split, Croatia, 15–17 September 2022; Springer: Berlin/Heidelberg, Germany, 2022; pp. 77–86. [Google Scholar]
- Gome, G.; Fein, Y.; Waksberg, J.; Maayan, Y.; Grishko, A.; Wald, I.Y.; Zuckerman, O. My First Biolab: A System for Hands-On Biology Experiments. In Proceedings of the Extended Abstracts of the 2019 CHI Conference on Human Factors in Computing Systems, Glasgow, UK, 4–9 May 2019; pp. 1–6. [Google Scholar]
- Gome, G.; Chak, B.; Tawil, S.; Shpatz, D.; Giron, J.; Brajzblat, I.; Weizman, C.; Grishko, A.; Schlesinger, S.; Shoseyov, O. Cultivation of Bovine Mesenchymal Stem Cells on Plant-Based Scaffolds in a Macrofluidic Single-Use Bioreactor for Cultured Meat. Foods 2024, 13, 1361. [Google Scholar] [CrossRef]
- Fein, Y.; Gome, G.; Zuckerman, O.; Erel, H. My first biolab: An inquiry-based learning system for microbiology exploration. In Proceedings of the 2020 ACM Interaction Design and Children Conference: Extended Abstracts, London, UK, 17–24 June 2020; pp. 292–295. [Google Scholar]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, R.; Giese, C. An overview on bioreactor design, prototyping and process control for reproducible three-dimensional tissue culture. In Drug Testing In Vitro: Breakthroughs and Trends in Cell Culture Technology; Wiley: Hoboken, NJ, USA, 2007; pp. 53–78. [Google Scholar]
- Haigh, J.; Schmidt, S.; Vicalvi, J.; Winterhalter, C. 17th Annual Report and Survey on Biopharmaceutical Manufacturing Capacity and Production; BioPlan Associates, Inc.: Rockville, MD, USA, 2020. [Google Scholar]
- Mahal, H.; Branton, H.; Farid, S.S. End-to-end continuous bioprocessing: Impact on facility design, cost of goods, and cost of development for monoclonal antibodies. Biotechnol. Bioeng. 2021, 118, 3468–3485. [Google Scholar] [CrossRef] [PubMed]
- Zohar, B.; Blinder, Y.; Epshtein, M.; Szklanny, A.A.; Kaplan, B.; Korin, N.; Mooney, D.J.; Levenberg, S. Multi-flow channel bioreactor enables real-time monitoring of cellular dynamics in 3D engineered tissue. Commun. Biol. 2019, 2, 158. [Google Scholar] [CrossRef] [PubMed]
- Gabetti, S.; Masante, B.; Cochis, A.; Putame, G.; Sanginario, A.; Armando, I.; Fiume, E.; Scalia, A.C.; Daou, F.; Baino, F.; et al. An automated 3D-printed perfusion bioreactor combinable with pulsed electromagnetic field stimulators for bone tissue investigations. Sci. Rep. 2022, 12, 13859. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, N.M.; Junne, S. Design, Applications, and Development of Single-Use Bioreactors. In Bioreactors; John Wiley Sons, Ltd.: Hoboken, NJ, USA, 2016; Chapter 9; pp. 261–294. [Google Scholar] [CrossRef]
- Milab. My First Biolab Video. YouTube Video. 2019. Available online: https://www.youtube.com/watch?v=1hQybiffh2Y (accessed on 4 April 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gome, G.; Benny, O.; Shoseyov, O.; Giron, J. Design Principles for Laser-Printed Macrofluidics. Inventions 2024, 9, 68. https://doi.org/10.3390/inventions9040068
Gome G, Benny O, Shoseyov O, Giron J. Design Principles for Laser-Printed Macrofluidics. Inventions. 2024; 9(4):68. https://doi.org/10.3390/inventions9040068
Chicago/Turabian StyleGome, Gilad, Ofra Benny, Oded Shoseyov, and Jonathan Giron. 2024. "Design Principles for Laser-Printed Macrofluidics" Inventions 9, no. 4: 68. https://doi.org/10.3390/inventions9040068
APA StyleGome, G., Benny, O., Shoseyov, O., & Giron, J. (2024). Design Principles for Laser-Printed Macrofluidics. Inventions, 9(4), 68. https://doi.org/10.3390/inventions9040068






